Research Article - Neuropsychiatry (2018) Volume 8, Issue 4
Distinct Plasma Cytokine Levels during Early Abstinence in Amphetamine-Dependent Women with and Without Psychosis
- Corresponding Author:
- San-Yuan Huang, M.D, Ph.D
Department of Psychiatry, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
Tel: 011-886-2-8792-7220
Fax: 011-886-2-8792-6715
Abstract
Objective
Amphetamine exposure is associated with significant effects on innate and adaptive immunity. The present study aimed to assess and compare the plasma levels of cytokines between amphetamine-dependent women, either with or without psychosis, and healthy controls.
Methods
We assessed the immune-cytokine markers in 79 female amphetamine addicts during early abstinence and in 49 healthy women to obtain reference values. Women with (AD-P, n=25) or without (AD-NP, n=54) psychosis were also identified. Multiplex magnetic bead assay was used to measure the plasma cytokine expression level simultaneously in all participants.
Results
We demonstrated an increase of T helper 1(Th1)- and Th2- related cytokines levels in both the entire AD cohort and AD-NP subgroup. A similar increase trend was also noted in the AD-P group; however, only three cytokines − interleukin (IL)-5, IL-10, and tumor necrosis factor (TNF)-α showed significant differences. Moreover, the cytokine profiles in the AD-P subgroup revealed decreased levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IFN-γ) and a shift toward Th2 responses when compared with the AD-NP subgroup.
Conclusion
Our results suggest that patients with AD showed immune system activation when compared to the control group, revealing an imbalance in the pro- and anti-inflammatory cytokines, and the existence of an immunological phenotype that may be associated with drug-related psychosis during early abstinence in AD. Future research should replicate and extend these results.
Keywords
Amphetamine Dependence; Psychosis; Cytokine; Immune Balance
Introduction
Amphetamine dependence (AD) is a chronic and relapsing brain disorder, associated with various factors [1], that constitutes a significant public health epidemic, societal burden, and an array of negative consequences for patients [2-4]. Amphetamine may adversely impact immunological responses, which can increase susceptibility to various infectious diseases and may underpin the mechanism for rapid progression from the human immunodeficiency virus (HIV)-positive stage to that of acquired immunodeficiency syndrome (AIDS) [5]. In contrast, chronic and acute amphetamine exposure is associated with exaggerated activation of the innate immune response in the brain [6,7]. Furthermore, substantial clinical evidence has indicated a link between central immune signaling and amphetamine addictive behavior, such as drug reward [8-10]. Circulating cytokines can cross the blood brain barrier, and central immune changes are reflected in peripheral circulation [11,12], which might be important in psychoneuroimmunology.
Recent studies have implied a causal relationship between methamphetamine (MA) and immune dysfunction in adult mammals [13]. In vitro studies revealed that MA could influence both cellular and humoral immunity [14,15]. Animal studies have demonstrated that MA administration produces immunomodulation in both innate and adaptive immunity [16-18] and modifies gene expression in immune cells [19]. MA also alters the expression of proinflammatory cytokines including TNF-α and IL-6 after lipopolysaccharide (LPS) stimulation in the mouse brain [20]. Nevertheless, in previous human studies examining the association between amphetamine and cytokine, adults in remission from MA dependence demonstrated a non-significant difference in plasma level of cytokines (i.e., IL-1β, IL-2, IL-6, IL-10, and TNF-α) compared with non-dependent controls [21]. The following study also showed no significant alteration in plasma level of IL-1βand IL-8 for the MA-active group or MA-remission group versus the control group [22]. Taken together, different cytokine profiles may occur in the early phase and late phases of AD abstinence. However, Miller and Goldsmith [23] assessed immune dysfunction, including peripheral immune cytokine levels, to be an immunophenotype across the entire clinical course in psychosis. Furthermore, recent studies suggested that immune-cytokine imbalances could be present in subjects who are at risk for developing psychosis [24,25], and may have a similar genetic background to amphetamine addicts with drug-induced psychosis [26,27]. To date, psychotic symptoms are among the well-known possible consequences of MA use, with an estimated prevalence of 10 to 60% in MA abusers [28-33]. Subsequently, methamphetamine-associated psychosis (MAP) has been considered as a pharmacological and environmental model of schizophrenia (SCZ) due to pronounced similarities in the clinical presentation, response to treatment and assumed neuromechanisms [34-37]. In addition, MAP and SCZ need not be considered as two separate phenomena, but as two phenomena interlinked in a dynamic way [26]. This relationship between MAP and SCZ may allow researchers to utilize MA use in studying the role of cytokines in SCZ. Intriguingly, an emerging theory of SCZ supposes disturbances of cytokines and inflammatory mediators, possibly originating in part from infectious exposures [38,39]. In line with a hypothesis for cytokine model of SCZ, repeated or chronic exposure to methamphetamine can be considered as an environmental factor [40] to elicit psychotic symptoms, potentially via the involvement of cytokine disturbances. Furthermore, previous studies have shown that repeated drug exposure induces alterations in peripheral immune factor expression that are associated with neuropsychiatric symptoms such as mood and cognitive impairments in patients with AD [21,22]. Taken together, these findings may be consistent with the premise that cytokines can be potent modulators of psychotic symptoms in patients with AD.
We applied a multiplex assay to detect a panel of 10 cytokines, providing a broader spectrum of in vivo changes in the cytokine profiles. This study aimed to investigate: (1) the alterations in plasma cytokines levels in patients with AD, and (2) the differences in plasma cytokines levels between non-psychosis AD patients (ADNP) and AD patients with psychosis (AD-P). In addition to cytokine levels, this study also examined Th2/Th1 cytokine ratios, which can determine the effect of amphetamine and associated drug-induced psychosis in shifting the immunity balance towards Th1 or Th2 response [41-43]. To the best of our knowledge, this is the first study to investigate such a broad range of cytokines to obtain a comprehensive picture of the inflammatory pattern in the early abstinence state among patients with AD.
We included only women in the present study because: (1) Previous cytokine findings are derived mostly from mixed genders with a limited female sample size [21,22] (2) Human and animal studies have suggested that women are more vulnerable to dependence or relapse on MA than men [44-49], and tend to display more severe psychiatric complications [49-53] as well as various psychotic symptoms [54]. (3) Recent studies indicated that the female MA user had dysregulation of hypothalamic-pituitary-adrenal (HPA) axis [52,55], which may be associated with vulnerability to develop psychosis [56,57].
Materials and Methods
▪ Participants
This study was performed in accordance with the 1994 Declaration of Helsinki (ethical laws pertaining to the medical profession). The research protocol was approved by the Institutional Review Board for the Protection of Human Subjects (TSGHIRB 096-05-073) at the Tri-Service General Hospital (TSGH; a medical teaching hospital belonging to the National Defense Medical Center in Taipei, Taiwan). All participants gave their written informed consent after having received a detailed description of the study procedures and were free to withdraw their participation at any time. Each participant was screened for psychiatric conditions by a welltrained psychologist using a Chinese version of the modified Schedule of Affective Disorder and Schizophrenia-Lifetime (SADS-L) [58,59] after initially evaluated by an attending psychiatrist.
The patient group consisted of 79 female patients with AD recruited from drug rehabilitation clinics and one general hospital in Northern Taiwan. The subjects were enrolled voluntarily in our study when they agreed to participate in drugabstinence treatment, consecutively followed in these medical institutions. AD patients were recruited less than 7 days after their last drug use, this period being defined as the early-abstinent state. To date, MA is used predominantly in the crystal form among those AD patients in Taiwan [50] and all of the patients inhaled or smoked MA in this study. The diagnosis of AD was confirmed on the basis of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text- Revision (DSM-IV-TR, American Psychiatric Association, 2000). All patients in this study met the DSM-IV-TR criteria for AD based on interviews and all available information with the help of (1) physicians’ medical records and hospital data and (2) a positive urine toxicology test for amphetamine on the day of registration. The exclusion criteria were as follows: (1) dependence on another substance, except nicotine; (2) major psychiatric disorders such as schizophrenia, bipolar disorder, and major depressive disorder; (3) recent use of medications such as psychotropics, steroids, antibiotics, nonsteroidal anti-inflammatory drugs or immunemodulatory drugs, either during the course of the study or within the month prior to enrollment; (4) general medical conditions associated with immune imbalances such as liver diseases, inflammatory or rheumatologic diseases, cardiovascular disease, respiratory diseases, or recent infections; (5) if women of childbearing age were pregnant or in the post-partum period. The control group consisted of 49 physically and psychiatrically healthy female volunteers enrolled from the community. In addition, there was no family history of psychiatric disorder or substance use disorder in the first-degree relatives of the control subjects.
