Review Article - Imaging in Medicine (2013) Volume 5, Issue 5
Diffusion tensor MRI as a biomarker in axonal and myelin damage
Wint Yan Aung1, Soe Mar2 and Tammie LS Benzinger*1,3
1Department of Radiology, Washington University, School of Medicine, 510 South Kingshighway Boulevard, St Louis, MO 63110, USA
2Department of Pediatric& Developmental Neurology, Washington University School of Medicine, St Louis, MO, USA
3Department of Neurological Surgery, Washington University School of Medicine, St Louis, MO, USA
Abstract
Keywords
acute and chronic CNS disorders; acute disseminated encephalomyelitis; Alzheimer’s disease; axial diffusivity; axonal injury; diffusion tensor imaging; leukodystrophy; MRI; multiple sclerosis; myelin damage; radial diffusivity
Principles of diffusion tensor imaging
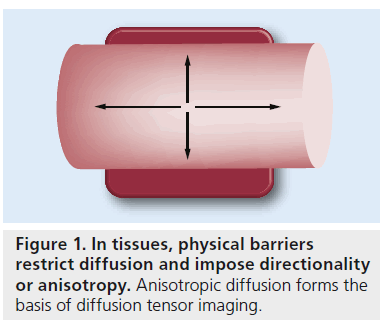
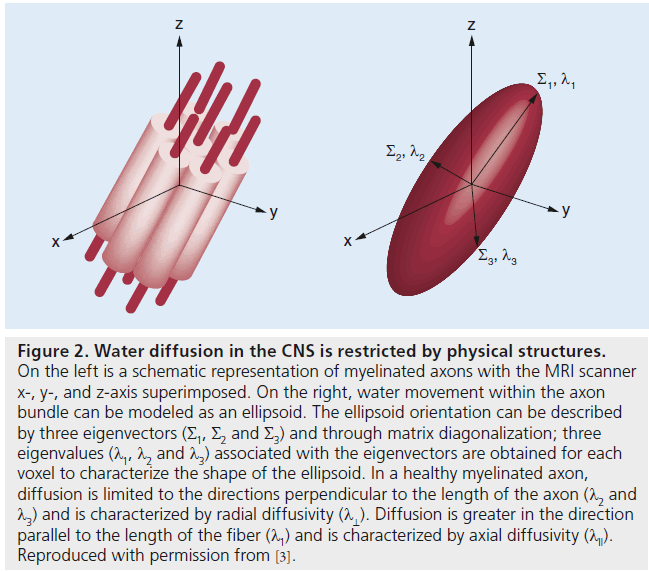
Diffusion tensor imaging (DTI) is a noninvasive, quantitative MRI technique that measures the rate and direction of movement of water molecules within tissues. In the CNS, axonal tracts and myelin present physical barriers that impose directionality or anisotropy on water diffusion (Figure 1) [1,2]. Applying magnetic field gradients allows the measurement of the rate and direction of the movement of water molecules in the direction of the magnetic field. Using the basis of diffusion, we can construct a 3D directional architecture of axon fibers and myelin in the CNS (Figure 2).
Diffusion is limited along certain directions in each image voxel. Diffusion in a voxel can be modeled as a 3D ellipsoid with a 3 × 3 matrix or the diffusion tensor (D), which can also be characterized by three eigenvectors (Σ1, Σ2 and Σ3).
Multiple summary parameters can be obtained from the diffusion tensor represented by an ellipsoid. The ellipsoid volume describes the mean diffusivity, which is a measure of the overall mean displacement of water molecules. The ellipsoid eccentricity describes fractional anisotropy (FA), which characterizes the fraction of molecular displacements that can be attributed to the orientation of the axon fiber (anisotropy). FA values vary between 0 for isotropic diffusion (diffusion in a sphere where molecular movement is equally restricted in all directions) and 1 (diffusion with infinite anisotropy) [3]. Another important parameter is the apparent diffusion coefficient (ADC), which takes into account the fact that restriction barriers, such as myelin and other cellular structures, may interfere with diffusion and, therefore, diffusion rates are only apparent [1,3,4].
These parameters can be used to deduce the general organization of fiber tracts. Often, disorganization of axon fiber architecture leads to unrestricted or isotropic diffusion and, as a result, reduced FA values. A low ADC value often indicates a well-organized structure while a high ADC indicates disorganization in structure. These anisotropy parameters can highlight interruption of diffusion along the axon fiber even before structural changes are obvious on other imaging modalities, making DTI a potentially useful tool for clinical application.
However, summary parameters allow for a simplified expression of water diffusion and are not specific to axon and myelin pathology [5,6]. A reduced anisotropy and high ADC do not allow us to discriminate between axonal and myelin damage. However, the directional architecture of axons and myelin in the CNS provides additional parameters that characterize water diffusion to the axonal tracts and underlying tissue structures. Through matrix diagonalization (a mathematical operation that allows orthogonal directions to coincide with the main diffusion directions; i.e., along which diffusion is the fastest), three eigenvalues (l1, l2 and l3), each associated with an eigenvector, are obtained for each voxel [7,8]. Eigenvalues can be used to characterize directional diffusivity describing water movement parallel to (axial diffusivity), and perpendicular to (radial diffusivity), axonal tracts.
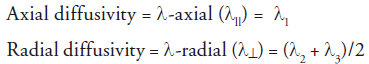
λ11 is the largest eigenvalue and is thought to characterize diffusion along the long axis of the axonal tract. Diffusion is greater in the direction parallel to the length of the fiber. λ⊥ is the average of the shorter two eigenvalues (λ2 + λ3) and is thought to characterize diffusion perpendicular to the long axis of fiber tract where diffusion is limited. Unlike summary parameters, such as ADC and FA, directional diffusivity takes anatomic details into account. Hence, it provides specific structural details regarding the anatomical status of axons and myelin.
Figure 2. Water diffusion in the CNS is restricted by physical structures. On the left is a schematic representation of myelinated axons with the MRI scanner x-, y-, and z-axis superimposed. On the right, water movement within the axon bundle can be modeled as an ellipsoid. The ellipsoid orientation can be described by three eigenvectors (Σ1, Σ2 and Σ3) and through matrix diagonalization; three eigenvalues (Λ1, Λ2 and Λ3) associated with the eigenvectors are obtained for each voxel to characterize the shape of the ellipsoid. In a healthy myelinated axon, diffusion is limited to the directions perpendicular to the length of the axon (l2 and l3) and is characterized by radial diffusivity (Λ⊥). Diffusion is greater in the direction parallel to the length of the fiber (Lambda1) and is characterized by axial diffusivity (Λ11). Reproduced with permission from [3].
DTI versus MRI in demyelinating diseases
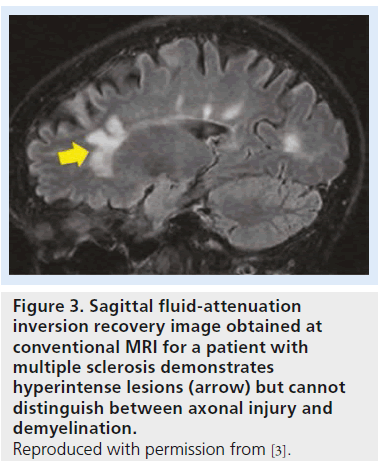
While MRI can help to identify demyelinating lesions, it is not capable of discriminating between demyelination and axonal injury. Demyelinating lesions are typically hyperintense on conventional T2-weighted images and fluidattenuation inversion recovery images. To some degree, we can discriminate between active and inactive lesions based on contrast enhancement. However, conventional MRI cannot predict whether T2 hyperintense lesions involve pure demyelination or a mixture of demyelination and axonal injury (Figure 3) [4]. Hence, while MRI has been an important diagnostic tool, it has been less satisfactory in terms of prognostic evaluation. In particular, with demyelinating diseases, such as multiple sclerosis (MS) and leukodystrophies, MRI provides important information for diagnoses but shows a weak correlation between burden of lesions, clinical manifestations and disability [9,10]. If acute axonal damage can be identified during a window of opportunity before clinical manifestations present, it could provide an opportunity for better management and have a positive impact on clinical outcome.
