Review Article - Interventional Cardiology (2022)
Distal radial access balloon aortic valvuloplasty
- Corresponding Author:
- Zoltán Ruzsa
Division of Invasive Cardiology,
Department of Internal Medicine,
University of Szeged,
Szeged,
Hungary,
E-mail: ruzsa.zoltan@med.u-szeged.hu
Received date: 28-Mar-2022, Manuscript No. FMIC-22-58625; Editor assigned: 30-Mar-2022, PreQC No. FMIC-22-58625 (PQ); Reviewed date: 15-Apr-2022, QC No. FMIC-22-58625; Revised date: 22-Apr-2022, Manuscript No. FMIC-22-58625 (R); Published date: 29-Apr-2022, DOI: 10.37532/1755- 5310.2022.14(S9).205
Abstract
Balloon Aortic Valvuloplasty (BAV) conducted through the radial artery is a new approach that may broaden the practical indications of aortic valvuloplasty due to the very low rate of vascular complications reported. Knowing the technique, which sheaths are compatible with the radial artery, which balloons are compatible with the respective sheaths is essential for the operator when embarking on a mini-BAV technique, but the learning curve is short and the benefits are many.
Keywords
Distal radial access • Snuffbox approach • Vascular complications • Balloon aortic valvuloplasty • Transcatheter aortic valve implantation • Frailty
Abbrevations
BAV: Balloon Aortic Valvuloplasty; TAVI: Transcatheter Aortic Valve Implantation; DRA: Distal Radial Access
Introduction
The relevance of Balloon Aortic Valvuloplasty (BAV) in the management of aortic stenosis has revived with the development of the Transcatheter Aortic Valve Implantation (TAVI) technique and taking into account the increasing frequency of severe symptomatic aortic stenosis in elderly and fragile individuals [1,2]. From palliative and bridge treatment to TAVI or surgical aortic valve replacement, the indications have been expanded to symptomatic improvement prior to consideration of definitive TAVI intervention [1,3]. The predominant access site for the BAV is the femoral artery, although the radial artery can safely accommodate 6-10 F sheaths with compatible balloons. Vascular complications during BAV remain the most common procedural complications despite smaller delivery systems and improved operator experience (comparable with that of TAVI) [2]. Subclavian and brachial access has been proposed to achieve fast mobilization and reduced hospital stays (crucial in this subpopulation) but is still detrimental in terms of vascular complications. Consequently, a transradial mini-invasive approach with rapid pacing through a 0.035 inch left ventricular support wire has been thoroughly encouraged, and promising results have been reported [4]. In recent years, a Distal Transradial Approach (DRA), also known as the snuffbox approach, has gained popularity among the interventional community due to several advantages: Minimal risk of hand ischemia due to blood flow preservation in the forearm, faster hemostasis, reduced nursing staff time, greater comfort and ergonomics for both the patient and the operator, preservation of the proximal forearm’s radial segment for future interventions or arterial graft harvesting, and possible lower radiation doses due to arm positioning on the pelvis [5-7]. Given these advantages, DRA competes with the conventional radial approach. Because data on a distal radial approach for BAV is reported only sporadically [8,9], we present our proof-of-concept, step-by-step description of the technique. This review complements the first 2 pilot studies conducted in our centers, making the transition from proximal radial access (the TRAV Study) to distal access (the DR-BAV Study) in BAV procedures [9,10].
Literature Review
The rationale
There are numerous instances in which independent BAV (not conducted immediately before or after TAVI) may be considered in the modern TAVI era. Radial BAV is used to “bridge” extreme or high-risk patients who are unable to undergo aortic valve intervention (TAVI or surgical aortic valve replacement) to a more stable clinical condition, allowing for a more thorough evaluation and treatment decision. Furthermore, BAV may have diagnostic value in low-gradient severe aortic stenosis with left ventricular impairment, allowing physicians to determine if the left ventricle is capable of recovering once the stenosis-induced afterload is removed [4]. Another reason for BAV’s global spread is its use as a bridge to decision before urgent major surgery in patients with an ambiguous prognosis. However, because of its limited duration and efficiency, BAV exposes patients to peri-procedural risks and vascular complications that must be considered. Logistical reasons such as limited health care resources/insurances resulting in an excessively long waiting list should not be forgotten. There are also many non-TAVI centers where the transfer to TAVI centers is difficult, either due to the patient’s clinical condition or the logistical reasons mentioned above.