All patients were interviewed face-to-face with a structured questionnaire to collect information regarding their socio-demographic characteristics, which included current age, BMI, employment status, marital status, cigarette smoking status and education level (number of years of schooling completed). Further investigation regarding their patterns of drug use included their main route of amphetamine administration, age of first amphetamine use, total duration of amphetamine use, and severity of dependence by using Severity of Dependence Scale (SDS) [60].
In addition, the patients were classified into two clinical subgroups: 25 patients with amphetamine-induced psychosis (AD-P group) and the 54 patients without psychosis (AD-NP group). Those who had a history of psychosis prior to amphetamine use and those where psychosis was closely related to other psychoactive drugs were excluded. Current psychotic symptoms were classified as any of the Brief Psychiatric Rating Scale items of suspiciousness, hallucinations or unusual thought content in the past month. The severity of psychotic symptom was also assessed by using Clinical Global Impression of Severity scale (CGI-S). We recruited AD patients free of major and minor psychiatric illnesses except mood and anxiety symptoms (subthreshold mood and anxiety disorders). Co-occurring mood and anxiety symptoms of the addicts by two subgroups were described below: AD-P 64.0% (16/25) versus AD-NP 33.3% (18/54).
▪ Blood collection and cytokine measurements
Peripheral blood samples were collected within 24 hours after recruitment from both healthy controls and the patients with AD. Venous blood samples were obtained by venipuncture and collected in EDTA-containing tubes (BD Vacutainer® K2E (EDTA) 18.0mg Plus Blood Collection Tube (10ml) Ref: 367525) between 0730 and 1000 AM after a night of fasting and bed rest. The same conditions were applied to all of the samples. The blood was placed on ice and centrifuged (3500 rpm for 15 min, at 4 °C) within 1.5 h. The plasma was collected and stored at − 80 °C freezer until the cytokine levels were measured. The samples had not been thawed before the cytokine analysis.
Plasma levels of Th1-related cytokines − interferon gamma (IFN-γ), TNF-α and IL-2, Th2-related cytokines − IL-4, IL-5, IL-6 and IL-10, and other cytokines − IL-1β, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF) − were simultaneously determined by multiplex immunoassay using the human ultrasensitive cytokine magnetic 10-Plex panel (Novex® by Life TechnologiesTM) with a Luminex analyzer (MAGPIX® systems) [61], according to the manufacturer’s instructions,. All analyses were performed in one batch using kits from the same production lot (Catalog#: LHC6004M). The sensitivity of detection (pg/mL) for each cytokine was as follows: IL-1β <0.05, IL-2 <0.1, IL-4<0.5, IL-6<0.1, IL-8<0.1, IL- 10<0.05, IFN-γ<0.5, TNF-α<0.05 and GMCSF< 0.05. Moreover, the inter-assay variation for each cytokine, using the multiplex kit, ranged from 7.0% to 9.8%. Standard curves were created from duplicate values, and all samples were analyzed as single determinations. In this study, we further calculated ratios among Th2 cytokines (IL-4, IL-6, IL-10) and Th1 cytokines (IL- 2, TNF-α, IFN-γ).
▪ Statistical analysis
Between-group differences in demographic and clinical characteristics were compared using Student’s t-test, chi-square test, and analysis of variance (ANOVA) when appropriate. Spearman’s rank correlation test was conducted to examine the association between cytokine concentrations and various variables such as age, body mass index (BMI), duration of AD, onset age of AD and CGI-S in patients with AD. Plasma cytokine levels were normalized through natural logarithmic transformation. Data points were reported as non-transformed raw values (means ± standard deviation [S.D.]), while the statistical analysis was conducted on the natural logarithmic transformed values. Multivariate analysis of covariance was conducted to assess for statistical differences on plasma cytokines levels as dependent variables with diagnostic groups as the main factors in the model, and age, BMI and mood/ anxiety symptoms as covariates to control the confounding effects. All statistical analyses were performed using SPSS statistical software, version 21.0 for Windows (SPSS Inc., Chicago, IL, USA) with the significance level set at p ≤ 0.05 (two tailed).
Results
▪ Demographic data and clinical characteristics of the study groups
Demographic and clinical characteristics of the AD and healthy control groups are shown in (Table 1). There was no significant difference in mean age and BMI between patients with AD and controls (p>0.05). Likewise, there were no significant differences in age or BMI in a threeway comparison among healthy controls, ADNP, and AD-P groups (p=0.143 for age; p=0.795 for BMI). Patients with AD also had significantly lower education attainment (years) than controls (9.73 ± 2.92 versus 14.54 ± 2.74, p<0.001), consistent with epidemiological features of patients with AD. Furthermore, there were no significant differences in the age, BMI, education level, age at onset, duration of AD, or severity of dependence scale [60] between the AD-NP and AD-P groups. Among the patients with AD, there were no significant associations between cytokine expression levels and clinical parameters such as age, BMI, age of onset, duration of AD, or severity of dependence scale (p ≥ 0.05 for all cytokines, as shown in (Table 2). Furthermore, IL-8 was positively correlated with psychotic symptoms severity (p=0.03) in AD-P group, but the correlation became insignificant after Bonferroni correction (p=0.005 was considered as significant (Table 2).
| Variable | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| HCs (n=49) | Total AD (n=79) | AD-NP (n=54) | AD-P (n=25) | pa | pb | pc | pd | |
| Age (years) | 35.33 ± 8.97 | 32.57 ± 9.28 | 32.80 ± 9.10 | 32.08 ± 9.84 | 0.101 | 0.159 | 0.158 | 0.752 |
| BMI (kg/m2) | 21.74 ± 2.81 | 21.58 ± 4.07 | 21.70 ± 4.07 | 21.34 ± 4.14 | 0.843 | 0.955 | 0.668 | 0.718 |
| Education (years) | 14.22 ± 2.99 | 9.96 ± 2.31 | 10.02 ± 2.46 | 9.84 ± 1.99 | <0.001 | <0.001 | <0.001 | 0.752 |
| Employment (Yes/No) | NA | 41/38 | 27/27 | 14/11 | NA | NA | NA | 0.620 |
| Marital status (S/M/D) | NA | 27/37/15 | 16/29/9 | 11/8/6 | NA | NA | NA | 0.198 |
| Cigarette smoking (Yes/No) | NA | 74/5 | 51/3 | 23/2 | NA | NA | NA | 0.649 |
| Age of onset | NA | 25.89 ± 8.43 | 25.91 ± 8.33 | 25.84 ± 8.83 | NA | NA | NA | 0.975 |
| Duration of drug use (years) | NA | 6.68 ± 8.06 | 6.89 ± 8.34 | 6.24 ± 7.55 | NA | NA | NA | 0.742 |
| SDS | NA | 4.82 ± 2.33 | 4.50 ± 2.18 | 5.24 ± 2.51 | NA | NA | NA | 0.344 |
Single/Married/Divorced.
aHealth controls vs. total AD.
bHealth controls vs. AD-NP.
cHealth controls vs. AD-P.
dAD-NP vs. AD-P.
Table 1: Demographic characteristics in female patients with amphetamine dependence and female healthy controls.
| IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-8 | IL-10 | GM-CSF | IFN-γ | TNF-α | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | -.005 | -.046 | -.070 | -.075 | -.099 | .064 | .008 | .005 | -.078 | -.010 |
| BMI | -.006 | -.093 | .013 | .036 | .055 | .124 | .035 | -.076 | .027 | .021 |
| Duration of drug use | .107 | -.015 | -.025 | -.180 | -.039 | -.165 | -.062 | -.062 | -.122 | -.077 |
| Onset age of drug use | -.038 | -.048 | -.042 | .073 | -.035 | .115 | .054 | -.008 | -.010 | -.026 |
| SDS | -.281 | -.036 | -.102 | -.152 | -.108 | -.204 | -.124 | -.122 | -.129 | -.083 |
| CGI-Sa | .366 | -.039 | .052 | .075 | -.360 | .527* | .311 | -.035 | .037 | .311 |
aCGI-S was used to evaluate the severity of psychotic symptoms in AD patients with psychosis.