DTI directional diffusivity has the potential to distinguish whether T2 hyperintense lesions involve pure demyelination, axonal damage or a mixture of both. In doing so, DTI directional diffusivity may provide valuable information on structural changes underlying demyelination, axonal damage and recovery. In addition, evaluating changes in DTI directional diffusivity and disability over time for prognostic and therapeutic decision-making purposes are promising potential applications of DTI.
Animal model & experiments
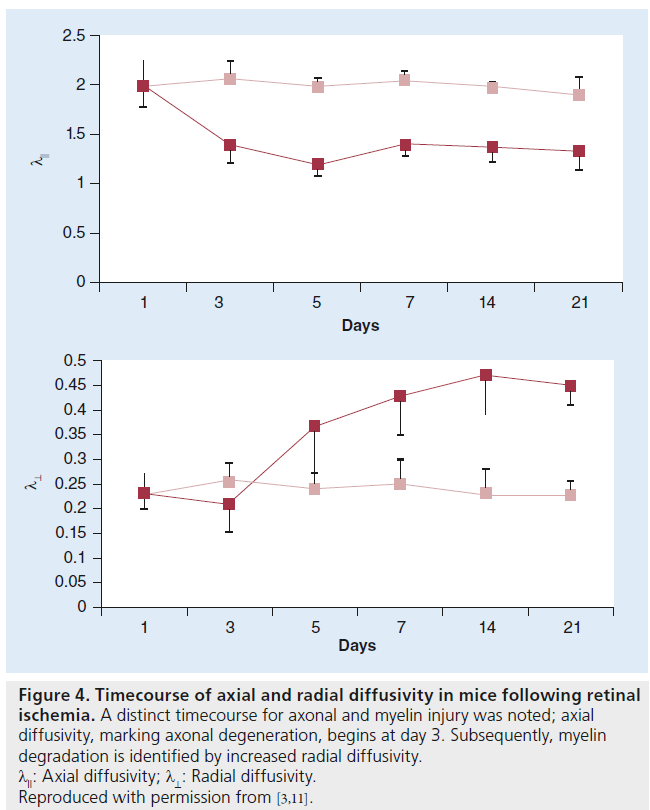
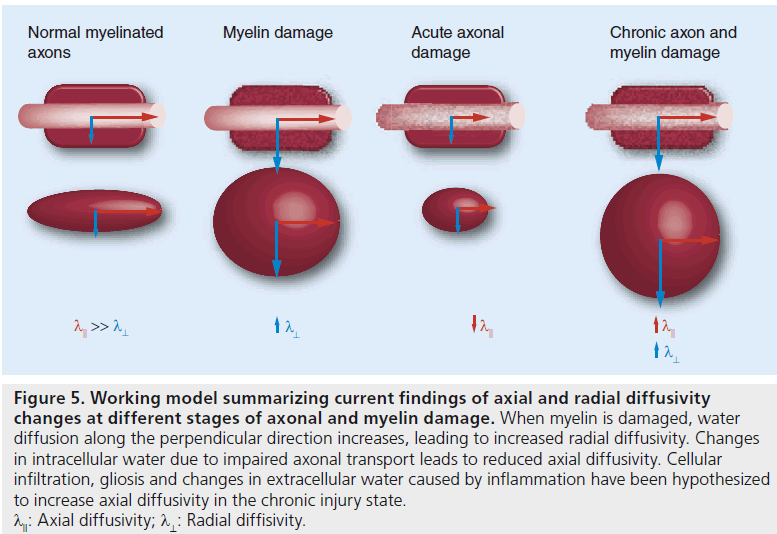
The use of λll and λ⊥ to model axonal and myelin injury has been established through numerous experiments in mice over the last decade. Using mouse optic nerve after retinal ischemia, Song et al. demonstrated that DTI diffusivity measures could differentiate between axonal and myelin damage [11]. In a mouse model of retinal ischemia, a timecourse assessment of DTI measurements demonstrated a decrease in λ11 at day 3 corresponding to axonal degeneration, which was confirmed by histology. An increase in λ⊥ was observed beginning at day 5 and was correlated with myelin degradation, again confirmed by histology [11]. A distinct timecourse for axonal and myelin injury was noted (Figure 4) [4].
Decreased λll has been attributed to fragmentation of axons, which creates barriers to the longitudinal movement of water. Accumulation of cellular debris, disordering of microtubule arrangement and filament aggregation leading to impaired axonal transport have been proposed as mechanisms that hinder water diffusion and, thereby, decrease λ11 [12–14]. Elevated λ⊥ is thought to be a consequence of myelin degradation, allowing increased water diffusion perpendicular to axons.
Using cuprizone treatment of C57BL/6 mice as a model of CNS acute demyelination, remyelination and axonal damage, a series of experiments have been conducted to further evaluate λ11 and λ⊥ , in vivo and ex vivo, with correlation to histological analysis [15–17]. Demyelination was quantified using Luxol fast blue staining, immunohistochemical examinations and electron microscopy, and was found to be consistent with increased λ⊥ . Axonal damage was evaluated by various immunochemical staining techniques for detecting nonphosphorylated neurofilaments and quantifying amyloid precursor protein-positive accumulations. Axonal damage, however, did not always correspond with λ11 throughout the pathologic process.
In these series of studies, mice were fed a diet of 0.2% cuprizone for 12 weeks followed by 12 weeks of recovery. Biweekly in vivo DTI results showed reduced λ11, indicative of axonal damage early on at 4 weeks into the diet. Axonal damage was confirmed by histology demonstrating axonal swellings, neurofilament dephosphorylation and reduced diameters. Increased λ⊥ corresponded to demyelination in the corpus callosum (CC) 6–12 weeks into the 0.2% cuprizone diet. This was followed by normalization of λ⊥ during remyelination in the recovery stage.
Some of the studies report that changes in axons and myelin indicated by histology did not always coincide with DTI diffusivity changes, despite the careful control of timing and pathologic process in the animal models. Often, histology revealed that demyelination begins early on at week 4, but this was not revealed by DTI. In another study looking at in vivo and ex vivo imaging of cuprizone-induced demyelination in the mouse CC, in vivo λ11 was found to detect axonal pathology early on and ex vivo λ⊥ was found to be more specific in conveying myelin damage that was consistent with histological evidence [18]. The authors have proposed that the sensitivity of λ⊥ in vivo may be masked by mechanisms of axonal injury, such as increased cell infiltration, which may restrict water diffusion, and cellular swelling [12,19], which could potentially reduce extracellular fluid and contribute to overall reduced diffusion. On the other hand, sensitivity of ex vivo λ⊥ may be less affected owing to an increase in permeability of the axon membrane as a result of fixation, reducing the barrier to water diffusion perpendicular to the axons [1].Again, while the timing of the pathologic process can be well controlled in animal models and may potentially resolve discrepancies between histology and DTI diffusivity changes, the challenge lies in translating the findings of mice studies to human diseases given the complexities and lack of control of timing in humans.
Figure 3.Sagittal fluid-attenuation inversion recovery image obtained at conventional MRI for a patient with multiple sclerosis demonstrates hyperintense lesions (arrow) but cannot distinguish between axonal injury and demyelination. Reproduced with permission from [3].
Nevertheless, the normalization of λ⊥ to baseline levels during periods of remyelination indicates that λ⊥ is an overall good measure of myelin damage. Changes in λ⊥ are not limited to demyelination; dysmyelination has also been found to correlate with increased λ⊥ [20,21].