With the constant improvements in TAVI technology and materials, BAV must follow. Serious adverse events are frequent after BAV, with 7% being related to vascular complications [11] and 2% occurring during temporary pacemaker implantation [12]. Theoretical elimination of these complications could be achieved by performing BAV via radial access without temporary PM implantation. The rationale for using the DRA over proximal radial access can be summarized into 3 reasons: (1) preservation of the forearm radial artery, (2) better ergonomics for the patient and operator and (3) presumably lower radiation doses and shorter procedure times. Kiemeneij, et al. reported that the distal radial artery was occluded in 1.5% of 62 patients and that the forearm radial artery was occluded in 0% of 62 patients who underwent a procedure involving a distal radial approach [5]. They considered that if the radial artery is occluded during the snuffbox DRA, antegrade flow through the superficial palmar arch is maintained. Therefore, a procedure using a distal radial approach does not cause forearm radial artery occlusion, which is of particular importance when performing BAV, as large sheaths are associated with a high vessel occlusion rate, Tumscitz, et al. reported a worrisome 20% radial occlusion rate at a 30-day followup in patients with proximal radial BAV [13]. Thus, a distal radial approach is considered an effective option from the viewpoint of radial artery preservation, but notably, the vessel diameter of the distal radial artery is smaller than that of the proximal radial artery, Norimatsu, et al. reported a difference in vessel diameter between the distal radial artery and proximal radial artery of 0.5 ± 0.4 mm and a distal-/proximal radial artery ratio of 0.8 ± 0.1 [14]. Nevertheless, a 7-8 Fr Glidesheath Slender can be inserted into the distal radial artery [15,16], although the vessel diameter should be evaluated using ultrasound prior to intervention. The versatility of the radial artery that can accommodate devices larger than its nominal diameter has been proven during BAV procedures, with 7-8 Fr sheaths and bulky balloons [9].
The technique
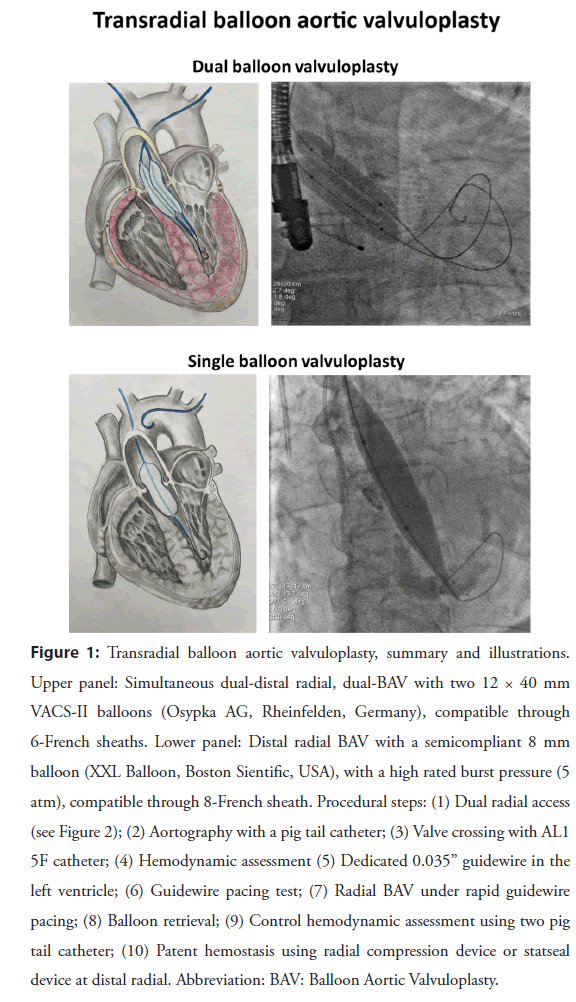
Puncture: After local anesthesia, both distal radial arteries were punctured under ultrasonography guidance with a dedicated transradial needle and sheath (Terumo Co., Japan, 5 F). The details of the puncture technique have been described elsewhere [5-7]. Aortography was performed using a pigtail catheter in a left lateral 30-degree view with 15 ml contrast through the left radial approach. The aortic valve was passed with a Terumo guidewire (Terumo IS, Tokyo, Japan) using an Amplatz left 1.0 or 2.0 diagnostic catheter (Cordis, Miami Lake, USA), and then the Terumo guidewire was replaced with a regular 260 cm 0.035” J-tip guidewire (Medtronic, Minneapolis, USA). The Amplatz catheter was changed to a diagnostic pigtail catheter, and the baseline pressure gradient was measured. After baseline pressure recordings, the Amplatz Super-Stiff or a Safari 0.035” 260 cm J-tip guidewire (Boston Scientific, Marlborough, USA) was advanced into the left ventricle, and then a 6 F radial sheath was exchanged for an 8 F short femoral sheath (Terumo IS, Tokyo, Japan). If resistance was encountered, the sheath could be inserted only 3-4 cm, which is sufficient for safe balloon retrieval. The balloon size was