*Indicates significance of p value<0.05; ** p value<0.01; *** p value<0.001
Table 2: Relationships between plasma cytokine levels and clinical parameters in female AD patients (Spearman’s rho).
▪ Comparison of plasma inflammatory cytokines between the AD and control groups
Plasma cytokine levels in the AD and healthy control groups are shown in (Table 3). After controlling for age and BMI, the total AD group had elevated levels of Th1-related cytokines and Th2-related cytokines. Similar cytokine level changes were also noted in the AD-NP and AD-P subgroups (Table 3 and Figures 1-3). Moreover, in comparison to the AD-NP subgroup, the AD-P subgroup had lower levels of IL-1β (p=0.003), IL-4 (p=0.040), IL-6 (p=0.013), IL-8 (p=0.021), and IFN-γ(p=0.003).
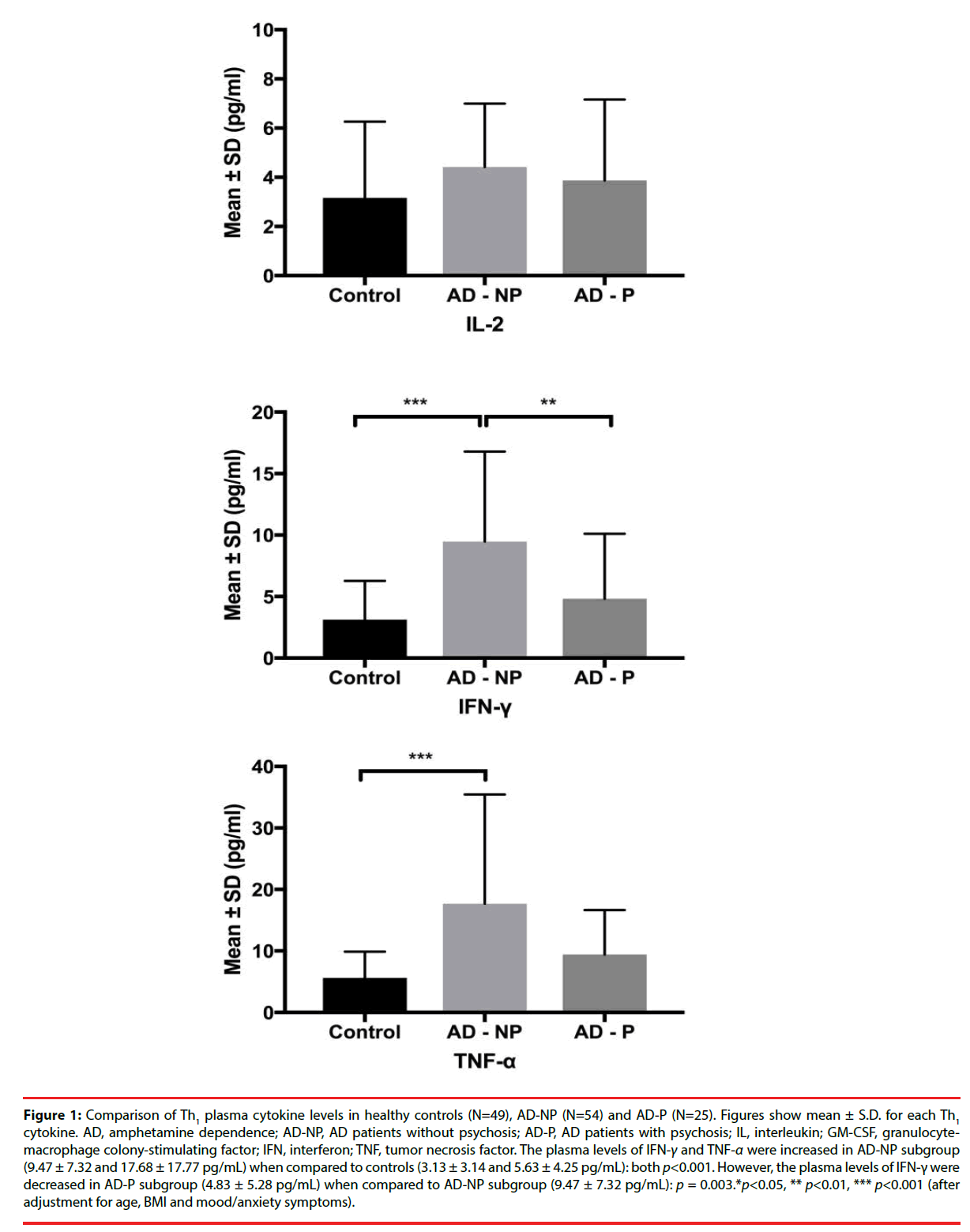
Figure 1: Comparison of Th1 plasma cytokine levels in healthy controls (N=49), AD-NP (N=54) and AD-P (N=25). Figures show mean ± S.D. for each Th1 cytokine. AD, amphetamine dependence; AD-NP, AD patients without psychosis; AD-P, AD patients with psychosis; IL, interleukin; GM-CSF, granulocytemacrophage colony-stimulating factor; IFN, interferon; TNF, tumor necrosis factor. The plasma levels of IFN-γ and TNF-α were increased in AD-NP subgroup (9.47 ± 7.32 and 17.68 ± 17.77 pg/mL) when compared to controls (3.13 ± 3.14 and 5.63 ± 4.25 pg/mL): both p<0.001. However, the plasma levels of IFN-γ were decreased in AD-P subgroup (4.83 ± 5.28 pg/mL) when compared to AD-NP subgroup (9.47 ± 7.32 pg/mL): p = 0.003.*p<0.05, ** p<0.01, *** p<0.001 (after adjustment for age, BMI and mood/anxiety symptoms).
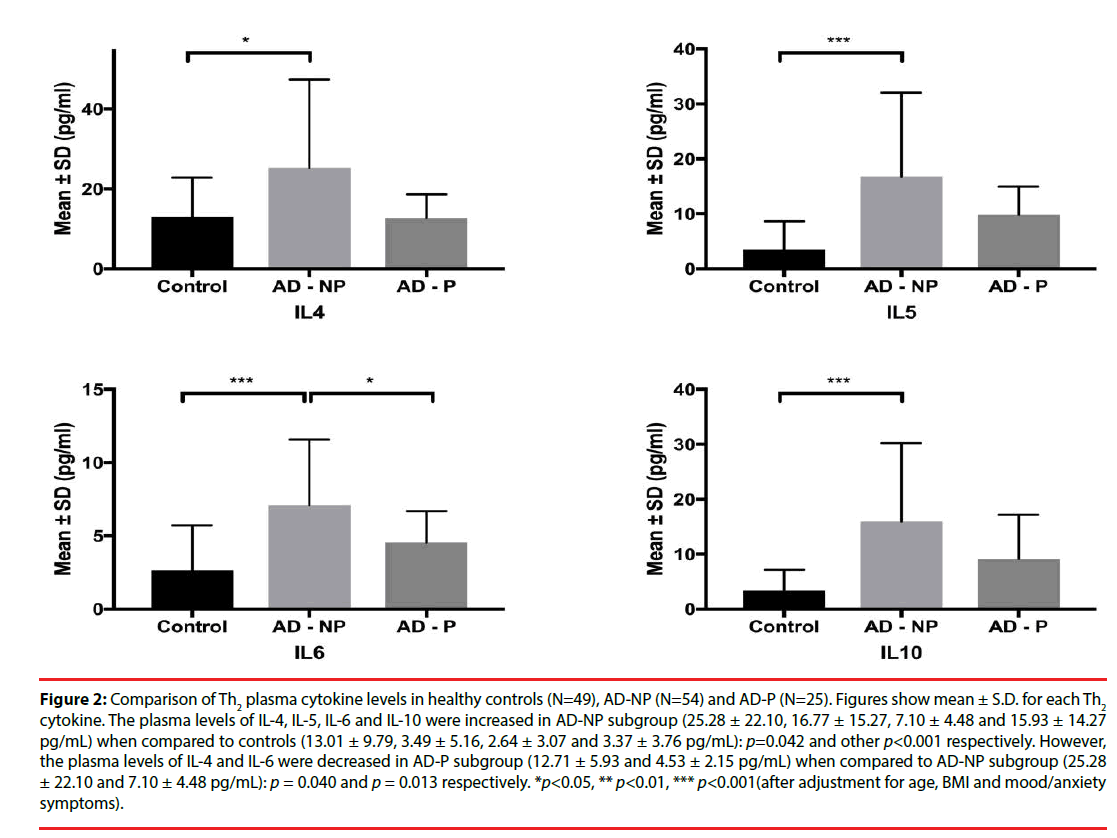
Figure 2: Comparison of Th2 plasma cytokine levels in healthy controls (N=49), AD-NP (N=54) and AD-P (N=25). Figures show mean ± S.D. for each Th2 cytokine. The plasma levels of IL-4, IL-5, IL-6 and IL-10 were increased in AD-NP subgroup (25.28 ± 22.10, 16.77 ± 15.27, 7.10 ± 4.48 and 15.93 ± 14.27 pg/mL) when compared to controls (13.01 ± 9.79, 3.49 ± 5.16, 2.64 ± 3.07 and 3.37 ± 3.76 pg/mL): p=0.042 and other p<0.001 respectively. However, the plasma levels of IL-4 and IL-6 were decreased in AD-P subgroup (12.71 ± 5.93 and 4.53 ± 2.15 pg/mL) when compared to AD-NP subgroup (25.28 ± 22.10 and 7.10 ± 4.48 pg/mL): p = 0.040 and p = 0.013 respectively. *p<0.05, ** p<0.01, *** p<0.001(after adjustment for age, BMI and mood/anxiety symptoms).