In the series of experiments described above, λ11 values consistently declined in the acute injury stage, but did not correlate with axonal atrophy during the chronic injury stage. An increase in in vivo measures of λ11 was found during chronic injury and the recovery stage. However, histochemical examinations consistently confirmed axonal regeneration and gain of normal morphology only during the stage of recovery following injury. While an increase in λ11 may reflect some degree of axonal recovery, even during the chronic demyelination stage, it is important to further investigate the precise mechanisms that entail axonal damage and repair. Cellular responses to CNS injury, such as gliosis, cellular infiltration and resolution of axonal swellings, during periods of chronic demyelination have been proposed as possible factors affecting λ11 and λ⊥ . Further studies involving different axonal injury models and careful timecourse histological quantification using fluorescence microscopy and immunostaining may be useful to understand the cellular mechanisms reflected by an increase in λ11. Possible mechanisms, such as myelin-associated glycoprotein activity, resolution of axonal swellings, astrogliosis and hypertrophy [22,23], associated with axonal recovery should be further investigated to understand potential implications of λ11 and λ⊥ measures.
Figure 4.Timecourse of axial and radial diffusivity in mice following retinal ischemia. A distinct timecourse for axonal and myelin injury was noted; axial diffusivity, marking axonal degeneration, begins at day 3. Subsequently, myelin degradation is identified by increased radial diffusivity. λ11: Axial diffusivity; λ⊥: Radial diffusivity. Reproduced with permission from [3,11].
In summary, the results of animal model studies suggest that λ⊥ is a good indicator of myelin damage. However, λ11 does not always correlate with axonal pathology. Currently, there is no animal model that describes demyelination and axonal damage independently, making it difficult to precisely discern the contributions with respect to pathology. We speculate that mechanisms associated with different stages of axonopathy may be attributed to the λ11 measure. Decreased λ11 has been associated with various mechanisms of axonal damage, such as axonal swelling [16,24], Wallerian degeneration [5,25,26] and diffuse axonal injury [12,27,28]. In animal experiments, λ11 was able to characterize axonal degeneration in the acute stage but could not demonstrate axonal damage in the chronic state. Based on the results of the animal models, it is conceivable that λ11 is a time-dependent measure reflective of different stages of axonal injury, which may be characterized by unique cellular mechanisms. The findings from animal models suggest that, in the context of demyelinating diseases, acute injury involving axonal swellings with minimal axonal damage may be able to indicate axonal involvement through λ11, while chronic diseases characterized by long-term axonal atrophy and degeneration may fail to do so. However, with all human studies, the challenge of lack of control of timing and the pathologic process is difficult to resolve and may not always demonstrate a clear-cut uniform answer. With this in mind, we will discuss the potential application of directional diffusivity in the context of acute demyelination disorders, such as acute disseminated encephalomyelitis (ADEM), chronic demyelinating diseases such as MS and leukodystrophies (Pelizaeus–Merzbacher disease [PMD]) and other neurodegenerative diseases such as Alzheimer’s disease (AD). Based on current DTI studies, we will also assess λ⊥ during the course of myelin damage and λ11 during the course of axonal injury in acute and chronic white matter diseases.
Acute CNS demyelination
MR markers for acute monophasic demyelinating disorders
DTI directional diffusivity may prove to be useful as a biomarker in differentiating between acute monophasic disorders and progressive disorders. The most common demyelinating disease in childhood represents ADEM, an acute monophasic disease associated with low rates of conversion to MS, a progressive disorder. ADEM is found to have an excellent recovery rate in the long-term follow-up [29]. MS is a progressive disorder and immunomodulatory treatments are used to reduce relapses and prevent permanent disability. It is difficult to differentiate between MS and ADEM early in the clinical process. MR markers involving CC long-axis perpendicular lesions and the presence of well-defined lesions are found to be specific, but not very sensitive for predicting conversion to MS after the first episode [30,31]. Recent studies have confirmed T2 lesions in periventricular white matter and the presence of T1 hypointense lesions are better predictors of MS diagnosis in children with acute demyelinating syndromes [32,33]. However, the MRI criteria have not yet been established that are predictive of disability in the long term. Similarly, spinal cord lesions have only shown a weak correlation with physical disability [34,35].
The distinction between MS and ADEM can be difficult, as no specific clinical feature exists for differentiating the two pathologies. Conventional MRI scans are not able to discriminate between the two diagnoses at the time of initial presentation. In addition, laboratory testing and physical examinations are not definitive. A final diagnosis is only made after months of follow-up, with the rate and number of relapses determining the final diagnosis – in that, if relapses occur that are disseminated with respect to space and time, MS is diagnosed [36]. At the same time, conversion to MS is uncertain from the initial diagnosis. Current MRI predictive criteria are not satisfactory in terms of distinguishing a first attack of MS from ADEM. Owing to the difficulty in identifying children at highest risk of developing MS after the first acute attack, disease-modifying treatments are typically not given. A lack of definitive diagnosis, however, can result in significant treatment delay for patients who will later be diagnosed with MS after many follow-ups. Predictive biomarkers to identify children at risk of conversion to MS may allow early treatment during a window of opportunity in which conversion to MS and progressive disability may be prevented.
DTI diffusivity as a potential biomarker
MS and ADEM are characterized by lesions with demyelination, inflammation and axonal injury. There are no exclusive clinical features within lesions that are specific for the pathologies. On the other hand, abnormalities in normalappearing white matter (NAWM) may point to distinct pathologic profiles for these disorders. In MS, axonal loss often occurs early in the course of the disease [37–39]. Many studies have also highlighted that axonal loss may be the primary determinant of irreversible neurological disability in MS patients [39–41].
In a recent retrospective DTI study looking at nonlesional white matter changes within central fibers of the CC genu and internal capsule in pediatric MS and monophasic demyelination disorders, Tillema et al. found lower FA values, increased λ⊥ and decreased λ11 compared with controls for the MS group early in the disease course, in less than 1 year (median duration from symptoms onset to DTI was 20 months) [42]. In the monophasic demyelination group, reduced FA values, increased λ⊥ and no difference in λ11 was observed (ADEM: median duration from symptoms onset to DTI was <1 week). Overall, DTI directional diffusivity appears to be consistent with pathologic profiles of axonal damage and demyelination for the disorders. Decreased λ11 in the MS group corresponds with the pathologic profile of MS characterized by early axonal loss. In the ADEM group, no change in λ11 was observed, consistent with limited axonal injury during the course of the disorder. The preliminary study suggests a unique λ11 pattern in pediatric MS, which may serve as a biomarker for predicting progression to MS after the first initial demyelinating event [4]. Further longitudinal studies are needed to confirm the application of DTI changes in these difficult clinical cases.
CNS demyelination: MS
DTI studies conducted in MS patients can help us understand the changes in white matter taking place in the context of chronic demyelination and axonal loss. In MS patients with low lesion loads and mean disease duration of 4 years [43], DTI–MRI of the brain revealed increased λ⊥ in both lesions and NAWM compared with healthy controls. λ11 was either increased or remained the same. In other DTI studies involving longer term relapse-remitting MS patients (disease duration of 3–9 years), increased λ⊥ has been observed in the CC and pyramidal tracts, both within lesions and NAWM [44–46], and correlated with progressive disability. Moreover, λ⊥ was shown to be a strong predictor of myelin status in postmortem human brain prior to, and after, fixation [47,48]. However, increased λ11 was found in these studies involving patients with longer disease duration.
Axonal atrophy is a pathologic feature of MS that is expected to occur early in the course of the disease. MRI studies have confirmed that CC atrophy is a common finding in MS [49–51], and histopathologic studies indicate early axonal injury, transection of axons passing through the CC and Wallerian degeneration over time [41,52]. As with mouse experiments during the chronic demyelinating state, current DTI studies in progressive MS patients with longer disease duration (over 3 years) did not demonstrate decreased λ11. Spinal cord CNS atrophy is another feature of MS expected to occur early in the disease process [53–55]. In a recent ex vivo imaging study of the spinal cord in subjects with long-standing secondary-progressive MS [48], higher λ11 and λ⊥ in MS lesions were found compared with age-matched controls. Histopathologic examination demonstrated a degree of demyelination that corresponded to high λ⊥ . The extent of axonal loss, however, was not found to correlate with observed λ11.
