Review Article - Pharmaceutical Bioprocessing (2017) Volume 5, Issue 4
Dipeptide synthesis and evaluation of antidiabetic activity of 4-hydroxyisoleucine from fenugreek seeds
- *Corresponding Author:
- Sridevi P
Department of Pharmaceutical Analysis
Sri Venkateshwara College of Pharmacy
Madhapur, Hyderabad, India
E-mail: sri_pingala@yahoo.co.in
Abstract
Fenugreek is one of the oldest medicinal plants, originating in India and Northern Africa. The hypoglycemic effects of fenugreek have been attributed to several mechanisms. Under In vitro conditions, the amino acid 4-hydroxyisoleucine(4-OHIL) in fenugreek seeds increased glucose-induced insulin release in human and rat pancreatic islet cells. It was observed that 4-OHILextracted from fenugreek seeds has insulin tropic activity. This amino acid appeared to act only on pancreatic beta cells, since the levels of somatostatin and glucagon were not altered.
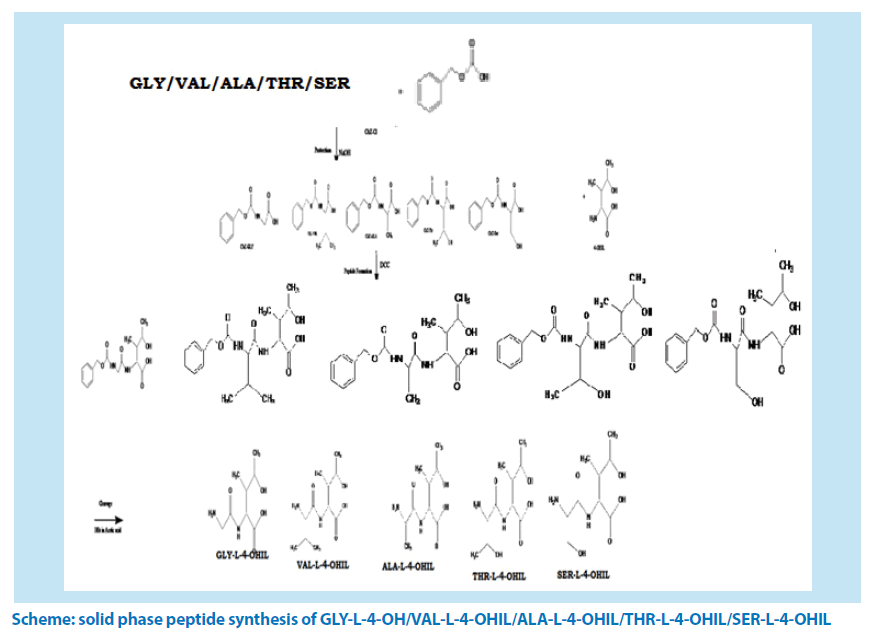
Experimental: In order to separate amino acids from methanol extract of fenugreek seeds, ion exchange chromatography method containing 225H cationic resin was used. Five dipeptides were prepared from 4-OHILnamely; GLY-L-4-OHIL, ALA-L-4-OHIL, SER-L-4-OHIL, VAL-L-4-OHIL, THR-L-4-OHIL using cbZ-Cl in protecting step, DCC in peptide forming step and HBr in acetic acid in deprotection step.
Results: Five dipeptides were confirmed by spectral data like IR, MASS, NMR and evaluated for anti-diabetic activity. The studies showed that only GLY-L-4-OHIL showed improved activity than the other derivatives and it is comparable with 4-OHIL.
Conclusion: Remarkable anti diabetic activity was observed when compared with standard drug and the parent compound 4-OHIL.
Keywords
fenugreek, isoleucine, somatostatin, dipeptides
Introduction
In many developed and developing countries the rise of lifestyle disorders like metabolic disorders has been increasing with the rise in economic development. Although these metabolic disorders can be controlled by increasing the physical activity and following proper diet, but the major concern is changing one’s usual life style. However, this problem may be addressed by taking functional foods which are having beneficial effects on several metabolic disorders. The use of functional foods is sometimes limited due to safety concerns [1].
Diabetes is a lifestyle disorder characterized by high levels of glucose in blood (hyperglycemia) [2]. This metabolic disorder is caused mainly due to insulin deficiency or insulin resistance or sometimes both [3].