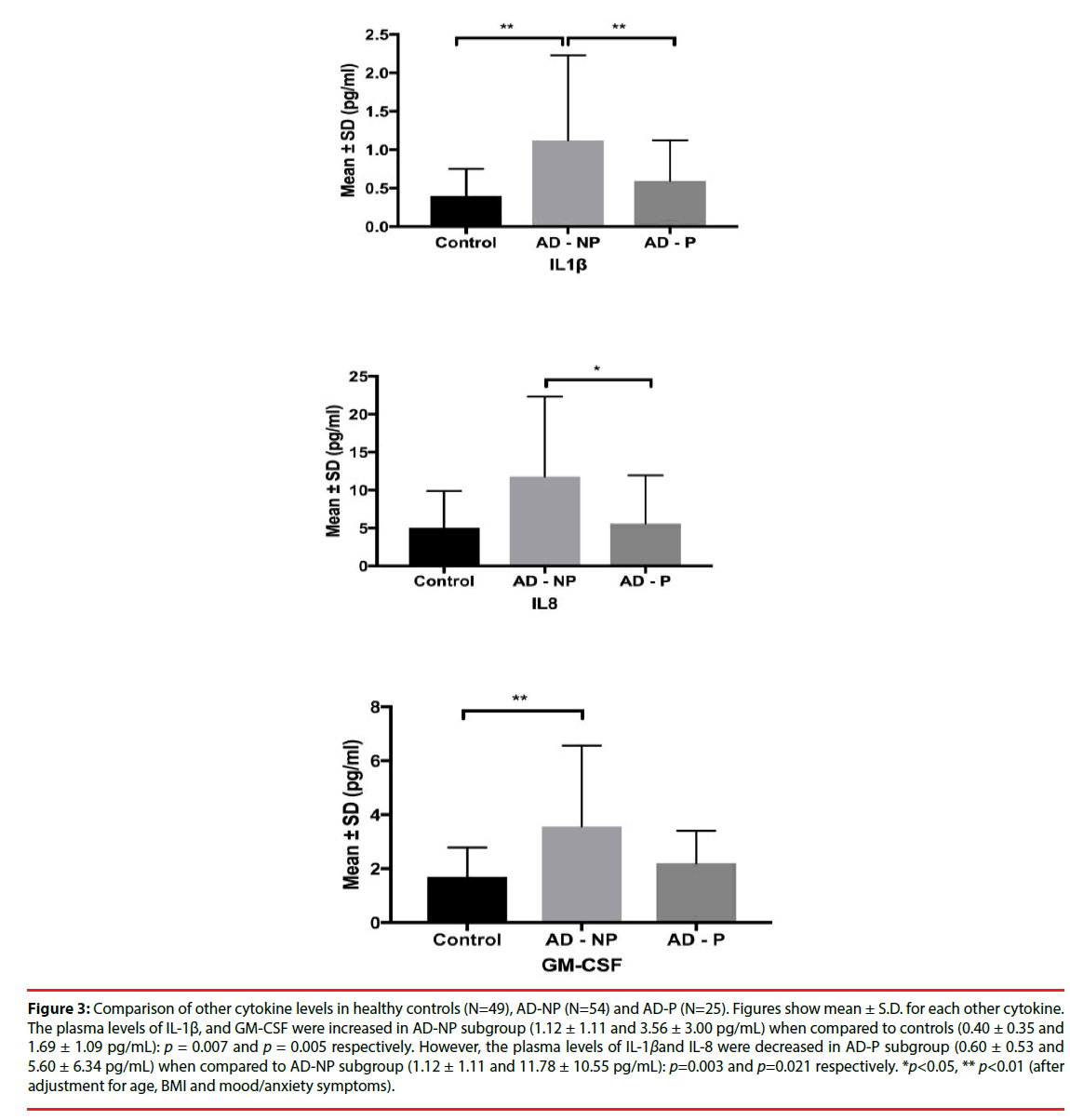
Figure 3: Comparison of other cytokine levels in healthy controls (N=49), AD-NP (N=54) and AD-P (N=25). Figures show mean ± S.D. for each other cytokine. The plasma levels of IL-1β, and GM-CSF were increased in AD-NP subgroup (1.12 ± 1.11 and 3.56 ± 3.00 pg/mL) when compared to controls (0.40 ± 0.35 and 1.69 ± 1.09 pg/mL): p = 0.007 and p = 0.005 respectively. However, the plasma levels of IL-1βand IL-8 were decreased in AD-P subgroup (0.60 ± 0.53 and 5.60 ± 6.34 pg/mL) when compared to AD-NP subgroup (1.12 ± 1.11 and 11.78 ± 10.55 pg/mL): p=0.003 and p=0.021 respectively. *p<0.05, ** p<0.01 (after adjustment for age, BMI and mood/anxiety symptoms).
| Immune factor | Control | AD patients (n=79) | pa | pb | pc | pd | ||
|---|---|---|---|---|---|---|---|---|
| (n=49) | Total (n=79) | AD-NP (n=54) | AD-P (n=25) | |||||
| Th1 cytokines | ||||||||
| IL-2 | 3.16 ± 3.10 | 4.25 ± 2.80 | 4.42 ± 2.57 | 3.87 ± 3.29 | 0.689 | 0.795 | 0.655 | 0.978 |
| IFN-γ | 3.13 ± 3.14 | 8.04 ± 7.06 | 9.47 ± 7.32 | 4.83 ± 5.28 | 0.003** | <0.001*** | 0.645 | 0.003** |
| TNF-α | 5.63 ± 4.25 | 15.14 ± 15.73 | 17.68 ± 17.77 | 9.43 ± 7.22 | <0.001*** | <0.001*** | 0.045* | 0.23 |
| Th2 cytokines | ||||||||
| IL-4 | 13.01 ± 9.79 | 21.41 ± 19.52 | 25.28 ± 22.10 | 12.71 ± 5.93 | 0.074 | 0.042* | 0.877 | 0.040* |
| IL-5 | 3.49 ± 5.16 | 14.62 ± 13.38 | 16.77 ± 15.27 | 9.80 ± 5.16 | <0.001*** | <0.001*** | 0.002** | 0.163 |
| IL-6 | 2.64 ± 3.07 | 6.31 ± 4.08 | 7.10 ± 4.48 | 4.53 ± 2.15 | <0.001*** | <0.001*** | 0.069 | 0.013* |
| IL-10 | 3.37 ± 3.76 | 13.84 ± 13.02 | 15.93 ± 14.27 | 9.13 ± 8.06 | <0.001*** | <0.001*** | 0.002** | 0.072 |
| Other cytokines | ||||||||
| IL-1β | 0.40 ± 0.35 | 1.00 ± 1.03 | 1.12 ± 1.11 | 0.60 ± 0.53 | 0.094 | 0.007** | 0.319 | 0.003** |
| IL-8 | 5.02 ± 4.87 | 10.06 ± 9.91 | 11.78 ± 10.55 | 5.60 ± 6.34 | 0.481 | 0.165 | 0.342 | 0.021* |
| GM-CSF | 1.69 ± 1.09 | 3.14 ± 2.65 | 3.56 ± 3.00 | 2.20 ± 1.20 | 0.006** | 0.005** | 0.183 | 0.189 |
*Indicates significance of p value<0.05; ** p value<0.01; *** p value<0.001
aTotal AD vs. HC,
bAD-NP vs. HC,
cAD-P vs. HC by Multivariate analysis of covariance with covariates as age and BMI.
dAD-NP vs. AD-P by Multivariate analysis of covariance with covariates as age, BMI and mood/anxiety symptoms.