The consistent findings of elevated λ⊥ in these studies suggest that λ⊥ has the ability to detect myelin damage. While λ11 appears to correlate with axonal involvement early during the course of MS (as demonstrated by studies during the pediatric population early in the disease course), it appears to lack the resolution needed to discern complex mechanisms involved in axonal damage and repair at the chronic stage. The complications of inflammation, cellular infiltration and gliosis may affect λ11, making it difficult to interpret whether increased λ11 truly demonstrates axonal recovery versus loss masked by effects on diffusivity attributed to confounding cellular responses. It is conceivable that λ11 is influenced by long-term changes and cellular responses in axons over the course of chronic, progressive demyelination; however, the effects of such cellular processes remain unclear.
Application of DTI in therapeutic & prognostic evaluations
DTI directional diffusivity may offer the potential to monitor therapeutic options and further our understanding of the disease process. In a DTI study looking at longitudinal changes in brain tissues in a group of patients with MS (mean disease duration: 11.9 years) starting natalizumab therapy, the authors found increased FA, decreased λ⊥ and no change in λ11 in gadolinium-enhancing lesions over the course of the year [56]. The reduction in λ⊥ may be indicative of remyelination. Natalizumab is an anti-inflammatory drug that prevents leukocyte adhesion to the capillary endothelial cells and, as a result, reduces leukocytes entering the CNS [57]. Natalizumab therapy has been shown to reduce conversion of gadolinium-enhancing lesions into T1 black holes, which are indicative of severe tissue damage comprising axonal loss, axonal swelling and demyelination [58]. It is thought that the anti-inflammatory effects of natalizumab would allow for more repair. In the study, higher λ⊥ at baseline predicted conversion to T1 black holes at 12 months. Similar predictions were demonstrated in another study that followed a cohort of 22 individuals (disease duration of 2–23 years) over 15 months [59]. On the other hand, in normal-appearing brain tissue, FA and λ11 demonstrated further decline over time, while no significant change in λ⊥ was observed. Hence, the decline in λ11 may suggest involvement of axonal loss and degeneration in normal-appearing brain tissue at the early stage before active lesions develop, possibly attributing to the progressive disability often observed in MS patients despite treatment.
Treatment with natalizumab demonstrates increased FA and reduced λ⊥ in gadoliniumenhancing lesions. This may be indicative of remyelination consistent with results from mouse studies. Identification of axonal damage in normal-appearing brain tissue illustrated by DTI, highlights its potential for evaluating treatment outcomes and drug efficacy. DTI directional diffusivity offers the potential for use as biomarker for prognostic purposes and monitoring treatment response.
Often, throughout the course of MS, progressive disability is observed despite the fact that no new lesions are detected on MRI. Conventional MRI cannot provide information on the degree of tissue injury at the cellular level. Progressive disability in MS, despite no new lesions, may suggest ongoing cellular processes around existing lesions or in NAWM that may be comprised of tissue damage involving myelin, axonal damage, or both. Imaging studies with histopathologic examinations have demonstrated axonal loss and microglial activation in areas of NAWM [60]. Diffusely abnormal white matter has been associated with axonal loss, decreased myelin and chronic fibrillary gliosis exemplified by astrocytic, microglial and oligodendrocytic hypertrophy [61,62]. These proposed mechanisms may lead to irreversible axonal loss involved in the disease progression and neurologic disability in MS patients. Early identification of these pathologic processes by DTI can aid clinicians in evaluating drug efficacy and making important decisions on therapeutic interventions.
In summary, λ⊥ is consistently found to be higher than baseline over the course of MS, while λ11 appears to change with chronicity of the disease. In the early stages of MS (disease duration less than 3 years), λ11 demonstrates a trend toward decreased values, but in patients with longer disease duration, increased λ11 is observed. The results are concordant with the findings from mouse experiments and the current working model (Figure 5).
Future directions
DTI directional diffusivity data could provide useful information on the pathology of MS at the cellular level and elucidate mechanisms involving damage, repair and functional plasticity. Since directional diffusivity can identify white matter changes with respect to myelin and axonal damage, it offers the potential for monitoring disease progression and predicting clinical outcome. Recently, lower λ⊥ at baseline has been found to be predictive of recovery after spinal cord relapse in patients with MS [63].
Studies have also highlighted that axonal loss may be the primary determinant of irreversible neurological disability in MS patients [39–41]. In a clinical setting, the ability to differentiate between axonal damage and demyelination can offer a valuable opportunity in monitoring therapeutic and drug-target response. Exploration of drugs and therapies that can aid axonal repair and prevent axonal damage may be a promising area of research for treatment. While the use of MRI in clinical trials of MS is well established, imaging methods, such as DTI, have the potential to serve as biomarkers and outcome measures of neuroprotective and reparative strategies in clinical trials of primary- and secondary-progressive MS. To further confirm axonal damage as a key determinant of neurologic disability, future studies should investigate changes in DTI directional diffusivity and disability as time progresses to identify any relationship with clinical outcome in MS patients. Changes in λ11 may be an important parameter linking axonal damage, predictive clinical outcome in MS and drug response in patients with MS.
Leukodystrophies: DTI directional diffusivity as a MR biomarker of axonal injury
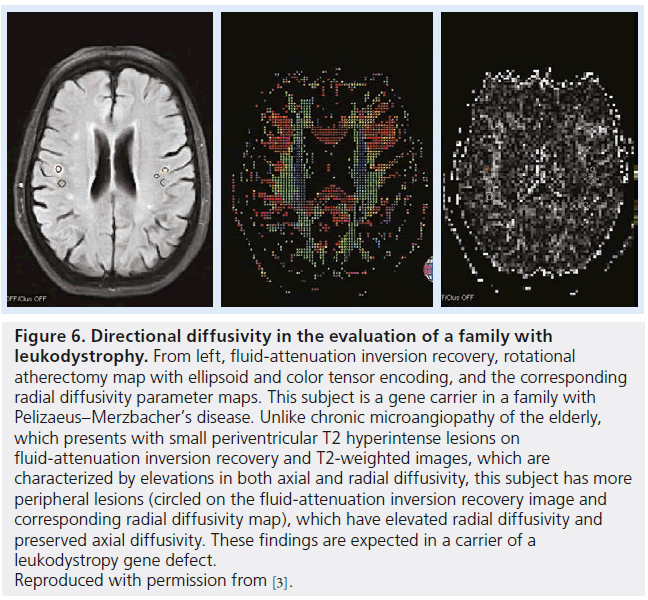
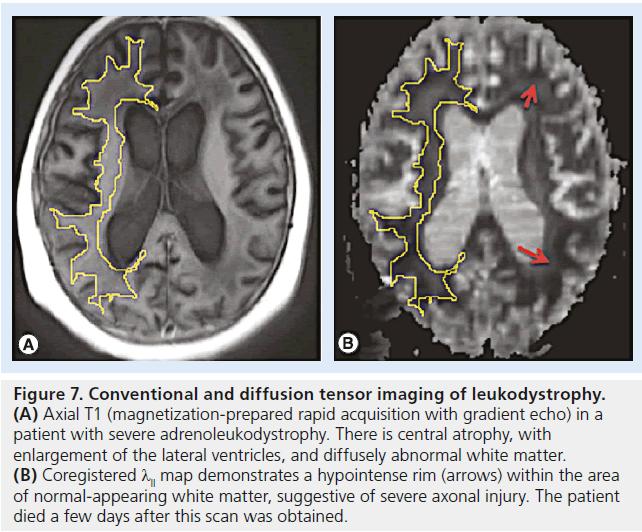
Leukodystrophies describe a set of inherited demyelinating disorders affecting myelin, leading to progressive deterioration in neurological status, disability and death [64]. A varying degree of axonal injury is observed and may correlate with progressive deterioration and neurologic signs (Figures 6 & 7) [65].