Fenugreek is the one of the oldest medicinal plants, originating in India and Northern Africa. As an annual plant, fenugreek grows to an average height of two feet. This plant is locally used in diabetic and non-diabetic people for reducing blood lipids and it also has antioxidant and anti-bacterial activity. This plant is used in therapy of atherosclerosis, rheumatism, sugar lowering, blood lipids lowering and appetizer [4]. The mechanisms by which fenugreek may lower blood glucose levels have not been well established in humans. The effects of fenugreek seeds are mainly due to the gum fraction, but do not exclude a longer term effect of other fenugreek components on glycemia. A novel amino acid derivative extracted from fenugreek seeds, 4-hydroxyisoleucine, stimulated glucose-dependent insulin release in isolated rat and human pancreatic islet cells. In a trial of acute effects in healthy volunteers, trigonelline reduced the early glucose response during an OGTT [5]. In diabetes subjects, fenugreek seeds acts as an anti-diabetic agent by bringing the glycemic control [3,6]. The amino acid present in this plant appear to act only on pancreatic beta-cells, since the levels of somatostatin and glucagon were not altered [4].
4-Hydroxyisoleucine
4-Hydroxyisoleucine (4-OHIL) is the most commonly found free amino acids in fenu-greek seeds. It occurs in two isomeric forms. The major isomer has a (2S, 3R, 4S) configuration which gives 90% of it in the seeds. While the minor isomer has a (2R, 3R, 4S) configuration and it possess both hypoglycemic & insulin tropic properties in vitro or in vivo. Due to this reason, it has becomea potential candidate for treating diabetes [7].
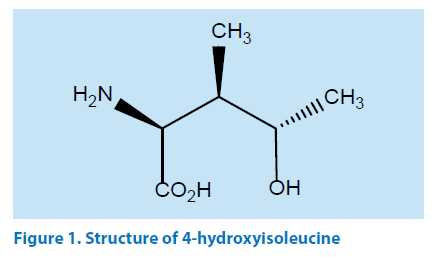
4-OHILis a branch amino acid extracted from the Fenugreek seeds which are having characteristic smell and bitter taste that of fenugreek seeds and leaves. The extracted natural active chemical is highly hygroscopic. The extract has several constituents. The active compound 4-OHIL is isolated by several purifications. The other constituent steroidal saponins (diosgenin, yamogenin, tigogenin, and neotigogenin) are also isolated separately from the extracts and mucilaginous fiber which are believed to be responsible for many of the beneficial effects fenugreek. The mucilage found in fenugreek does not dissolve but instead swells when mixed with fluids. Since the body cannot digest the mucilage from fenugreek it is believed to be an effective laxative. Medicinally, it was used for the treatment of wounds, abscesses, arthritis, bronchitis and digestive problems. The studies have confirmed the presence of 4-OHILin fenugreek seeds in two diastroisomers: the major one being the (2S, 3R and 4S) configuration, representing about 90% of the total content of 4-OHIL, and the minor one being the (2R, 3R and 4S) configuration. The major isomer is presently interesting with respect to experimental evidence indicating its ability to stimulate glucose-induced insulin secretion in micro molar concentrations. Some studies have also shown that the natural analogue of 4-OHIL is more effective as an anti-diabetic agent than a synthetic version [8,9]. The structure of 4-OHIL is depicted in Figure 1.
Biological uses of 4-OHIL
4-OHILin fenugreek extract plays a valuable role in insulin promotion and glucose regulation which may help to reduce body fat [10]. It has nutrient partitioning effect, which means it helps to shuttle nutrients to muscle cells preferentially over fat cells [11]. It appears to be glucose dependent. Higher is the blood glucose level, greater is the insulin promoting response elicited by 4-OHIL. By helping to regulate insulin needs, 4-OHIL works as an adaptogen. It is a non-protein amino acids isolated from fenugreek seeds. 4-OHIL will be more efficient to creatine in the muscle cells to enhance muscle strength and lean muscle mass and increase strength and size of muscle cells.It contains hormone precursors that can increase milk production in nursing mothers and it is widely used for insufficient lactation. 4-OHIL indirectly helps to lower the levels of triglycerides [12]. It has been used to treat bronchitis and asthma. It is also considered as a good herbal remedy for sore throat and coughs. It has been used to promote hair growth both in women and men [13]. It has been used for skin irritation, such as ulcers, boils, eczema, dandruff and cellulite.4-OHIL may help stimulate the secretion of insulin, reduce insulin resistance, and decrease blood sugar levels in diabetes patients [14].
Pharmacological uses of 4-OHIL
4-OHILshowed remarkable Anti diabetic activity, Antiplasmodic activity, Hypolipidemic activity, Immunological activity, Antibacterial activity, Anthelmintic activity, Anti-inflammatory, analgesic activity and Antioxidant activity [15-18].
Experimental
Materials and methods
The wavelength of the compounds was measured by UV-VIS spectrophotometer (UV- -Pharma Spec 1700 Shimadzu) [19].
The HNMR Spectra were obtained on a Bruker DRX-400MHZ Spectrometer in CDCl3 using TMS as internal standard and chemical shifts are expressed in δ scale [20].