Table 3: Multiplex cytokine analysis of plasma (pg/ml) in female patients with amphetamine dependence (AD) and controls.
▪ Difference in Th2/Th1 cytokine ratios between the AD and control groups
We further analyzed Th2/Th1 cytokine ratios after controlling for age and BMI. Table 4 reveals the changes in the ratios of Th1 and Th2 cytokine levels among patients with AD and controls. In comparison to the control group, the total AD cohort had significantly lower Th2/Th1 ratios of IL-4/IFN-γ(p=0.02), IL-4/TNF-α(p=0.001), and IL-5/IFN-γ(p=0.011) but significantly higher Th2/Th1 ratios of IL-5/IL-2 (p<0.001), IL-5/ TNF-α(p=0.006), IL-6/IL-2 (p<0.001), IL-10/IL-2 (p<0.001), IL-10/ TNF-α(p=0.002), and IL-10/IFN-γ(p=0.005). AD subgroups also had similar cytokines expression patterns in addition to only a decreasing trend in the ratio of IL-4/IFN-γand IL-5/IFN-γ. However, the ratios of IL-4/IFN-γ(p=0.049), IL-5/IFN-γ(p=0.010) and IL-6/IFN-γ(p=0.004) were significantly higher in the AD-P subgroup compared to the AD-NP subgroup.
| Control (n=49) | AD patients (n=79) | pa | pb | pc | pd | |||
|---|---|---|---|---|---|---|---|---|
| Total (n=79) | AD-NP (n=54) | AD-P (n=25) | ||||||
| Th2/Th1 | ||||||||
| IL-4/IL-2 | 4.55 ± 1.73 | 5.42 ± 4.70 | 6.01 ± 5.41 | 4.10 ± 1.91 | 0.331 | 0.171 | 0.903 | 0.083 |
| IL-4/IFN-γ | 6.48 ± 8.42 | 3.57 ± 3.60 | 3.02 ± 1.85 | 4.80 ± 5.78 | 0.020* | 0.003** | 0.515 | 0.049* |
| IL-4/ TNF-α | 2.64 ± 1.39 | 1.85 ± 1.78 | 1.93 ± 2.04 | 1.69 ± 0.98 | 0.001** | 0.002** | 0.001** | 0.890 |
| IL-5/IL-2 | 1.33 ± 2.57 | 3.65 ± 2.05 | 3.85 ± 2.23 | 3.21 ± 1.51 | <0.001*** | <0.001*** | 0<0.001*** | 0.179 |
| IL-5/IFN-γ | 5.29 ± 2.92 | 2.62 ± 2.96 | 2.20 ± 1.65 | 3.58 ± 4.65 | 0.011* | 0.082 | 0.007** | 0.010* |
| IL-5/ TNF-α | 0.76 ± 1.57 | 1.26 ± 0.66 | 1.24 ± 0.66 | 1.29 ± 0.65 | 0.006** | 0.016* | 0.036* | 0.589 |
| IL-6/IL-2 | 0.94 ± 0.86 | 1.71 ± 0.90 | 1.78 ± 0.96 | 1.53 ± 0.72 | <0.001*** | <0.001*** | 0.003** | 0.206 |
| IL-6/IFN-γ | 1.23 ± 1.71 | 1.17 ± 1.09 | 0.91 ± 0.42 | 1.76 ± 1.75 | 0.246 | 0.828 | 0.060 | 0.004** |
| IL-6/ TNF-α | 0.48 ± 0.33 | 0.60 ± 0.33 | 0.58 ± 0.33 | 0.64 ± 0.33 | 0.491 | 0.689 | 0.471 | 0.585 |
| IL-10/IL-2 | 1.43 ± 1.68 | 3.51 ± 2.33 | 3.68 ± 2.16 | 3.15 ± 2.70 | <0.001*** | 0<0.001*** | 0<0.001*** | 0.100 |
| IL-10/IFN-γ | 1.75 ± 3.41 | 2.30 ± 1.98 | 2.03 ± 1.65 | 2.92 ± 2.51 | 0.005** | 0.047* | 0.002** | 0.058 |
| IL-10/ TNF-α | 0.69 ± 0.46 | 1.10 ± 0.51 | 1.11 ± 0.53 | 1.06 ± 0.46 | 0.002** | 0.005** | 0.016* | 0.787 |
* Indicates significance of p value <0.05; ** p value <0.01; *** p value <0.001
a Total AD vs. HC, b AD-NP vs. HC, c AD-P vs. HC by Multivariate analysis of covariance with covariates as age and BMI.
d AD-NP vs. AD-P by Multivariate analysis of covariance with covariates as age, BMI and mood/anxiety symptoms.
Table 4: Difference in ratios of Th2/Th1 cytokines between patients with amphetamine dependence (AD) and controls.
Discussion
This study aimed to examine the immunecytokine effect of amphetamine by simultaneous analysis of 10 cytokines, providing an extended scope with which to elucidate the target cytokines of amphetamine. The first major finding of this study was that chronic exposure to amphetamine had a significant stimulating effect on several peripheral cytokine levels. Out of the entire AD cohort, when compared to healthy controls, the pro-inflammatory cytokines showing elevated levels were IL-6, IFN-γ, TNF-α, and GM-CSF, while the only anti-inflammatory cytokines showing elevated levels was IL-5 and IL-10 in the view of innate immunity. A similar cytokine pattern, along with a significantly increased level of IL-1β and IL-4 was also observed in the ADNP patients. Findings from our study revealed greater elevated levels across all the studied proand anti-inflammatory cytokines in the AD cohort when compared to healthy controls. To our knowledge, this is the first demonstration of a generalized peripheral inflammatory state with compensatory anti-inflammatory activation in AD. However, further studies are needed to determine the cellular source of the pro- and anti-inflammatory cytokines and the underlying mechanisms that drive these changes.
Various in vivo and in vitro studies have revealed that MA directs microglia and astrocytes to presume a pro-inflammatory phenotype, some of which may be brain-specific [8]. Several lines of evidence indicate that activated microglia increased the production of TNF-α, IL-1β, and IL-6, which are involved in pro-inflammatory processes, contributing to stimulant-induced neuroinflammation and neurodegeneration [62,63]. In an astrocytic cell line, both acute and chronic exposure to MA produce upregulated levels of IL-6 and IL-8 mRNA/ protein levels, resulting in an inflammatory response that inhibits neurogenesis in the brain [64]. In line with this evidence, higher proinflammatory cytokines (IL1β, IL-6, IFN-γ, TNF-α, and GM-CSF) within our entire AD and AD-NP groups could potentially reflect MA-induced neuroinflammation through the expression of pro-inflammatory mediators. Furthermore, the simultaneous elevation of antiinflammatory cytokines (IL-4, IL-5 and IL-10) in those patients may support the perspective that activation of microglia and astrocytes are normal compensatory reactions to brain injury [21,65], implying an enhanced and complex inflammatory milieu in the brain.
The report by Harms et al. [18] demonstrates that MA also has the ability to disrupt immune homeostasis and impact key subsets of leukocytes, including natural killer cells (NK), dendritic cells (DCs), monocytes, macrophages, and T cell memory populations; hence, MA may impact the innate and adaptive arms of immunity. Moreover, a portfolio of cytokines is essential for the role of macrophages as sentries for the innate immune system that mediate the transition from innate to adaptive immunity [66]. Indeed, MAtreated macrophages in tissue culture displayed increased levels of pro-inflammatory cytokine TNF-α, whereas similar cells stimulated with lipopolysaccharide (LPS) showed increased amounts of IL-1β and IL-8 in addition to TNF-α [67]. Recently, it was reported that MA altered the expression of pro-inflammatory cytokines such as TNF-α and IL-6 upon LPS stimulation in mouse brains [20]. Taken together, our data showed increased plasma levels of IL-1β, IL- 6, TNF-α, and IL-10 throughout the entire AD and AD-NP groups; this further suggests that MA alters the production of both proinflammatory and anti-inflammatory cytokines in the periphery via microphage activation [68].