Animal studies of PMD
Several DTI studies involving mouse models of various forms of leukodystrophy have been performed to characterize pathological changes in white matter. Among the many forms of leukodystrophies, PMD is the most widely studied as it is well exemplified in mouse models. Using transgenic mice with the duplicated PIP1 gene as a model of PMD, Ruest and coworkers compared ex vivo DTI findings with histology in three brain regions involving the anterior commissure, CC and hippocampal fimbria [66]. PMD is an X-linked recessive disorder caused by mutations associated with PLP1 that encodes two proteolipid proteins in oligodendrocytes, PLP and DM20. PLP is required for the production and stabilization of myelin while DM20 may be involved in oligodendrocyte differentiation and survival. Among the mutations associated with PMD, gene duplication has been found to be the most common cause. Mutations present a broad range of clinical severity and white matter pathology, primarily involving myelin loss, dysmyelination and a variable degree of axonal damage [67,68]. In the experiment, both t-value maps and tract-based spatial statistics voxel-based analyses demonstrated loss of anisotropy and increased water diffusion along, and perpendicular to, white matter tracts – that is, decreased FA, increased λ11 and λ⊥ diffusivities compared with the wild-type.
Figure 5.Working model summarizing current findings of axial and radial diffusivity changes at different stages of axonal and myelin damage. When myelin is damaged, water diffusion along the perpendicular direction increases, leading to increased radial diffusivity. Changes in intracellular water due to impaired axonal transport leads to reduced axial diffusivity. Cellular infiltration, gliosis and changes in extracellular water caused by inflammation have been hypothesized to increase axial diffusivity in the chronic injury state. lll: Axial diffusivity; l^: Radial diffisivity.
Figure 6.Directional diffusivity in the evaluation of a family with leukodystrophy. From left, fluid-attenuation inversion recovery, rotational atherectomy map with ellipsoid and color tensor encoding, and the corresponding radial diffusivity parameter maps. This subject is a gene carrier in a family with Pelizaeus–Merzbacher’s disease. Unlike chronic microangiopathy of the elderly, which presents with small periventricular T2 hyperintense lesions on fluid-attenuation inversion recovery and T2-weighted images, which are characterized by elevations in both axial and radial diffusivity, this subject has more peripheral lesions (circled on the fluid-attenuation inversion recovery image and corresponding radial diffusivity map), which have elevated radial diffusivity and preserved axial diffusivity. These findings are expected in a carrier of a leukodystropy gene defect. Reproduced with permission from [3].
Increased λ⊥ was correlated with demyelination in all of the three brain regions and was confirmed by antimyelin basic protein staining and electron microscopy. Subsequently, further immunohistology analyses confirmed axonal damage (antiamyloid precursor protein-positive accumulations) and increased astrocytosis (anti- GFAP or GFAP accumulations). Increased λ11 may be reflective of the finding that amyloid precursor protein accumulations were not as extensive due to the time-dependent manner of axonal degeneration [69].
Figure 7.Conventional and diffusion tensor imaging of leukodystrophy. (A) Axial T1 (magnetization-prepared rapid acquisition with gradient echo) in a patient with severe adrenoleukodystrophy. There is central atrophy, with enlargement of the lateral ventricles, and diffusely abnormal white matter. (B) Coregistered λll map demonstrates a hypointense rim (arrows) within the area of normal-appearing white matter, suggestive of severe axonal injury. The patient died a few days after this scan was obtained.
Based on the work of Harsan and coworkers, it is conceivable that increased λ11 detected in PMD mouse models may be attributed to astrogliosis and hypertrophy [23]. In their jimpy PLP-mutant mouse model, Harsan et al. demonstrated irreversible dysmyelination with increased astrocytic activity in mouse brains. In comparing DTI–MRI parameters of jimpy mouse brain with dysmyelinated brain of oligo-TTK transgenic mice with little astrocyte hypertropy, dramatic increases in λ⊥ and λ11 were observed in the jimpy mouse brain. Astrocytes are major regulators of water content in the brain via AQP4 [70], and its cytoplasmic domains can extend along the axonal tracts. Hence, it is possible that astrocyte hypertrophy attributed to gliosis may contribute to increased water diffusion and, thereby, lead to changes in λ11, and even λ⊥ , parameters to some degree.
Application & future research
DTI directional diffusivity measurements may prove to be useful biomarkers in monitoring axonal pathology in patients with leukodystrophies, regardless of the genetic etiology and, thus, provide important early diagnostic and prognostic information. Directional diffusivity as a MR biomarker also offers the potential to monitor and evaluate myelin therapies under development. In addition, changes in directional diffusivity may be used to differentiate between progressive white matter disorders, such as leukodystrophies, and static white matter disorders, such as periventricular leukomalacia.
Alzheimer’s disease
AD is a neurodegenerative disorder classically characterized as a gray matter disease, with accumulation of misfolded proteins in the extracellular space forming amyloid plaques and within neurons forming neurofibrillary tangles. Volumetric reduction in gray matter regions of AD brains is significant in patients with AD [71,72], and recent studies have shown that this reduction may occur early in the disease process, as it has been found in adults with family history of AD [73,74].
Recently, DTI directional diffusivity studies have revealed white matter involvement in AD bringing a new perspective into the pathology of AD, which has previously been discussed primarily as a gray matter disease in the literature. Early degeneration of the temporal lobe structures has been commonly cited in the neurodegenerative process of AD [72,75]. Involvement of white matter in temporal, parietal and frontal regions, as well as in the CC, has been noted and, hence, are often associated with manifestations of impairment in attentive, executive, language and visuospatial functions in patients with AD [76–78]. In a DTI study of NAWM in patients with mild (early) AD, mild cognitive impairment and controls [79], increased λ⊥ and reduced λ11 were observed in higher cortical regions (temporal, parietal and frontal NAWM) compared with the healthy, age-matched controls. The changes reflected in these higher cortical regions correlated with declined cognitive function. In high-risk AD groups, increased λ⊥ and limited decreased λ11 have been observed, suggesting compromised structural integrity in white matter tracts, particularly in connections to the medial temporal lobes [80,81]. These findings suggest that decreases in axonal and myelin integrity are conceivably associated with degeneration of white matter tracts.
Findings in λ⊥ changes early in the disease process provide additional evidence for the hypothesis of myelin involvement at the onset of AD. In post-mortem studies, increased levels of b-amyloid peptides, which are cytotoxic to oligodendrocytes, have been detected, accompanied by reductions in white matter cholesterol, protein levels and myelin proteins [82,83]. Bartzokis and authors have put toward the myelin hypothesis of AD, which proposes that AD is initially a disease of demyelination in which b-amyloid and tau aggregates are the by-product of homeostatic repair [84,85]. Current DTI findings of reduced λ⊥ in AD patients support the hypothesis of white matter involvement and myelin damage.
In longer term studies involving AD patients with longer duration of disease, increased λ11 was often reported over time [86,87]. Increased λ⊥ with disease progression appears to be a rather consistent trend observed among AD subjects, regardless of the duration of AD disease. Increased λ11 may represent a later stage of white matter damage, marked by microglia activation and fluid shifts. Similar to previous studies described in this article, the mechanisms for increased λ11 observed with progression of white matter pathologies remain relatively unclear and several hypotheses have been proposed.
Astrogliosis is a known pathologic feature of AD that has been shown to be associated with b-amyloid deposition and oligodendrocyte death [88–90]. In animal studies, astrogliosis has been associated with increased λ11. Reactive astrogliosis, accompanied by accumulation of cellular debris and cytoplasmic extensions in the pathogenesis of AD, appears to be a contributing factor to increased λ113 over time, although further research is warranted.