selected by computed tomography angiography measurements or by echocardiographic measurements in urgent cases (balloon to annulus diameter ratio 0.8). The procedure is usually performed with 16-22 mm low-profile compliant balloons (in our center, 16- 22 mm × 4 cm, 8 F-compatible VACS II balloons, Osypka AG, Rheinfelden, Germany, OR an 18 mm × 4 cm × 120 cm XXL Balloon Dilation Catheter, Boston Scientific) (illustrative example in Figure 1). BAV was performed under rapid temporary high-rate (180-220 bpm) right ventricular dual-lead stimulation: The distal stiff 0,035-inch guidewire core served as the negative lead and a needle placed in the subcutaneous tissue of the groin served as the positive lead. Per our local protocol, we usually prefer a 16- 18 mm diameter balloon because semicompliant balloon material enables some variation in the diameter above or below the nominal level. Thus, the balloon diameter was modulated with manual inflation to achieve a balloon-to-annulus ratio of 1:1, which was confirmed by complete sealing of the valvular orifice and aortic pulse abrogation. After balloon removal, pressure measurements with a pig tail catheter were performed again. Target reduction of the mean gradient was >30%. A second balloon dilatation was performed at the operator’s discretion. Usually, at least 2 inflations are required to achieve a significant gradient drop. The compliant balloon is somewhat slippery, and a stiffer wire can help stabilize the balloon by pushing on the wire against the left ventricle to anchor the balloon and perform a longer pacing run before inflating the balloon. A cocktail of 5000 UI of unfractioned heparin and 250 mcg nitroglycerine was administered directly into the radial artery through the sheath.
Figure 1: Transradial balloon aortic valvuloplasty, summary and illustrations. Upper panel: Simultaneous dual-distal radial, dual-BAV with two 12 × 40 mm VACS-II balloons (Osypka AG, Rheinfelden, Germany), compatible through 6-French sheaths. Lower panel: Distal radial BAV with a semicompliant 8 mm balloon (XXL Balloon, Boston Sientific, USA), with a high rated burst pressure (5 atm), compatible through 8-French sheath. Procedural steps: (1) Dual radial access (see Figure 2); (2) Aortography with a pig tail catheter; (3) Valve crossing with AL1 5F catheter; (4) Hemodynamic assessment (5) Dedicated 0.035” guidewire in the left ventricle; (6) Guidewire pacing test; (7) Radial BAV under rapid guidewire pacing; (8) Balloon retrieval; (9) Control hemodynamic assessment using two pig tail catheter; (10) Patent hemostasis using radial compression device or statseal device at distal radial. Abbreviation: BAV: Balloon Aortic Valvuloplasty.
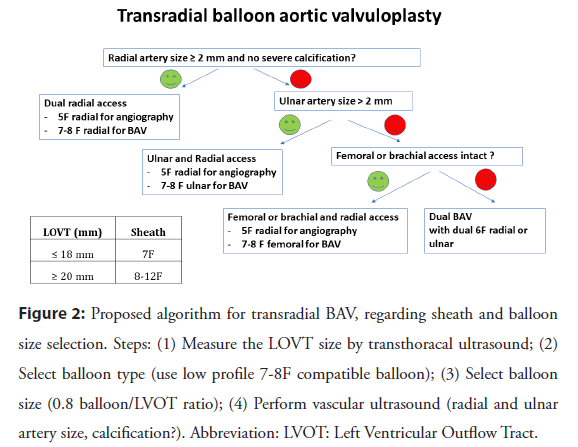
Figure 2: Proposed algorithm for transradial BAV, regarding sheath and balloon size selection. Steps: (1) Measure the LOVT size by transthoracal ultrasound; (2) Select balloon type (use low profile 7-8F compatible balloon); (3) Select balloon size (0.8 balloon/LVOT ratio); (4) Perform vascular ultrasound (radial and ulnar artery size, calcification?). Abbreviation: LVOT: Left Ventricular Outflow Tract.
Postoperative treatment: After the procedure, the sheath was immediately removed, and hemostasis was achieved by applying one hemostatic StatSeal disc (Biolife, LLC, USA) with a compressive bandage for 4 hours. The patient was examined overnight in the intensive care unit.