FAB mass spectra were recorded on a JEOL SX 102/DA 6000 mass spectrometer using Argon/Xenon (6KV, 10Ma) as the FAB gas. EI mass spectra were recorded on JEOL JMS- -D-300 spectrometer with the ionization potential of 70 eV and ES mass on Quantro- -II, micromass instrument. Silica gel (60-120 mesh) for column chromatography was used. Room temperature mentioned were in the range between 20-400C unless stated otherwise [21-22].
Scheme for the extraction and isolation of amino acid (4-OHIL) from fenugreek seeds [23]:
Powdered seeds
+
50% methanol
+
Keep it for over night
+
Methanol layer is transferred to column having 225H cationic resin (ion exchange chromatography)
+
Wash with 2N Hcl, water, ammonium hydroxide
4-OHIL was identified by ninhydrin reagent using TLC spot test
Scheme: solid phase peptide synthesis of GLY-L-4-OH/VAL-L-4-OHIL/ALA-L-4-OHIL/THR-L-4-OHIL/SER-L-4-OHIL
Solid-phase dipeptide synthesis
Reagents and conditions of the above mentioned scheme are showed in Table 1.
| Synthetic cycle | Reagents | Conditions |
|---|---|---|
| Protection step | Glycine,Alanine,Threonine,Serine,Valine,Cbz-Cl, dioxane | 0-5°C,1hr |
| Peptide forming step | 4-OHIL,DCC,DMF | 25°C, 18hrs |
| De-protection step | HBr in acetic acid, NaOH | 25°C,1hr |
Table 1. Reagentsand conditions of dipeptide synthesis
General procedure
Step I: protection
Gly/Val/Ser/Ala/Thr is dissolved in water and sodium bicarbonate (2eq) using a magnetic stirrer with heating and the mixture was cooled to 5°C and then Cbz-Cl (1.5eq) in paradioxane was added slowly. The resulting mixture is stirred at 0°Cfor 1hour and allowed to warm at room temperature. Then water is added and aqueous layer is extracted 2 times with ethylacetate. The organic layer is back extracted twice with saturated sodium bicarbonate solution. The aqueous layers acidified to a pH of 1 with 10% HCl and extracted three times with ethylacetate and the organic layers were dried and concentrated in vaccum [24,25].
Step II
CarbobenzoxyGly/Val/Ser/Ala/Thrwas neutralized. A solution containing 2.34 gm of 4-OHILin 20 ml of di-methyl formamide was added and shaken for 10 minutes for penetration of the reactant into solid. A solution of 2.31 gm of Dicyclohexylcarbodimide in 4.6 ml of di-methyl formamide was then added and stirred for 18 hours at 25°C, then filtered and washed. Then 3 ml of acetic anhydride and 1 ml of triethylamine in 20 ml of di-methyl formamide was added. After 2 hours, the resin was filtered and washed 4 times each with DMF, ethanol and acetic acid to remove excess reagents and bi-products. A hydrolysate of dried sample of the product showed CarbobenzoxyGly/Val/Ser/Ala/Thr4-OHIL [26].
Step III: The deprotection step (Gly/Val/Ser/ Ala/Thr 4-OH Isoleucine)
CarbobenzoxyGly/Val/Ser/Ala/Thr4-OHILwas decarbobenzoxylated with HBr in acetic acid. The mixture was added to a round bottomed flask equipped with a stirrer and cooled in an ice bath during cleavage process. The peptide resin was slowly added under inert atmosphere andtemperature, it is allowed to proceed for room temperature with stirring for 3 hours. The mixture is filtered and it was saponified by shaking for 1 hour at 25°C in a solution of 40.5 ml of ethanol and 4.5 ml of 2N aqueous NaOH. The filtrate was neutralized with HCl and saponification step was repeated on the resin with more ethanolic sodium hydroxide for another hour. The resin was washed with ethanol and water and neutralized filtrates were combined. Finally Gly/Val/Ser/Ala/Thr4-OHILwas formed [27].
In vitro anti-diabetic activity
Non-enzymatic glycosylation of haemoglobin assay
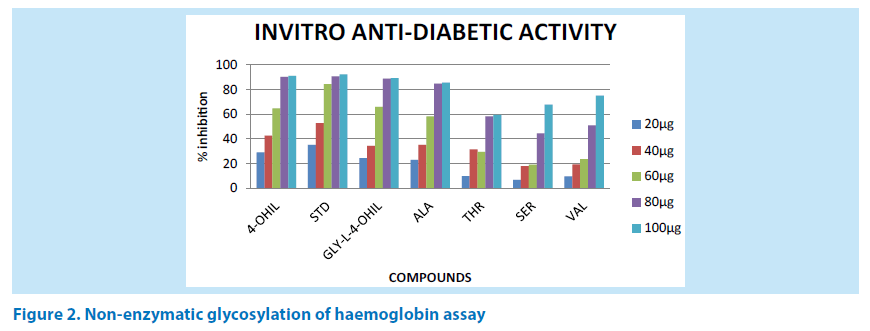
Anti-diabetic activity of 4-OHIL and its dipeptides like Gly-L-4-OHIL, Val-L-4- OHIL, ALA-L-4-OHIL, Thr-L-4-OHIL, Ser-L- 4-OHILwere investigated by estimating degree of non-enzymatic haemoglobin-glycosylation, measured colorimetrically at 520 nm. Glucose (2%), haemoglobin (0.06%) and Sodium azide (0.02%) solutions were prepared in phosphate buffer 0.01 M to get pH 7.4. 1 ml each of the above solution was mixed and 1 ml of each concentrations of 4-OHIL and dieptides were added to above mixture. Mixture was incubated in dark place at room temperature for 72 h [28-31]. The degree of glycosylation of haemoglobin was measured colorimetrically at 520 nm. Alpha- Tocopherol (Trolax) was used as a standard drug for assay. % inhibition was calculated by using the formulae (Figure 2).