Application & future research
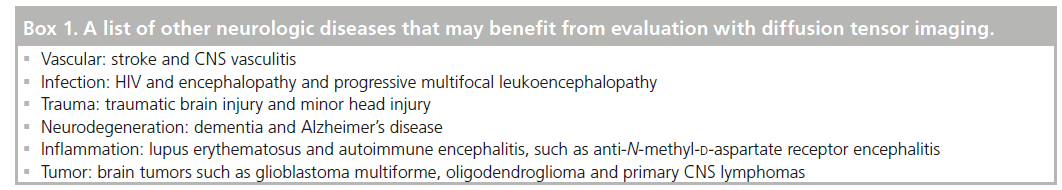
Progressive increases in λ⊥ have been correlated with the severity of dementia [86,91]. On the contrary, axonal loss is commonly believed to be the primary determinant of irreversible disability. At present, λ11 does not appear to have the power to discern different underlying mechanisms involved in axonal damage and repair at the chronic stage. Since the detection of axonal damage by λ11 remains flawed at chronic stages, we cannot exclusively rely on λ11 to assess axonal damage. Indeed, axonal damage including impaired axonal transport, swelling and accumulation of cellular debris have been confirmed even at early stages of AD in both mouse models and humans [92]. Further refinement of the meaning of λ11 is warranted to apply it as a measure of axonal damage in the context of confounding factors of cellular infiltration and inflammation (Box 1) [93].
Limitations of anatomic correlates of DTI
Since water tends to diffuse along axonal tracts, measurement of diffusion anisotropy within white matter can provide anatomical information on axonal architecture. However, there are some limitations to the current diffusion tensor model. The tensor model attempts to average diffusion data and assume that one long axis represents the fiber orientation within the diffusion ellipsoid. This seems possible when the ellipsoid consists of a fiber population that follows a similar directionality. In situations where fibers branch or crossover, the longest axis may no longer represent the orientation of any fibers depending on the angle and ratios of fiber branching (e.g., fanning of pyramidal projection fiber bundles, crossing of adjacent fiber bundles of cingulum and body of CC).
Diffusion may be hindered in all directions due to these heterogeneous fiber orientations within a voxel and may appear to be isotropic or low anisotropy. Consequently, areas of white matter where two or more heterogenous fiber populations pass within the same pixel may appear to be hypointense on DTI. It is important to note that these effects can impact upon the diffusion tensor and its parameters to some degree. In an attempt to resolve crossing fibers, higher order models and methods have been proposed [94,95]. However, these approaches require long acquisition times, which make them difficult to accomplish on clinical systems. The long scan time, acquisition methods and heavy computational load to acquire the data make them difficult to apply in clinical systems [94–96].
In addition, Wheeler-Kingshott and authors suggest that when comparing patients with healthy controls, it may be necessary to analyze the direction of eigenvectors together with the assessment of eigenvalues (i.e., axial and radial diffusivity) and compare how eigenvectors are aligned with underlying tissue structures, making sure the eigenvalues represent the same physical information [93].
Finally, in areas of tissue partial volume where white and gray matter or white matter and cerebrospinal fluid (CSF) reside in the same pixel, additional diffusion bias is introduced, which may affect anisotropy of neighboring white matter [97–100]. For instance, the CC is often prone to partial volume effects from neighboring CSF. Hence, in a disease such as MS where CC thinning can occur, bias attributed by partial volume effects from neighboring CSF may lead to overestimation of structural loss if partial volume effects are not corrected for. Various methods of CSF suppression have been introduced including fluid-attenuated inversion recovery diffusion-weighted imaging–MRI [101], and a bitensor method used to correct free water contamination of standard DTI-acquisition schemes, possibly differentiating the contribution of the free water compartment from that of the tissue compartment (Box 2) [102].
Potential barriers to implementation of DTI in clinical practice
DTI relies upon the performance of the diffusion gradients encoded on the MRI scanners. Performance in single-center studies, such as those discussed in this manuscript, has been robust. However, there have been relatively few multicenter studies published due to a lack of cross-vendor hardware standardization, protocol (software) standardization and a widely accepted diffusion phantom standard [95]. A further barrier is added when pediatric studies are concerned. As myelination of the white matter changes during childhood and adolescence, diffusion measurements in white matter also vary with age, thus, reference cohorts of normal participants are required [103].
Conclusion & future perspective
In summary, DTI plays an important role in identifying subtle white matter changes with respect to axon and myelin damage that are not distinguishable using conventional MRI. DTI directional diffusivity can offer the potential to identify pathologic changes in axons and myelin and, hence, convey the underlying pathology and structural and functional adaptations before gross anatomical changes become apparent on conventional MRI.
Particularly in the acute and early disease phase, λ11 and λ⊥ have the capacity to characterize axonal and myelin damage, providing a landscape of white matter pathogenesis and degeneration. However, λ11 does not appear to have the resolution to discern underlying complex mechanisms involved in axonal damage and repair at the chronic stage. We expect that future advances will improve the resolution to overcome the current imaging limitations and will be able to distinguish axonal repair versus axonal damage in the chronic stages as we further understand the effects on diffusivity induced by complex processes of cellular infiltration and gliosis.

Financial & competing interests disclosure
TS Benzinger has received research funding from Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly for her research in PET imaging of Alzheimer’s disease. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
*of interest
** of considerable interest
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 15(7–8), 435–455 (2002). nn A technical review on the relationship between diffusion parameters and the underlying microstructure of neural fibers.
- Beaulieu C, Allen PS. Water diffusion in the giant axon of the squid: implications for diffusion-weighted MRI of the nervous system. Magn. Reson. Med. 32(5), 579–583 (2005).
- Basser PJ. New histological and physiological stains derived from diffusion-tensor mr images. Ann. NY Acad. Sci. 820(1), 123–138 (2006).
- Benzinger TL, Cross AH, Xu J, Naismith R, Sun SW, Song SK. Directional diffusivity as a magnetic resonance (MR) biomarker in demyelinating disease. Proc. SPIE 6759, 67590O (2007).
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20(3), 1714–1722 (2003).
- Tyszka JM, Readhead C, Bearer EL, Pautler RG, Jacobs RE. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage 29(4), 1058–1065 (2006).
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 8(7), 333–344 (1995).
- Xue R, van Zijl P, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn. Reson. Med. 42(6), 1123–1127 (1999).
- Filippi M, Rocca MA, Barkhof F et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 11(4), 349–360 (2012).
- Filippi M, Agosta F. Imaging biomarkers in multiple sclerosis. J. Magn. Reson. Imaging 31(4), 770–788 (2010).
- Sun SW, Liang H-F, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage 32(3), 1195–1204 (2006).&Investigation of in vivo and ex vivo diffusion tensor parameters to characterize axonal and myelin injury in mice with retinal ischemia.
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. Am. J. Neuroradiol. 23(5), 794–802 (2002).
- Beaulieu C, Does MD, Snyder RE, Allen PS. Changes in water diffusion due to Wallerian degeneration in peripheral nerve. Magn. Reson. Med. 36(4), 627–631 (2005).
- Dijkhuizen RM, de Graaf RA, Tulleken KA, Nicolay K. Changes in the diffusion of water and intracellular metabolites after excitotoxic injury and global ischemia in neonatal rat brain. J. Cereb. Blood Flow Metab. 19(3), 341–349 (1999).
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 55(2), 302–308 (2006).
- Song SK, Yoshino J, Le TQ et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26(1), 132–140 (2005).
- Xie M, Tobin JE, Budde MD et al. Rostro-caudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J. Neuropathol. Exp. Neurol. 69(7), 704–716 (2010).
- Zhang J, Jones MV, McMahon MT, Mori S, Calabresi PA. In vivo and ex vivo diffusion tensor imaging of cuprizone-induced demyelination in the mouse corpus callosum. Magn. Reson. Med. 67(3), 750–759 (2011).
- Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 13(3), 329–339 (2003).