Per the center’s protocol, distal radial puncture is performed under ultrasound guidance regardless of radial access expertise (due to a higher success rate and faster and safer cannulation). Ultrasound can also be used to determine whether the radial artery is sufficiently large to accommodate the necessary sheath and check for calcifications that can block balloon delivery. Additional lidocaine should be given before large sheath exchange, as spasm is the main impediment restraining large delivery systems. Long sheaths that reach the brachial artery are also a practical solution for preventing radial spasms and balloon retraction. Nitroglycerin can be used in selected patients but should be avoided in patients with critical aortic stenosis and marginal blood pressure.
The size of the balloon is very important to sheath size selection and is determined by the size of the annulus. The size of the delivery sheath can be decreased with the use of peripheral balloons or with a decreasing balloon size. Operators can use semicompliant (Tyshak II, NuMED, USA; XXL Balloon, Boston Sientific, USA) or noncompliant aortic balloons (Z-Med, NuMED, USA; Maxi LD Balloon, Cordis, Florida, USA). Semicompliant balloons tend to have a lower profile and therefore require smaller vascular access sheaths, which helps reduce vascular complications in the elderly population. The trade-off, however, is that these balloons have less predictable inflation diameters than noncompliant balloons and have lower rated burst pressures. Dedicated BAV balloons have a larger profile, but peripheral balloons can be used in smaller sheaths. Smaller balloons can have smaller hemodynamic effects than larger balloons. In our study, the 0.8 annulus-to-balloon ratio was safe and had a satisfactory clinical effect, but the hemodynamic effect was not always improved. In cases where there is not enough decrease of the gradient and an upgrade to a valvuloplasty balloon requiring a vascular sheath >10 F is required, then crossover to femoral access is mandatory or dual balloon valvuloplasty can be used with 2 balloons. In some cases, the use of sheathless 7 F guides or long sheaths allows balloon manipulation when delivery and retraction are anticipated to be difficult. The decision to use such long catheters is based on US examination, and small-caliber and diseased arteries are prone to additional spasm and device entrapment. The proposed algorithm is summarized on Figure 2.
Discussion
Distal radial access: Less occlusion, longer learning curve
Radial artery cannulation via DRA was initially used by anesthesiologists for perioperative patient monitoring [17] and more recently for retrograde radial artery recanalization of radial artery occlusion [18]. Because radial artery occlusion represents one of the few remaining limitations of the radial arterial approach and this risk increases with larger sheaths, the perspective of puncturing the permeable distal segment of the radial artery and re-open the occluded proximal radial artery should be also kept in mind [19]. The vessel at the anatomical snuffbox is roughly 80% of the size of the radial artery at the forearm with mean diameter is 2.4 mm [7,14], and is more tortuous. Relevant drawbacks of the DRA are the more challenging puncture of a smaller artery and the higher rate of failure to advance the wire and subsequent arterial cannulation. The learning curve with distal radial access is navigable, but there are nuances to the technique that should be appreciated in the interest of patients’ safety. At least 100 cases are needed to consistently maintain a high success rate of >96% [7,20]. This is of particular importance if the aim is to push DRA in more difficult scenarios such as STEMI or unstable, high-risk cases [21]. Marked improvement can be felt within the 50 cases, which is encouraging for a novice operator [22].
Radial complications in BAV
The SOFTLY II safety data revealed an extremely low rate of vascular complications: Zero of 330 patients had serious vascular problems, while one of 330 VARC-2 patients had mild vascular complications [4]. One patient underwent minor vascular surgery as a result of balloon rupture and trapping in the radial artery. The surgical removal was carried out in the catheterization laboratory and had no effect on the in-hospital stay or the necessity for blood cell transfusions. As a result, caution should be taken to avoid balloon rupture during radial BAV. The DRBAV safety data reported no intra or periprocedural complications (balloon entrapment or compartment syndrome requiring surgical intervention) nor Valve Academic Research Consortium (VARC) 2 bleeding complications occurred in the study population. Only minor VARC-2 bleeding was reported (3 local hematomas not requiring surgery). At the 30-day echocardiographic follow-up, the radial artery was patent in 94% of the patients, and no patient showed symptoms related to radial artery occlusion [9].
Conclusion
In most patients, mini-invasive distal radial balloon aortic valvuloplasty can be performed as a safer alternative to the traditional femoral access. Given the short learning curve and extra benefits of this novel technique, it has the potential to become a new standard of care in the future. Furthermore, transradial BAV technologies may progress in the future, allowing the use of thinner sheaths and larger balloons. Of course, some patients will simply not have sufficiently large radial arteries for an 8-Fr sheath, and the ultimate question will be whether DR-BAV is a safer bridge to SAVR or TAVI than the femoral approach when feasible. Randomized studies are needed to verify this potential benefit. studies will help to gain better understanding of this important aspect of this infection that has represented an unprecedented challenge for the entire healthcare community.