Figure 2. Non-enzymatic glycosylation of haemoglobin assay
AT-A0 /A0 × 100
AT = Abs of test
A0 = Abs of control
All the tests were performed in triplicate (n=3)
In vivo anti-diabetic activity of 4-ohil and gly-l-4-ohilin alloxan induced diabetic rats
Experimental design
Diabetes was induced by a single injection of Alloxan 60 mg/kg b.w. to rats fasting for at least 16 h through the tail vein in freshly prepared 10 mmol/l sodium citrate, pH 4.5. Blood glucose levels were measured daily 3 days prior and 7 days after alloxan administration [32]. Development of diabetes mellitus was proven by sustained hyperglycaemia and glycosuria (diabetic rats had glycaemia >250 mg/dl) [33,34].
Group separation
The rats were randomly divided into 5 groups (n = 6) as follows
Group I: Normal control animals I that received saline solution, i.p for 21 days.
Groups II: Diabetic control animals treated with alloxan( Sod CMC, i.p) for 3days and were left untreated for 21 days.
Group III: (Standard) diabetic rats treated with Standard drug Glipizide (5 mg/kg. P.o. daily) for 21 days.
Group IV: Diabetic rats treated with 4-OHIL 200 mg/kg p.o. for 21 days.
Group V: Diabetic rats treated with 4-OHIL 400 mg/kg p.o. for 21 days
Group VI: Diabetic rats treated with 200 mg/kg of GLY-L-4-OHIL p.o. for 21 days.
Group VII: Diabetic rats treated with 400 mg/ kg of GLY-L-4-OHILp.o. for 21 days.
The rats developed diabetes within 2 days after alloxanisation as evidenced by sustained hyperglycaemia and glycosuria. Only those animals with blood glucose level above 250 mg/dl were included in the study. Every week (from 1st week to 3rd week) on 1st, 7th, 14th, and 21st day, blood samples were collected by retro-orbital puncture under light ether anesthesia, then the serum was separated by centrifugation at 2000rpm for 15 min in a cooling centrifuge and blood glucose levels were measured [35,36]. On 21st day, glucose level was finally measured (Figure 3).
The values are expressed as Mean ± S.E.M and n=6 in each group. *P<0.05 (significant as compared with normal control), P<0.0001 for diabetic control vs normal control, P<0.05 for normal control vs standard, P<0.05 for normal control vs 4-OHIL, P<0.05 for normal control vs GLY-L-4-OHIL. All comparisons were made by One Way Anova followed by Dunnet’s t test.
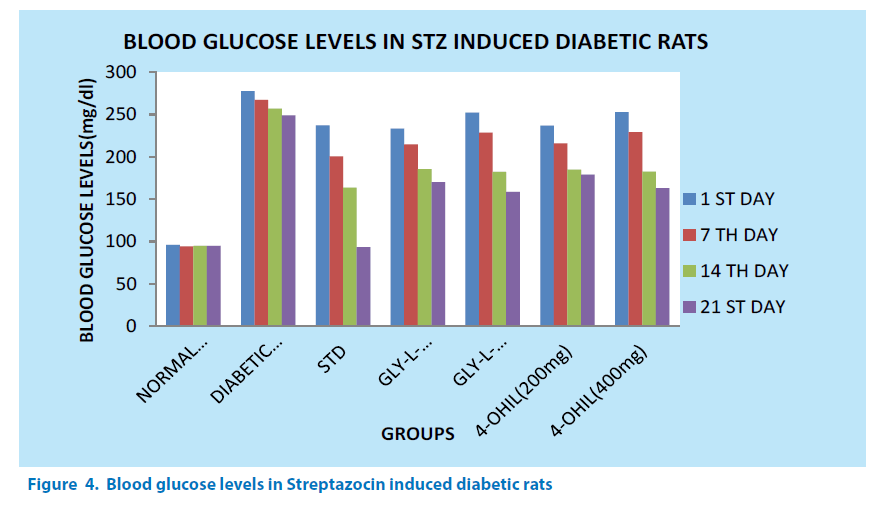
Streptozocin induced diabetes in rats
Experimental study
Diabetes was induced experimentally in rats by a single intraperitoneal injection of a freshly prepared solution of streptozotocin (STZ), at a dose of 80 mg/kg body weight in 0.1 m cold citrate buffer of pH 4.5. After 1, 7, 21 days blood was collected with aseptic precautions from the tail vein of the rats under ether anesthesia and blood glucose levels were determined using Glucometer [37]. Animals were considered to be diabetic if the blood glucose levels were above 250 mg/dl, and those animals only were used for the experimental analysis [38]. Diabetes was developed and stabilized in STZ-treated rats over a period of 7 days; control rats were given citrate buffer 4.5 [39,40]. The animals were followed up to 21 days to check the time required for 4-OHIL and GLY-L-4-OHIL to produce peak hypoglycemic activity (Figure 4).