- Harsan LA, Poulet P, Guignard B et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J. Neurosci. Res. 83(3), 392–402 (2006).
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17(3), 1429–1436 (2002).
- Fruttiger M, Montag D, Schachner M, Martini R. Crucial role for the myelin-associated glycoprotein in the maintenance of axon-myelin integrity. Eur. J. Neurosci. 7(3), 511–515 (2006).
- Harsan LA, Poulet P, Guignard B, Parizel N, Skoff RP, Ghandour MS. Astrocytic hypertrophy in dysmyelination influences the diffusion anisotropy of white matter. J. Neurosci. Res. 85(5), 935–944 (2007).& Examination of astrocytic hypertrophy in dysmyelination and its influence on directional diffusivity.
- Kim JH, Loy DN, Wang Q et al. Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J. Neurotrauma 27(3), 587–598 (2010).
- Liu M, Gross DW, Wheatley BM, Concha L, Beaulieu C. The acute phase of Wallerian degeneration. Longitudinal diffusion tensor imaging of the fornix following temporal lobe surgery. Neuroimage, 74, 128–139 (2013). nn Investigation of diffusivity changes in acute and chronic stages following temporal lobe surgery.
- Xie M, Wang Q, Wu TH, Song SK, Sun SW. Delayed axonal degeneration in slow Wallerian degeneration mutant mice detected using diffusion tensor imaging. Neuroscience 197, 339–347 (2011).
- Li J, Li XY, Feng DF, Gu L. Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur. J. Neurosci. 33(5), 933–945 (2011).
- Bennett RE, Mac Donald CL, Brody DL. Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci. Lett. 513(2), 160–165 (2012).
- Mar S, Lenox J, Benzinger T, Brown S, Noetzel M. Long-term prognosis of pediatric patients with relapsing acute disseminated encephalomyelitis. J. Child Neurol. 25(6), 681–688 (2010).
- Mikaeloff Y, Suissa S, Vallée L et al. First episode of acute CNS inflammatory demyelination in childhood: prognostic factors for multiple sclerosis and disability. J. Pediatr. 144(2), 246–252 (2004).
- Neuteboom R, Boon M, Berrevoets CC et al. Prognostic factors after a first attack of inflammatory CNS demyelination in children. Neurology 71(13), 967–973 (2008).
- Verhey LH, van Pelt-Gravesteijn ED, Ketelslegers IA et al. Validation of MRI predictors of multiple sclerosis diagnosis in children with acute CNS demyelination. Mult. Scler. Relat. Disord. 2(3), 193–199 (2013).
- Verhey LH, Branson HM, Shroff MM et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol. 10(12), 1065–1073 (2011).
- Stankiewicz J, Neema M, Alsop D et al. Spinal cord lesions and clinical status in multiple sclerosis. A 1.5 T and 3 T MRI study. J. Neurol. Sci. 279(1), 99–105 (2009).
- Verhey LH, Branson HM, Makhija M, Shroff M, Banwell B. Magnetic resonance imaging features of the spinal cord in pediatric multiple sclerosis. a preliminary study. Neuroradiology 52(12), 1153–1162 (2010).
- Dale R, Branson J. Acute disseminated encephalomyelitis or multiple sclerosis: can the initial presentation help in establishing a correct diagnosis? Arch. Dis. Child. 90(6), 636–639 (2005).
- Coles AJ, Cox A, Le Page E et al. The window of therapeutic opportunity in multiple sclerosis. J. Neurol. 253(1), 98–108 (2006).
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Brück W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain 125(10), 2202–2212 (2002).
- Schirmer L, Antel JP, Brück W, Stadelmann C. Axonal loss and neurofilament phosphorylation changes accompany lesion development and clinical progression in multiple sclerosis. Brain Pathol. 21(4), 428–440 (2011).
- Tallantyre EC, Bø L, Al-Rawashdeh O et al. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult. Scler. 16(4), 406–411 (2010). nn Degree of axonal degeneration is associated with clinical disability.
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338(5), 278–285 (1998).
- Tillema J, Leach J, Pirko I. Non-lesional white matter changes in pediatric multiple sclerosis and monophasic demyelinating disorders. Mult. Scler. J. 18(12), 1754–1759 (2012).
- Roosendaal S, Geurts J, Vrenken H et al. Regional DTI differences in multiple sclerosis patients. Neuroimage 44(4), 1397–1403 (2009).
- Giorgio A, Palace J, Johansen-Berg H et al. Relationships of brain white matter microstructure with clinical and MR measures in relapsing-remitting multiple sclerosis. J. Magn. Reson. Imaging 31(2), 309–316 (2010).
- Henry RG, Oh J, Nelson SJ, Pelletier D. Directional diffusion in relapsing-remitting multiple sclerosis: a possible in vivo signature of Wallerian degeneration. J. Magn. Reson. Imaging 18(4), 420–426 (2003).
- Kern KC, Sarcona J, Montag M, Giesser BS, Sicotte NL. Corpus callosal diffusivity predicts motor impairment in relapsing – remitting multiple sclerosis: a TBSS and tractography study. Neuroimage 55(3), 1169–1177 (2011).
- Schmierer K, Wheeler-Kingshott CA, Tozer DJ et al. Quantitative magnetic resonance of post-mortem multiple sclerosis brain before and after fixation. Magn. Reson. Med. 59(2), 268–277 (2008).
- Klawiter EC, Schmidt RE, Trinkaus K et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 55(4), 1454–1460 (2011). nn Investigation of diffusion tensor imaging with histochemical staining for demyelination and axonal damage in ex vivo multiple sclerosis spinal cords. Highlights the potential implication of radial diffusivity in the clinical assessment of demyelination.
- Audoin B, Ibarrola D, Malikova I et al. Onset and underpinnings of white matter atrophy at the very early stage of multiple sclerosis–a two-year longitudinal MRI/MRSI study of corpus callosum. Mult. Scler. 13(1), 41–51 (2007).
- Pelletier J, Suchet L, Witjas T et al. A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing–remitting multiple sclerosis. Arch. Neurol. 58(1), 105–111 (2001).
- Juha M, Leszek S, Sten F et al. Progression of non-age-related callosal brain atrophy in multiple sclerosis: a 9-year longitudinal MRI study representing four decades of disease development. J. Neurol. Neurosurg. Psych. 78(4), 375–380 (2007).
- Dziedzic T, Metz I, Dallenga T et al. Wallerian degeneration: a major component of early axonal pathology in multiple sclerosis. Brain Pathol. 20(5), 976–985 (2010).
- Deluca G, Ebers G, Esiri M. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain 127(5), 1009–1018 (2004).
- Evangelou N, Deluca G, Owens T, Esiri M. Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. Brain 128(1), 29–34 (2005).
- Gilmore CP, Deluca GC, Bö L et al. Spinal cord neuronal pathology in multiple sclerosis. Brain Pathol. 19(4), 642–649 (2009).
- Fox R, Cronin T, Lin J et al. Measuring myelin repair and axonal loss with diffusion tensor imaging. Am. J. Neuroradiol. 32(1), 85–91 (2011). & Investigation of longitudinal changes in brain lesional and nonlesional white matter. Higher radial diffusivity within Gd-enhancing lesions at baseline predicts conversion to the T1 black holes.
- Selewski D, Shah G, Segal B, Rajdev P, Mukherji S. Natalizumab (Tysabri). Am. J. Neuroradiol. 31(9), 1588–1590 (2010).
- Fisher E, Chang A, Fox RJ et al. Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann. Neurol. 62(3), 219–228 (2007).
- Naismith R, Xu J, Tutlam N et al. Increased diffusivity in acute multiple sclerosis lesions predicts risk of black hole. Neurology 74(21), 1694–1701 (2010).
- Moll NM, Rietsch AM, Thomas S et al. Multiple sclerosis normal-appearing white matter: pathology–imaging correlations. Ann. Neurol. 70(5), 764–773 (2011).