Although the mechanisms underlying the interplay between cells of the adaptive immune system and MA are currently unclear, previous animal studies firmly established that MA adversely affects adaptive responses [16-18,69]. Our data showed upregulated levels of both Th1 cytokines (IFN-γ and TNF-α) and Th2 cytokines (IL-5, IL-6, and IL-10) in AD. This suggests an activated adaptive immune system, which further supports a pattern of more general cytokine involvement. In our additional analysis of the Th2/Th1 ratio between AD patients and controls, the predominance of higher Th2/Th1 ratios (IL6/IL2, IL10/IL2, IL10/IFN-γ, and IL10/ TNF-α) in AD patients indicates a shift towards Th2 response. In contrast, a lower Th2/ Th1 ratio (IL4/IFN-γ and IL4/ TNF-α) in patients with AD, despite the trend of increased IL-4 levels, might indicate a possible diverse role for IL-4 within MA-induced adaptive immunity. Nevertheless, changes in Th2/Th1 cytokine ratios in AD patients were not consistent either; hence no consistent and convincing conclusion was made. For example, while the ratio of IL-5/ IL-2 or IL-5/TNF-αwas higher, the IL-5/IFN-γ ratio was lower. Hence, these differences cannot be used to distinguish between a Th1 or Th2 polarization within this population.
The second major finding of this study was that subgroup analysis revealed a significant downregulated plasma level of IFN-γ, IL-1β, IL-4, IL-6, and IL-8 in the AD-P subgroup compared with the AD-NP subgroup. Two possible hypotheses to explain the distinct difference in immune profiles when being compared between the two subgroups, with regard to the role of dopamine (DA) in the immune system, are discussed as follows. (A) DA regulates T cells directly; amphetamine acts to increase the release of and sustain extracellular concentrations of neurotransmitters including dopamine (DA) [70]. DA is an important regulator linking the nervous and immune systems [71]. Sarkar, et al. [72], in their review of focusing on DA mediated regulation of T cell functions, indicated the unique interactions between DA and T cells, where DA activates naïve or resting T cells, but inhibits activated T cells. For example, DA is reported to induce cytokine secretion in resting T cells [73]. Stimulation of DA D2 and D1/ D5 receptors induces IL-10 secretion, while stimulation of DA D3 and D1/D5 receptors increases the secretion of TNF-α [73]. In contrast to activating resting T cells, stimulation of DA D2/D3 receptors in activated T cells has also been shown to inhibit secretion of IL-2, IFN-γ, and IL-4 [74]. In line with this evidence, our result, which shows increased levels of IL-10 and TNF-α in both the entire AD cohort and the AD-NP subgroup, may indicate the immune state as being the result of DA activating the resting T cells. On the other hand, the AD-P subgroup had a lower level of IFN-γ and IL-4 than the AD-NP subgroup, which suggests that hyperactive DA transmission was involved in the pathology of psychosis [75], and may further inhibit activated T cells. The latter model may be also supported by the finding that patients with schizophrenia show increased amphetamineinduced synaptic DA concentration in the mesolimbic areas [76]. In addition, changes in the status of DA concentrations and/or receptors, particularly in the T cells are associated with dysregulated immune functions in patients with schizophrenia [77,78]. Taken together, these findings suggest that the DA-mediated changes in the immune system are also linked to the etiology of amphetamine-induced psychosis. (B) Neurotransmitters regulate peripheral cytokines through “cortisol” levels; central DA neurotransmission plays a key role in the pathogenesis of schizophrenia [79]. Moreover, DA promotes the secretion of corticotrophinreleasing hormone (CRH) in the hypothalamus [80], resulting in downstream secretion of cortisol. Higher cortisol levels provide negative feedback to the peripheral immune system to suppress the production of pro-inflammatory cytokines [81], and may also play an important role in causing a shift from cellular (Th1) to humoral (Th2) immune responses [82]. Taken together, this evidence supports our finding that decreased plasma levels of pro-inflammatory cytokines (IL- 1β, IL-6, and IFN-γ (Table 3) and higher Th2/ Th1 ratio (IL-4/IFN-γ, p=0.049; IL-5/IFN-γ, p=0.026; IL-6/IFN-γ, p=0.003 (Table 4) in the AD-P group, when compared to the AD-NP group, indicates that excessive brain DA levels in patients with psychosis, which subsequently activates the hypothalamic–pituitary–adrenal (HPA) axis to regulate peripheral cytokine levels through cortisol levels.
However, an interesting and perplexing question then arises as to whether the similar immunecytokine changes were found in the entire AD cohort or AD-NP subgroup, especially when both amphetamine [83,84] and amphetamineinduced DA release [70], which subsequently stimulated the HPA axis and initiated the secretion of cortisol. However, the results in the present study, which show increased pro-inflammatory cytokine levels and an inconclusive Th1 or Th2 shift in AD-NP patients in comparison with the healthy controls, are not in agreement with the latter hypothesis of DA activating the HPA axis to suppress pro-inflammatory cytokines and to a shift toward Th2 response [81,82]. Due to the discrepancy in the assumed immunological changes between the two compared groups (AD-NP vs. healthy control and AD-P vs. healthy control), we focused on the possible role of the HPA axis and manifestation of druginduced psychosis in addition to the effect of DA on cortisol levels. Recently, substantial studies investigating possible links between the HPA axis abnormalities and the development of psychosis indicated that the presence of HPA axis hyperactivity; a blunted HPA axis response to stress appears to be a part of the biological vulnerability to psychosis [56,57]. Furthermore, a review of studies to explore stress abnormalities in individuals at risk for psychosis suggested a shared environmental risk factor and genetic predisposition between HPA axis function and psychosis [85]. Combining our findings and these studies makes intuitive sense to support the proposition that individuals who are vulnerable to develop psychosis may have HPA axis impairment − DA-induced dysregulated hyper activation of the HPA axis as well as impaired function of negative feedback, ultimately, generated over-outflow secretion of cortisol, which perhaps accounts for the characteristic alternation in cytokine profiles in AD patients with psychosis. Although the two above-mentioned hypotheses need to be verified by further precise molecular and animal studies, the presence of drug-related psychosis emerges as a key component in elucidating the cytokine imbalance in patients with AD that is responsible, to some extent, for the immuneinflammatory deviations described here.
However, in comparison to the control group, the AD-P group had a significantly increased level of pro-inflammatory cytokines (TNF-α) and anti-inflammatory cytokines (IL- 5 and IL-10), as well as the preponderance of higher Th2/Th1 cytokine ratios. This possibly strengthens the presumption that high levels of pro-inflammatory cytokines in psychosis could be related to an over-activation of Th1 activity [86], resulting in an imbalance in the Th1/Th2 cytokines ratio and a shift towards the Th2 response [87]. This is not surprising in view of the fact that the expression of some immune markers may vary with the clinical status of patients; in other words, there appear to be separate groups of state and trait markers [23-25]. Combining this evidence with our results, we may suppose that AD patients with psychosis as a clinical immune-phenotype are characterized with some dysregulated cytokine trait markers. Our pilot finding may also provide insight for following studies to accomplish more sensitive and accurate immune-endocrine or metabolic profiling, and screening and identification of potential biomarkers by utilizing combinations of gas chromatography-quadrupole time-offlight mass spectrometry (GC/Q-TOF-MS) and liquid chromatography-quadrupole time-offlight mass spectrometry (LC/Q-TOF-MS) [88].
The results of the present study should be considered in the context of several limitations. First, based on the nature of the current crosssectional study and lack of investigating possible shared risk genes to develop amphetamineinduced psychosis and psychosis, it is challenging to either explore the causal relationships within the psycho-immuno-endocrine context or elucidate the question of whether the transient or prolonged psychosis has greater genetic similarity to schizophrenia. To gain convincing results, further longitudinal studies with appropriate sample sizes and enrolling psychosis patients are required. Second, despite the modest sample size in the entire AD cohort (n=79), the number of individuals recruited in psychosis subgroups was relatively small, which may reduce the power to detect an association. Third, the early life childhood trauma could be a common predisposing factor between the psychosis and substance abuse [89]; furthermore, either a history of childhood trauma or recent stressful life-events contribute to the pro-inflammatory state, which is in part associated with psychosis [90]; therefore, the confounding effect of past and recent stressors should not be overlooked when interpreting our results. Fourth, although we controlled for potential confounding factors such as age and BMI, inflammation including raised pro-inflammatory cytokines and acute phase proteins may play a role in smoking [91]. We did not exclude cigarette smokers from our study patients. Because of the high prevalence of smoking among patients with AD, it would have been difficult to exclude smokers, for whom the distinction may be of little clinical significance. Lastly, although our study investigated a broad range of peripheral plasma immune proteins, there are a number of cellular sources for the cytokines we measured in addition to Th1 and Th2 cells. Thus, further well-designed studies exploring additional biomarkers and functional pathways of interest are needed to recognize the cellular source of inflammatory cytokines and the underlying mechanisms that drive these changes. In addition, although a single-gender study may reduce the risk of introducing confounding variables into the analysis, our results cannot be generalized to male patients with AD.