Group separation
The rats were randomly divided into 5 groups (n = 6) as follows
Group I : Normal control animals I that received citrate buffer (4.5), i.p for 21 days [41,42].
Groups II: Diabetic control animals treated with Streptozocin (Sod CMC, i.p) for 3 days and were left untreated for 21 days.
Group III: (Standard) diabetic rats treated with Standard drug Glibenclamide (25 mg/ kg. P.o. daily) for 21 days.
Group IV: Diabetic rats treated with 4-OHIL 200 mg/kg p.o. for 21 days.
Group V: Diabetic rats treated with 4-OHIL 400 mg/kg p.o. for 21 days
Group VI: Diabetic rats treated with 200 mg/ kg of GLY-L-4-OHIL p.o. for 21 days.
Group VII: Diabetic rats treated with 400 mg/ kg of GLY-L-4-OHIL p.o. for 21 days.
The values are expressed as Mean ± S.E.M and n=6 in each group. *P<0.05 (significant as compared with normal control), P<0.05 for diabetic control vs normal control, P<0.05 for diabetic control vs standard, P<0.05 for diabetic control vs 4-OHIL, P<0.05 for Diabetic control vs GLY-L-4-OHIL. All comparisons were made by One Way Anova followed by Dunnet’s t test.
Results
Physical and chromatographic data of Isoleucine derivatives are mentioned in Table 2 and their spectral data is mentioned below [43-47].
| S. No | Compound | Melting point | %yield | Rf Value |
|---|---|---|---|---|
| 1. | 4-OHIL | 52°C | 68% | 0.8 |
| 2. | GLY-4-OHIL | 54°C | 72% | 0.7 |
| 3. | Val–L-4-OHIL | 52°C | 69% | 0.9 |
| 4. | Ala–L-4-OHIL | 58°C | 62% | 0.8 |
| 5. | Thr–L-4-OHIL | 61°C | 59% | 0.9 |
| 6. | Ser–L-4-OHIL | 59°C | 66% | 0.9 |
Table 2. Physical and thin layer chromatography data of dipeptides
Spectral data
4-OHIL
IR(KBr): 1641cm-1(COOH), 3290cm-1(OH), 3074 cm-1(N-H)
HNMR(200MZ) (solvent: CDCl3) δppm:11.084(d,1H,COOH), 1.41 (d,3H,CH-CH 3), 1.06(d,3H,CH-CH3), 2.31(d,2H,NH2), 2.07(d,1H,OH), 3.48(m,1H,CH-CH3), 3.68(m,1H,CH-NH2), 2.54(m,1H,CH-CH3).
GLY-L-4-OHIL
IR(KBr): 3315.60cm-1(N-H), 1742.39cm-1(C=O), 1660cm-1(C=O), 2960.77cm-1(OH), 1365.19cm-1(C-O), 1460.25cm-1(C-H bending).
HNMR: (200MZ) (solvent: CDCl3) δppm:1.21(d,3H,CH-CH3), 1.06(d,3H,CH-CH 3)2.01 (d, 1H, NH),2.01 (d, 1H, OH), 3.38(m,1H,CH-CH3),3.54 (t,2H,COCH2NH2),8.0(d,1H, CONH), 2.60 (m,1H,CH-CH3),11.00 (s,1H,COOH),4.45 (m, 1H,CHNH).
Mass (m/z): 202.
VAL-L-4-OHIL
IR(KBr): 3315.60cm-1(N-H), 1742.39cm-1(C=O), 1660cm-1 (C=O), 2978cm-1(CH3), 2960.77 cm-1(OH),1365.19cm-1(C-O), 1460.25cm-1(C-H bending).