References
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 143(5): 35-71 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Dall'Ara G, Tumscitz C, Grotti S, et al. Contemporary balloon aortic valvuloplasty: Changing indications and refined technique. Catheter Cardiovasc Interv. 97(7): 1033-42 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Piayda K, Wimmer AC, Sievert H, et al. Contemporary use of balloon aortic valvuloplasty and evaluation of its success in different hemodynamic entities of severe aortic valve stenosis. Catheter Cardiovasc Interv. 97(1): 121-29 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Tumscitz C, Di Cesare A, Balducelli M, et al. Safety, efficacy and impact on frailty of mini-invasive radial balloon aortic valvuloplasty. Heart. 107(1): 874-80 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 13(7): 851-57 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Achim A, Szűcsborus T, Sasi V, et al. Distal radial secondary access for transcatheter aortic valve implantation: The minimalistic approach. Cardiovasc Revasc Med. S1553-8389(21)00748-X (2021).
[CrossRef] [Google Scholar] [PubMed]
- Achim A, Kákonyi K, Jambrik Z, et al. Distal radial artery access for coronary and peripheral procedures: A multicenter experience. J Clin Med. 10(24): 5974 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Di Cesare A, Tonet E, Campo G, et al. Snuffbox approach for balloon aortic valvuloplasty: A case series. Catheter Cardiovasc Interv. 97(5): E743-E747 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Achim A, Szűcsborus T, Sasi V, et al. Safety and feasibility of distal radial balloon aortic valvuloplasty: The DR-BAV study. JACC Cardiovasc Interv. 15(6): 679-681 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Molnár L, Papp R, Szigethi T, et al. Safety and feasibility of Transradial Aortic Valve Valvuloplasty (TRAV study). Postepy Kardiol Interwencyjnej. 17(4): 381-388 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Kleczynski P, Kulbat A, Brzychczy P, et al. Balloon aortic valvuloplasty for severe aortic stenosis as rescue or bridge therapy. J Clin Med. 10(20): 4657 (2021).
[CrossRef] [Google Scholar] [PubMed]
- McCann P. A review of temporary cardiac pacing wires. Indian Pacing Electrophysiol J. 7(1): 40-9 (2007).
[Google Scholar] [PubMed]
- Tumscitz C, Campo G, Tebaldi M, et al. Safety and feasibility of Transradial mini-invasive balloon aortic valvuloplasty: A pilot study. JACC Cardiovasc Interv. 10(13): 1375-77 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Norimatsu K, Kusumoto T, Yoshimoto K, et al. Importance of measurement of the diameter of the distal radial artery in a distal radial approach from the anatomical snuffbox before coronary catheterization. Heart Vessels. 34(10): 1615-20 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Gasparini GL, Garbo R, Gagnor A, et al. First prospective multicentre experience with left distal transradial approach for coronary chronic total occlusion interventions using a 7 Fr Glidesheath Slender. EuroIntervention. 15: 126-28 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Saito S, Hajime I, Hosokawa G, et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv. 46(2): 173-78 (1999).
[CrossRef] [Google Scholar] [PubMed]
- Amato JJ, Solod E, Cleveland RJ. A “second” radial artery for monitoring the perioperative pediatric cardiac patient. J Pediatr Surg. 12(5): 715-7 (1977).
[CrossRef] [Google Scholar] [PubMed]
- Babunashvili A, Dundua D. Recanalization and reuse of early occluded radial artery within 6 days after previous transradial diagnostic procedure. Cathet Cardiovasc Intervent. 77(4): 530-6 (2011).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Sanz-Sánchez J, Regazzoli D, Petriello G, et al. The last broken barrier: Retrograde radial artery recanalization prior to transradial coronary interventions. Cardiovasc Revasc Med. 28S: 125-126 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Roh JW, Kim Y, Lee OH, et al. The learning curve of the distal radial access for coronary intervention. Sci Rep. 11(1): 13217 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Achim A, Marc M, Ruzsa Z. Surgical turned-downed CHIP cases-can PCI save the day? Front Cardiovasc Med 9: 872398 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Bach I, Patel P, Nisar T, et al. Abstract 1122â€Â000162: Distal radial access learning curve spanning eighty attempts: A singleâ€ÂCenter, singleâ€Âoperator experience. Stroke Vasc Neurol. 1(S1): e12061 (2021).