Conclusion
Our study supports preliminary evidence of both activated innate and adaptive immune systems in patients with AD. Moreover, the distinct cytokine profiles between AD-P and AD-NP subgroups suggest the existence of an immunological phenotype associated with drugrelated psychosis during early abstinence in AD. Replication of our results is warranted to verify these findings, and further longitudinal studies are required to measure the alteration in cytokines levels in AD subjects with a long period of drug abstinence.
Conflict of Interest
All authors declare no conflict of interest.
Acknowledgments
Funding for this study was provided by Ministry of Science and Technology, Taiwan (MOST- 103-2325-B-016-001, MOST-104-2314- B-016-012-MY3) (SYH), National Science Council (NSC-101-2325-B-016-003) (SYH), Tri- Service General Hospital (TSGH-C103-133, TSGH-C104-129, TSGH-C105-124) (SYH), and (TSGH-C105-125, TSGH-C106-108, TSGH-C107-112) (SCK) and Medical Affairs Bureau, Ministry of National Defense, Taiwan, (MAB-104-073) (SYH). These funding agencies played no role in the study design, collection, analysis or interpretation of data, the writing of the report, or the decision to submit the paper for publication. We also like to thank Miss Yun-Hsin Lin for her assistance in the preparing this manuscript.
References
- Kendler KS, Jacobson KC, Prescott CA, et al. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry 160(4), 687-695 (2003).
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu. Rev. Public. Health 31, 385-398 (2010).
- Meredith CW, Jaffe C, Ang-Lee K, et al. Implications of chronic methamphetamine use, a literature review. Harv. Rev. Psychiatry 13, 141-154 (2005).
- Courtney KE, Ray LA. Methamphetamine, an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug. Alcohol. Depend 143,11-21 (2014).
- Passaro RC, Pandhare J, Qian HZ, et al. The Complex Interaction Between Methamphetamine Abuse and HIV-1 Pathogenesis. J. Neuroimmune. Pharmacol 10(3), 477-486 (2015).
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities, classical and emerging mechanisms. Ann. N. Y. Acad. Sci 1187,101-121 (2010).
- Wisor JP, Schmidt MA, Clegern WC. Cerebral microglia mediate sleep/wake and neuroinflammatory effects of methamphetamine. Brain. Behav. Immun 25(4), 767-776 (2011).
- Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse, mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol. Ther 134(2), 219-245 (2012).
- Loftis JM, Huckans M. Substance use disorders, psychoneuroimmunological mechanisms and new targets for therapy. Pharmacol. Ther 139(2), 289-300 (2013).
- Stolyarova A, Thompson AB, Barrientos RM, et al. Reductions in frontocortical cytokine levels are associated with long-lasting alterations in reward valuation after methamphetamine. Neuropsychopharmacology 40(5), 1234-1242 (2015).
- Ghosh A, Birngruber T, Sattler W, et al. Assessment of blood-brain barrier function and the neuroinflammatory response in the rat brain by using cerebral open flow microperfusion (cOFM). PLoS One 9, e98143 (2014).
- Banks WA. Blood-brain barrier transport of cytokines, a mechanism for neuropathology. Curr. Pharm. Des 11(8), 973-984 (2005).
- Salamanca SA, Sorrentino EE, Nosanchuk JD, et al. Impact of methamphetamine on infection and immunity. Front. Neurosci 8, 445 (2014).
- House RV, Thomas PT, Bhargava HN. Comparison of immune functional parameters following in vitro exposure to natural and synthetic amphetamines. Immunopharmacol. Immunotoxicol 16(1), 1-21 (1994).
- Iwasa M, Maeno Y, Inoue H, et al. Induction of apoptotic cell death in rat thymus and spleen after a bolus injection of methamphetamine. Int. J. Legal. Med 109(1), 23-28 (1996).
- In SW, Son EW, Rhee DK, et al. Methamphetamine administration produces immunomodulation in mice. J. Toxicol. Environ. Health 68(23), 2133-2145 (2005).
- Peerzada H, Gandhi JA, Guimaraes AJ, et al. Methamphetamine administration modifies leukocyte proliferation and cytokine production in murine tissues. Immunobiology 218(8), 1063-1068 (2013).
- Harms R, Morsey B, Boyer CW, et al. Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS One 7, e49897 (2012).
- Mahajan SD, Hu Z, Reynolds JL, et al. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells, implications for HIV-1 pathogenesis. Mol. Diagn. Ther 10, 257-269 (2006).
- Buchanan JB, Sparkman NL, Johnson RW. A neurotoxic regimen of methamphetamine exacerbates the febrile and neuroinflammatory response to a subsequent peripheral immune stimulus. J. Neuroinflammation 7, 82 (2010).
- Loftis JM, Choi D, Hoffman W, et al. Methamphetamine causes persistent immune dysregulation, a cross-species, translational report. Neurotox Res 20(1), 59-68 (2011).
- Huckans M, Fuller BE, Chalker AL, et al. Plasma Inflammatory Factors Are Associated with Anxiety, Depression, and Cognitive Problems in Adults with and without Methamphetamine Dependence, An Exploratory Protein Array Study. Front. Psychiatry 6, 178 (2015).
- Miller BJ, Goldsmith DR. Towards an Immunophenotype of Schizophrenia, Progress, Potential Mechanisms, and Future Directions. Neuropsychopharmacology. 42(1), 299-317 (2017).
- Stojanovic A, Martorell L, Montalvo I, et al. Increased serum interleukin-6 levels in early stages of psychosis, associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology 41, 23-32 (2014).
- Zeni-Graiff M, Rizzo LB, Mansur RB, et al. Peripheral immuno-inflammatory abnormalities in ultra-high risk of developing psychosis. Schizophr. Res 176, 191-195 (2016).
- Bramness JG, Gundersen OH, Guterstam J, et al. Amphetamine-induced psychosis--a separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC. Psychiatry 12, 221 (2012).
- Grant KM, LeVan TD, Wells SM, et al. Methamphetamine-associated psychosis. J. Neuroimmune. Pharmacol 7(1), 113-139 (2012).
- McKetin R, McLaren J, Lubman DI, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction 101(10), 1473-1478 (2006).
- Glasner-Edwards S, Mooney LJ. Methamphetamine psychosis, epidemiology and management. CNS. Drugs 28(12), 1115-1126 (2014).
- Srisurapanont M, Ali R, Marsden J, et al. Psychotic symptoms in methamphetamine psychotic in-patients. Int. J. Neuropsychopharmacol 6(4), 347-352 (2003).
- Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction 101 Suppl 1, 76-88 (2006).
- Farrell M, Marsden J, Ali R, et al. Methamphetamine, drug use and psychoses becomes a major public health issue in the Asia Pacific region. Addiction 97, 771-772 (2002).
- Mahoney JJ, Kalechstein AD, De La Garza R, et al. Presence and persistence of psychotic symptoms in cocaine- versus methamphetamine-dependent participants. Am. J. Addict 17(2), 83-98 (2008).
- Breen MS, Uhlmann A, Nday CM, et al. Candidate gene networks and blood biomarkers of methamphetamine-associated psychosis, an integrative RNA-sequencing report. Transl. Psychiatry 6, e802 (2016).
- Bousman CA, Glatt SJ, Everall IP, et al. Genetic association studies of methamphetamine use disorders, A systematic review and synthesis. Am. J. Med. Genet. B Neuropsychiatr. Genet 150b, 1025-1049 (2009).
- Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front. Hum. Neurosci 8, 537 (2014).
- Srisurapanont M, Arunpongpaisal S, Wada K, et al. Comparisons of methamphetamine psychotic and schizophrenic symptoms, a differential item functioning analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 35(4), 959-964 (2011).