HNMR(200MZ)(solvent:CDCl3)δppm:8.1(- d,1H,CONH),11.1(s,1H,COOH),0.96(- d,3H,1XCH3), 1.21(d,3H,1XCH3), 1.413(d,3H, CH3),1.594(d,3H,1XCH3), 2.412(d, 2H, NH2), 2.17(d, 1H, OH), 2.746(m, 1H, CH), 3.74(m, 1H, 1XCH), 3.32(m, 1H, 1XCH), 3.66(d, 1H, 1XCH), 4.384 (t,1H,CH).
MASS (m/z): 246 [M+1].
ALA-L-4-OHIL
IR(KBr): 3315.60cm-1(N-H), 1718.39cm-1(C=O),1660 cm-1 (C=O), 2978 cm-1(CH3), 2960.77 cm-1(OH), 1365.19cm-1(C-O), 1460.25cm-1(C-H bending).
HNMR(200MZ)(solvent:CDCl3)δppm:8.1(- d,1H,CONH),11.1(s,1H,COOH),1.06(d,3H,1X-CH 3), 1.21(d,3H,1XCH3), 1.413(d,3H, CH3), 1.594(d,3H,1XCH3), 2.17(d, 1H, OH), 2.68(m, 1H, CH), 3.74(m, 1H, 1XCH), 3.38(d, 1H, 1XCH), 4.45 (t,1H,CH ),2.0(d, 2H, NH2), 1.28(d,3H,1X-CH 3).
MASS (m/z): 218[M+1].
Thr-L-4-OHIL
IR(KBr): 3315.60cm-1(N-H), 1715.39cm-1(C=O),1660 cm-1 (C=O), 2978 cm-1(CH3) 2960.77 cm-1(OH),1365.19cm-1(C-O), 1460.25cm-1(C-H bending).
HNMR(200MZ): (solvent:CDCl3) δppm:8.2(d,1H,CONH),11.1(s,1H,COOH), 0.91(d,3H,1XCH3), 1.21(d,3H,1XCH3), 1.3(d,3H, CH3 ), 2.1(m, 1H, CH), 2.4(d, 1H, OH), 2.5(d, 1H, OH), 2.7(d, 2H,NH2), 3.6(t, 1H, 1XCH), 3.38(m, 1H, 1XCH), 3.8(m, 1H, 1XCH), 4.45 (t,1H,CH ).
MASS (m/z): 248[M+1].
SER-L-4-OHIL
IR(KBr): 3315.60cm-1(N-H), 1742.39cm-1(C=O),1660 cm-1 (C=O), 2978 cm-1(CH3), 2960.77 cm-1(OH), 1365.19cm-1(C-O), 1460.25cm-1(C-H bending).
HNMR(200MZ)(solvent:CDCl3)δppm:8.0(- d,1H,CONH),11.0(s,1H,COOH),0.96(d,3H,1XCH 3), 1.21(d,3H,1XCH3), 2.412(d, 2H, NH2), 2.0(d, 1H, OH), 2.0(d, 1H, OH), 2.68(m, 1H, CH), 3.65(m, 1H, 1XCH), 3.38(m, 1H, 1XCH), 4.384 (t,1H,CH ), 4.03 (t,2H,CH2).
Mass (m/z): 333[M+2].
Discussion
Six Dipeptides from 4-OHILwere synthesized by fallowing the scheme; they are 4-OHIL, GLY-4-OHIL, Val–L-4-OHIL, Ala–L-4-OHIL, Thr–L-4-OHIL, Ser–L-4-OHIL [48-50].
The purified compounds were undergone with physical and spectral analysis and their structural configuration is conformed with Infra-red spectroscopy, Nuclear Magnetic Resonance spectroscopy and Mass spectroscopy.
The evaluation of Isoleucine derivatives were done with in vitro and in vivo anti diabetic activity [51], they are
i. Non-enzymatic glycosylation of haemoglobin assay
ii. Alloxan induced diabetic rats
iii. Streptozocin - induced diabetic rats
Statistical analysis reveals that all the experimental results were expressed as MEAN ± S.D where n=6 the results were subjected to one way ANOVA followed by Dunnetts test for multiple comparisons. The statistical significance of different values with P<0.05 was considered significant.
Out of all the derivatives prepared GLY-L-4- OHIL showed prominent anti diabetic activity when compared with standard and control.
Conclusion
Six dipeptides were synthesized from 4-OH Isoleucine with glycine, Threonine, Serine and Valine using Cbz chloride as protecting agent, DCC and DMF are used in peptide forming step and Hbr in acetic acid is used in deprotection step. Finally formed dipeptides were confirmed by spectral data (IR, MASS and HNMR). The formed dipeptides were evaluated for Anti-diabetic activity in different animal models. In vitro studies revealed that compared to all other dipeptides GLY-L-4- OHIL showed improved activity, then it was evaluated for in vivo studies which showed remarkable anti diabetic activity when compared with standard drug and the parent compound 4-OH Isoleucine.