- Laule C, Vavasour IM, Leung E et al. Pathological basis of diffusely abnormal white matter: insights from magnetic resonance imaging and histology. Mult. Scler. J. 17(2), 144–150 (2011).
- Seewann A, Vrenken H, van der Valk P et al. Diffusely abnormal white matter in chronic multiple sclerosis: imaging and histopathologic analysis. Arch. Neurol. 66(5), 601–609 (2009).
- Freund P, Wheeler-Kingshott C, Jackson J, Miller D, Thompson A, Ciccarelli O. Recovery after spinal cord relapse in multiple sclerosis is predicted by radial diffusivity. Mult. Scler. 16(10), 1193–1202 (2010).
- Lyon G, Fattal-Valevski A, Kolodny EH. Leukodystrophies: clinical and genetic aspects. Top. Magn. Reson. Imaging 17(4), 219–242 (2006).
- Mar S, Noetzel M. Axonal damage in leukodystrophies. Pediatr. Neurol. 42(4), 239–242 (2010).
- Ruest T, Holmes WM, Barrie JA et al. High-resolution diffusion tensor imaging of fixed brain in a mouse model of Pelizaeus–Merzbacher disease: comparison with quantitative measures of white matter pathology. NMR Biomed. 24(10), 1369–1379 (2011).
- Regis S, Biancheri R, Bertini E et al. Genotype–phenotype correlation in five Pelizaeus–Merzbacher disease patients with PLP1 gene duplications. Clin. Genet. 73(3), 279–287 (2008).
- Sima AA, Pierson CR, Woltjer RL et al. Neuronal loss in Pelizaeus–Merzbacher disease differs in various mutations of the proteolipid protein 1. Acta Neuropathol. 118(4), 531–539 (2009).
- 9 Garbern JY, Yool DA, Moore GJ et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125(3), 551–561 (2002).
- Nicchia G, Nico B, Camassa L et al. The role of aquaporin-4 in the blood–brain barrier development and integrity: studies in animal and cell culture models. Neuroscience 129(4), 935–944 (2004).
- Jack C, Shiung M, Gunter J et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62(4), 591–600 (2004).
- Juottonen K, Laakso M, Insausti R et al. Volumes of the entorhinal and perirhinal cortices in Alzheimer’s disease. Neurobiol. Aging 19(1), 15–22 (1998).
- Berti V, Mosconi L, Glodzik L et al. Structural brain changes in normal individuals with a maternal history of Alzheimer’s. Neurobiol. Aging 32(12), 2325.e17–e26 (2011).
- Honea R, Swerdlow R, Vidoni E, Goodwin J, Burns J. Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology 74(2), 113–120 (2010).
- Galton CJ, Patterson K, Graham K et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology 57(2), 216–225 (2001).
- Agosta F, Scola E, Canu E et al. White matter damage in frontotemporal lobar degeneration spectrum. Cerebral Cortex 22(12), 2705–2714 (2012).
- Duning T, Warnecke T, Mohammadi S et al. Pattern and progression of white-matter changes in a case of posterior cortical atrophy using diffusion tensor imaging. J. Neurol. Neurosurg. Psych. 80(4), 432–436 (2009).
- Hampel H, Teipel S, Alexander G, Pogarell O, Rapoport S, Möller HJ. In vivo imaging of region and cell type specific neocortical neurodegeneration in Alzheimer’s disease. Perspectives of MRI derived corpus callosum measurement for mapping disease progression and effects of therapy. Evidence from studies with MRI, EEG and PET. J. Neural. Transm. 109(5–6), 837–855 (2002).
- Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer’s disease. Ann. NY Acad. Sci. 1097(1), 259–264 (2007). & Significant changes in frontal, temporal and parietal white matter diffusion tensor parameters in mild cognitive impairment and Alzheimer’s disease patients demonstrate an association with cognitive function.
- Gold BT, Powell DK, Andersen AH, Smith CD. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer’s disease. Neuroimage 52(4), 1487–1494 (2010).
- Smith CD, Chebrolu H, Andersen AH et al. White matter diffusion alterations in normal women at risk of Alzheimer’s disease. Neurobiol. Aging 31(7), 1122–1131 (2010).
- Lee J-T, Xu J, Lee J-M et al. Amyloid-b peptide induces oligodendrocyte death by activating the neutral sphingomyelinase–ceramide pathway. J. Cell Biol. 164(1), 123–131 (2004).
- 3 Roher AE, Weiss N, Kokjohn TA et al. Increased Ab peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry 41(37), 11080–11090 (2002).
- Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 32(8), 1341–1371 (2011).
- Bartzokis G, Lu PH, Mintz J. Human brain myelination and amyloid beta deposition in Alzheimer’s disease. Alzheimers Dement. 3(2), 122–125 (2007).
- Acosta-Cabronero J, Alley S, Williams GB, Pengas G, Nestor PJ. Diffusion tensor metrics as biomarkers in Alzheimer’s disease. PloS ONE 7(11), e49072 (2012). &Diffusivity changes appear to be stage specific in Alzheimer’s disease; increased axial and mean diffusivity are observed in early stages followed by increased radial diffusivity with disease progression and severity.
- Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 133(2), 529–539 (2010).
- Pike C, Cummings B, Monzavi R, Cotman C. b-amyloid-induced changes in cultured astrocytes parallel reactive astrocytosis associated with senile plaques in Alzheimer's disease. Neuroscience 63(2), 517–531 (1994).
- Santillo AF, Gambini JP, Lannfelt L et al. In vivo imaging of astrocytosis in Alzheimer’s disease: an 11C-L-deuteriodeprenyl and PIB PET study. Eur. J. Nucl. Med. Mol. Imaging 38(12), 2202–2208 (2011).
- Steele ML, Robinson SR. Reactive astrocytes give neurons less support: implications for Alzheimer’s disease. Neurobiol. Aging 33(2), 423.e1–e13 (2012).
- Zhang Y, Schuff N, Du AT et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain 132(9), 2579–2592 (2009).
- Stokin GB, Lillo C, Falzone TL et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307(5713), 1282–1288 (2005).
- Wheeler-Kingshott CA, Cercignani M. About ‘axial’ and ‘radial’ diffusivities. Magn. Reson. Med. 61(5), 1255–1260 (2009).
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn. Reson. Med. 65(6), 1532–1556 (2011).
- Chung HW, Chou MC, Chen CY. Principles and limitations of computational algorithms in clinical diffusion tensor MR tractography. Am. J. Neuroradiol. 32(1), 3–13 (2011). & Explanation of obstacles in implementing advanced diffusion tensor imaging techniques as clinical tools.
- Poupon C, Roche A, Dubois J, Mangin JF, Poupon F. Real-time MR diffusion tensor and Q-ball imaging using Kalman filtering. Med. Image Anal. 12(5), 527–534 (2008).
- Papadakis NG, Martin KM, Mustafa MH et al. Study of the effect of CSF suppression on white matter diffusion anisotropy mapping of healthy human brain. Magn. Reson. Med. 48(2), 394–398 (2002).
- Falconer JC, Narayana PA. Cerebrospinal fluid-suppressed high-resolution diffusion imaging of human brain. Magn. Reson. Med. 37(1), 119–123 (1997).
- Shimony JS, Mckinstry RC, Akbudak E et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis1. Radiology 212(3), 770–784 (1999).
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 23(7), 803–820 (2010). nn Identifies important steps in diffusion MRI analysis and associated pitfalls.
- Chou MC, Lin YR, Huang TY et al. FLAIR diffusion-tensor MR tractography: comparison of fiber tracking with conventional imaging. Am. J. Neuroradiol. 26(3), 591–597 (2005).
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med. 62(3), 717–730 (2009).
- Hermoye L, Saint-Martin C, Cosnard G et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29(2), 493–504 (2006). &Shows that reference cohorts of normal participants are needed as myelination of white matter changes during childhood and adolescence.