- Girgis RR, Kumar SS, Brown AS. The cytokine model of schizophrenia, emerging therapeutic strategies. Biol. Psychiatry 75(4), 292-299 (2014).
- Kneeland RE, Fatemi SH. Viral infection, inflammation and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 42, 35-48 (2013).
- Dean K, Murray RM. Environmental risk factors for psychosis. Dialogues. Clin. Neurosci 7(1), 69-80 (2005).
- Kidd P. Th1/Th2 balance, the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev 8(3), 223-246 (2003).
- Levandowski ML, Viola TW, Prado CH, et al. Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug. Alcohol. Depend 167, 140-148 (2016).
- Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox. Res10 (2), 131-148 (2006).
- Brecht ML, O'Brien A, von Mayrhauser C, et al. Methamphetamine use behaviors and gender differences. Addict. Behav 29(1), 89-106 (2004).
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 172(4), 443-449 (2004).
- Ruda-Kucerova J, Amchova P, Babinska Z, et al. Sex Differences in the Reinstatement of Methamphetamine Seeking after Forced Abstinence in Sprague-Dawley Rats. Front. Psychiatry 6, 91 (2015).
- Reichel CM, Chan CH, Ghee SM, et al. Sex differences in escalation of methamphetamine self-administration, cognitive and motivational consequences in rats. Psychopharmacology (Berl) 223(4), 371-380 (2012).
- Holtz NA, Lozama A, Prisinzano TE, et al. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug. Alcohol. Depend 120(1), 233-237 (2012).
- Dluzen DE, Liu B. Gender differences in methamphetamine use and responses, a review. Gend. Med 5, 24-35 (2008).
- Lin SK, Ball D, Hsiao CC, et al. Psychiatric comorbidity and gender differences of persons incarcerated for methamphetamine abuse in Taiwan. Psychiatr. Clin. Neurosci 58(2), 206-212 (2004).
- Polcin DL, Buscemi R, Nayak M, et al. Gender Differences in Psychiatric Symptoms among Methamphetamine Dependent Residents in Sober Living Houses. Addict. Disord. Their. Treat 11(2), 53-63 (2012).
- King G, Alicata D, Cloak C, et al. Psychiatric symptoms and HPA axis function in adolescent methamphetamine users. J. Neuroimmune. Pharmacol 5(4), 582-591 (2010).
- Simpson JL, Grant KM, Daly PM, et al. Psychological Burden and Gender Differences in Methamphetamine-Dependent Individuals in Treatment. J. Psychoactive. Drugs 48, 261-269 (2016).
- Mahoney JJ, Hawkins RY, De La Garza R, et al. Relationship between gender and psychotic symptoms in cocaine-dependent and methamphetamine-dependent participants. Gend. Med 7(5), 414-421 (2010).
- Zuloaga DG, Johnson LA, Agam M, et al. Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine. J. Neurochem 129(3), 495-508 (2014).
- Shah JL, Malla AK. Much ado about much, stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr. Res 162(1)0, 253-260 (2015).
- Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology 38(5), 603-611 (2013).
- Endicott J, Spitzer RL. A diagnostic interview, the schedule for affective disorders and schizophrenia. Arch. Gen. Psychiatry 35(7), 837-844 (1978).
- Merikangas KR, Stevens DE, Fenton B, et al. Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychol. Med 28(4), 773-788 (1998).
- Gossop M, Darke S, Griffiths P, et al. The Severity of Dependence Scale (SDS), psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 90(5), 607-614 (1995).
- Yen CH, Ho PS, Yeh YW, et al. Differential cytokine levels between early withdrawal and remission states in patients with alcohol dependence. Psychoneuroendocrinol 76, 183-191 (2017).
- Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation, emerging evidence of their interconnection. Neurotox. Res 23, 174-88 (2013).
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J. Neuroimmune. Pharmacol 3, 203-217 (2008).
- Shah A, Silverstein PS, Singh DP, et al. Involvement of metabotropic glutamate receptor 5, AKT/PI3K signaling and NF-kappaB pathway in methamphetamine-mediated increase in IL-6 and IL-8 expression in astrocytes. J. Neuroinflammation 9, 52 (2012).
- Neumann H. Control of glial immune function by neurons. Glia 36, 191-199 (2001).
- Arango DG, Descoteaux A. Macrophage cytokines, involvement in immunity and infectious diseases. Front. Immunol 5, 491 (2014).
- Liu X, Silverstein PS, SinghV, et al. Methamphetamine increases LPS-mediated expression of IL-8, TNF-alpha and IL-1beta in human macrophages through common signalling pathways. PLoS. One 7, e33822 (2012).
- Burns A, Ciborowski P. Acute exposure to methamphetamine alters TLR9-mediated cytokine expression in human macrophage. Immunobiol 221, 199-207 (2016).
- Martinez LR, Mihu MR, Gacser A, et al. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J. Infect. Dis 200, 131-141 (2009)
- Sulzer D, Sonders MS, Poulsen NW, et al. Mechanisms of neurotransmitter release by amphetamines, a review. Prog. Neurobiol 75, 406-433 (2005)
- Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol 102, 113-124 (2000).
- Sarkar C, Basu, B, Chakroborty, D, et al. The immunoregulatory role of dopamine, an update. Brain. Behav. Immun 24, 525-528.
- Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J. Neuroimmunol. 169, 161-171 (2005).
- Ghosh MC, Mondal AC, Basu S, et al. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol 3, 1019-1026 (2003).
- Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia, a review. Schizophr. Bull 2, 19-76 (1976).
- Breier A, Su TP, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations, evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. USA94, 2569-2574 (1997).
- Ilani T, Strous RD, Fuchs S. Dopaminergic regulation of immune cells via D3 dopamine receptor, a pathway mediated by activated T cells. FASEB. J 18, 1600-1602 (2004).
- Boneberg EM, von Seydlitz E, Propster K, et al. D3 dopamine receptor mRNA is elevated in T cells of schizophrenic patients whereas D4 dopamine receptor mRNA is reduced in CD4+ -T cells. J. Neuroimmunol 173, 180-187 (2006).
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia, version III--the final common pathway. Schizophr Bull 35, 549-562 (2009).
- Calogero AE, Gallucci WT, Chrousos GP, et al. Catecholamine effects upon rat hypothalamic corticotropin-releasing hormone secretion in vitro. J. Clin. Invest 82, 839-846 (1988).
- Watkins LR, Nguyen KT, Lee JE, et al. Dynamic regulation of proinflammatory cytokines. Adv. Exp. Med. Biol. 461, 153-178 (1999).
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci 1024, 138-46 (2004).
- Madden LJ, Flynn CT, Zandonatti MA, et al. Modeling human methamphetamine exposure in nonhuman primates, chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacol 30, 350-359 (2005).
- Harris DS, Reus VI, Wolkowitz OM, et al. Altering cortisol level does not change the pleasurable effects of methamphetamine in humans. Neuropsychopharmacol 28, 1677-1684 (2003).
- Aiello G, Horowitz M, Hepgul N, et al. Stress abnormalities in individuals at risk for psychosis, a review of studies in subjects with familial risk or with "at risk" mental state. Psychoneuroendocrinol 37, 1600-13 (2012).
- Crespo-Facorro B, Carrasco-Marin E, Perez-Iglesias R, et al. Interleukin-12 plasma levels in drug-naive patients with a first episode of psychosis, effects of antipsychotic drugs. Psychiatry .Res 158, 206-16 ( 2008).
- Schwarz MJ, Chiang S, Muller N, et al. T-helper-1 and T-helper-2 responses in psychiatric disorders. Brain. Behav. Immun 15, 340-370 (2001).
- Zhang B, Zhang H, Du C, et al. Metabolic responses of the growing Daphnia similis to chronic AgNPs exposure as revealed by GC-Q-TOF/MS and LC-Q-TOF/MS. Water. Res 114, 135-143 (2017).
- Ng QX, Yong BZJ, Ho CYX, et al. Early life sexual abuse is associated with increased suicide attempts, an update meta-analysis. J. Psychiatr .Res. 99, 129-141 (2018).
- Di Nicola M, Cattaneo A, Hepgul N, et al. Serum and gene expression profile of cytokines in first-episode psychosis. Brain. Behav. Immun 31, 90-95 (2013).
- Nunes SO, Vargas HO, Prado E, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci. Biobehav. Rev 37, 1336-1345 (2013).