References
- Muraki E, Yukie H, Chiba H et al. Dose-dependent effects, safety and tolerability of fenugreek in diet-induced metabolic disorders in rats. Lipid. Health. Dis. 10, 240 (2011).
- Sai YRKM, Dattatreya A, Anand SY et al. Biomarkers of internal origin and their significance in diabetes and diabetic complications. J. Diab. Metab. R1, 001 (2011).
- Adapa D, Sarangi TK. A Review on diabetes mellitus: complications, management and treatment modalities. J. Med. Health. Sci. 4(3), (2015).
- Ortiz A, Sansinenea E. Cyclic dipeptides: secondary metabolites isolated from different microorganisms with diverse biological activities. Curr. Med. Chem. 24(25), 2773-2780 (2017).
- Neelakantan N, Narayanan M, Souza RJ et al. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr. J. 13, 7 (2014).
- Sharma RD. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. Nutr. Res. 6, 1353-1364 (1986).
- Milne PJ, Hunt AL, Rostoll K et al. The biological activity of selected cyclic dipeptides. J. Pharm. Pharmacol. 50(12), 1331-1337 (1998).
- Wang X, Lin M, Xu D et al. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules. 22, 2069 (2017).
- Paul R, Choudhury A, Choudhury S et al. Cholesterol in pancreatic β-cell death and dysfunction: underlying mechanisms and pathological implications. Pancreas. J. 45(3), 317-324 (2016).
- Ortiz A, Sansinenea E. Cyclic dipeptides: secondary metabolites isolated from different microorganisms with diverse biological activities. Curr. Med. Chem. 24(25), 2773 -2780 (2017).
- Avalos-Soriano A, De la Cruz-Cordero R, Rosado JL et al. 4-Hydroxyisoleucine from Fenugreek (Trigonella foenum-graecum): effects on insulin resistance associated with obesity. Molecules. 21(11), 1596 (2016).
- Siodłak D. α, β-Dehydroamino acids in naturally occurring peptides. Amino. Acids. 47(1), 1-17 (2015).
- Al‐Habori M, Raman A. Antidiabetic and hypocholesterolaemic effects of fenugreek. Phytotherapy. Res. 12(4), 233-242(1998).
- Broca C, Gross R, Petit P et al. 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am. J. Physiol. Endocrinol. Metab. 277(4), E617-E623 (1999).
- Verma N, Usman K, Patel N et al. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonellafoenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes. Food. Nutr. Res. 60, 10 (2016).
- Avalos-Soriano A, De la Cruz-Cordero R, Rosado JL et al. 4-Hydroxyisoleucine from Fenugreek (Trigonellafoenum-graecum): effects on insulin resistance associated with obesity. Molecules. 21(11), E1596 (2016).
- Sauvaire Y, Petit P, Broca C et al. 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes. 47(2), 206-210 (1998).
- Sauvaire Y, Petit P, Broca C et al. 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes. 47, 206-210 (1998).
- Ajabnoor MA, Tilmisany AK. Effect of Trigonellafoenumgraceum on blood glucose levels in normal and alloxan-diabetic mice. Ethnopharmacol. 22, 45-49 (1988).
- Amin R, Abdul-Ghani AS, Suleiman MS. Effect of Trigonellafeonumgraecum on intestinal absorption. Diabetes. 36, 211ª (1987).
- Stark A, Madar Z. The effect of an ethanol extract derived from fenugreek (Trigonellafoenum-graecum) on bile acid absorption and cholesterol levels in rats. Br. J. Nutr. 69, 277-287 (1993).
- Sauvaire Y, Ribes G, Baccou JC et al. Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids. 26, 191-197 (1991).
- Varshney IP, Sharma SC. Saponins and sapogenins: part XXXII. Studies on Trigonellafoenum-graecum Linn. seeds. J. Ind. Chem. Soc. 43, 564-567 (1966).
- Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br. J. Nut. 55, 643-649 (1986).
- Gopalan C, Ramasastri BV, Balasubramaniyam SC. Nutritive value of Indian food. Nat. Inst. Nutr.
- Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur. J. Clin. Nutr. 44(4), 301-306 (1990).
- Vijaykumar MV, Singh S, Chhipa RR et al. The hypoglycaemicactivit of fenugreek seed extract is mediated through the stimulation of an insulin signaling pathway. Br. J. Pharmcol. 146, 41-48 (2005).
- Mohammad S, Taha A, Akhtar K et al. In vivo effect of Trigonellafoenumgraecumonthe expression of pyruvate kinase, phosphoenolpyruvatecarboxykinase and distribution of glucose transporter (GLUT4) in alloxan-diabetic rats. Can. J. Physiol. Pharmacol. 84(6), 647-654 (2006).
- Ribes G, Sauvaire Y, Da Costa C et al. Anti-diabetic effects of subfractions from fenugreek seeds in diabetic dogs. Proc. Soc. Exp. Biol. Med. 182(2), 159-166 (1986).
- Hamden K, Jaouadi B, Carreau S et al. Potential protective effect on key steroidogenesis and metabolic enzymes and sperm abnormalities by fenugreek steroids in testis and epididymis of surviving diabetic rats. Arch. Physiol. Biochem. 116(3), 146-155 (2010).
- Kumar SG, Shetty AK, Salimath PV. Modulatory effect of fenugreek seed mucilage and spent turmeric on intestinal and renal disaccharidases in streptozotocin induced diabetic rats. Plant. Food. Human. Nutr. 60(2), 87-91 (2005).
- Haiyan Li, Lin C, Duan X et al. The effect of disease knowledge training on pregnancy outcome in gestational diabetes. Biomed. Res. 28(22), 10128-10131 (2017).
- Gupta A, Gupta R, Lal B. Effect of Trigonellafoenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J. Assoc. Physicians. Ind. 49: 1057-1061 (2001).
- Zhenzhen Qu, JiangtaoYu, Kun Ma et al. Characteristics of bacterial infection in patients with diabetic foot and its relationship with degree of vascular lesions in lower extremities. Biomed. Res. 28(22), 10181-10185(2017).
- Sharma RD, Raghuram TC. Hypoglycaemic effect of fenugreek seeds in non-insulin dependent diabetic subjects. Nutr. Res. 10, 731-739 (1990).
- Wan-Jun K, Shi L, Shi Y et al. Analysis on distribution, drug resistance and risk factors of multi drug resistant bacteria in diabetic foot infection. Biomed. Res. 28(22), 10186-10190 (2017).
- Song R, Chen X, Zhao Z. Protective effect of vitamin B1 on diabetic peripheral neuropathy (DPN) and its mechanism. Biomed. Res. 28(14), 6210-6215 (2017).
- Neeraja A, Rajyalakshmi P. Hypoglycemic effect of processed fenugreek seeds in humans. J. Food. Sci. Technol. 33, 427-430 (1996).
- Yang L, Zhang X, Ma X et al. Correlation between 25-hydroxy-Vitamin-D and senile diabetic peripheral neuropathy in type 2 diabetes. Biomed. Res. 28(14), 6286-6289 (2017).
- Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and lipids in type I diabetes. Eur. J. Clin. Nutr. 44, 301-306 (1990).
- Haoyun Li, Zhang C, He L et al. Association of extracellular superoxide dismutase Ala40Thr, Arg213Gly, and Leu53Leu polymorphisms with risk of type 2 diabetes mellitus in a Chinese population. Biomed. Res. 28(14), 6392-6400 (2017).
- Prasanna M. Hypolipidemic effect of fenugreek: a clinical study. Ind. J. Pharm. 32, 34-36 (2000).
- Chaolin Li, Huafeng Li, Lujie Zhao et al. Thyroid stimulating hormone (TSH) and free thyroxine concentrations are associated with lipids metabolism in euthyroid new-onset type 2 diabetic subjects. Biomed. Res. 28(14), 6484-6491(2017).
- Chia-Ling J, Chuan-Chuan H, Yu-Shan T et al. The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase IV inhibitor for the management of type 2 diabetes. Biomedicine. 5(3), 14 (2015).
- Jiang N, Zhang S, Zhu J et al. Hypoglycemic, hypolipidemic and antioxidant effects of peptides from red deer antlers in streptozotocin-induced diabetic mice. Tohoku. J. Exp. Med. 236, 71-79 (2015).
- Mapelli C, Natarajan SI, Jean-Philippe M et al. Eleven amino acid glucagon-like peptide-1 receptor agonists with antidiabetic activity. J. Med. Chem. 52(23), 7788-7799 (2009).
- Sathiavelu A, Sangeetha S, Archit R et al. In Vitro anti-diabetic activity of aqueous extract of the medicinal plants Nigella sativa, Eugenia jambolana, Andrographispaniculata and Gymnemasyl vestre. Int. J. Drug. Dev. Res. 5(2), 323-328 (2013).
- Sattar NA, Hussain F, Iqbal T et al. Determination of in vitro antidiabetic effects of Zingiber officinale Roscoe. Brazilian. J. Pharm. Sci. 48(4), (2012).
- Susanna P, Shetty K, Obeid T. In Vitro assays of anti-diabetic and anti-hypertensive potential of some traditional edible plants of qatar. J. Med. Active. Plants. 4(3), 22-29 (2015).
- Pal-Bhadra VRSM, Arora N, Santosh SP et al. Target sites for microRNA expressed in pancreatic islets in Type 2 diabetes mellitus associated genes. Online. J. Bioinformatics. 11(2), 224-243 (2010).
- Duddela S, Sekhar PN, Padmavati GV et al. Probing the structure of human glucose transporter 2 and analysis of protein ligand interactions. Med. Chem. Res. 19(8), 836-853 (2010).