Perspective - Interventional Cardiology (2013) Volume 5, Issue 2
Does catheter ablation of atrial fibrillation eliminate the need for anticoagulation?
- Corresponding Author:
- Prashanthan Sanders
Centre for Heart Rhythm Disorders
(CHRD), University of Adelaide & Royal
Adelaide Hospital, Adelaide, Australia
Tel: +61 8 8222 2723
Fax: +61 8 8222 2722
E-mail: prash.sanders@adelaide.edu.au
Abstract
Keywords
ablation, anticoagulation, atrial fibrillation, cessation, sinus rhythm
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice. Its prevalence has increased over the last few decades and is projected to rise further by 2.5-fold by the middle of the 21st century. This has been associated with an exponential increase in the number of hospitalizations and economic costs [1]. AF is responsible for one-sixth of all strokes and is associated with a fivefold increased stroke risk in the general population [2]. Furthermore, evidence suggests that cardioembolic strokes are more severe and are twice as likely to be fatal [3]. Anticoagulation remains to be the cornerstone in the management of AF.
Although catheter ablation is performed for symptomatic AF, there are emerging data that catheter ablation may modify the natural history of AF [4–6]. However, the long-term results of catheter ablation are sobering, with present guidelines recommending continuation of anticoagulation after catheter ablation based on stroke risk score [7–9]. Despite these guidelines, the practice of anticoagulation following successful catheter ablation for AF differs across centers.
Rationale behind stopping anticoagulation after successful catheter ablation for AF
There are two potential rationales for the cessation of anticoagulation after ablation. First, elimination of AF after successful ablation results in the maintenance of sinus rhythm and, therefore, presumably reverses the heightened stroke risk. Second, there is an established risk of major bleeding with anticoagulation therapy [10,11].
Furthermore, the patients deemed at a higher stroke risk are often at a greater risk of major bleeding [12]. Therefore, the recommendation for the cessation of anticoagulation is based on the alteration of this risk–benefit determination after AF ablation.
Although extremely promising, this approach has certain weaknesses. It is based on the assumption that elimination of clinical AF will eliminate the risk of stroke. However, there are a number of confounding factors that need to be considered. First, there is increasing evidence that argues in favor of the compounding effect of the prothrombogenic conditions that frequently coexist with AF. Second, the definition of successful ablation differs across centers, based on varying degrees of intermittent or continuous monitoring. Indeed, there is evidence that even in the highly symptomatic patients undergoing ablation for AF, approximately 30–40% will subsequently have only asymptomatic recurrence [13,14]. Finally, the jury is still out on how much AF burden is required to significantly elevate stroke risk [15].
Current guidelines for anticoagulation after catheter ablation for AF
The European Society of Cardiology guidelines on follow-up after AF ablation recommends that the guidelines for anticoagulation at the time of cardioversion for AF should apply to patients with AF at the time of ablation (if AF termination is sought at the time of ablation) [8,9,16]. It is also recommended that catheter ablation should be performed on oral anticoagulation or heparin. Low molecular weight or unfractionated heparin should be utilized to bridge the resumption of systemic anticoagulation. The anticoagulation should continue for at least 3 months and discontinuation of warfarin is not recommended in patients at high risk of stroke.
Current practice of anticoagulation after catheter ablation for AF
A survey across Canadian centers brings forth the current practice for anticoagulation after ablation for AF [17]. For patients with a CHADS2 score of ≤1, warfarin was continued for at least 3 months by most physicians (89% for paroxysmal and 94% for persistent AF), but rarely beyond a year (6% for paroxysmal and 3% for persistent AF) after a successful ablation. However, in patients with CHADS2 score of ≥2, warfarin was discontinued in only 11% (persistent AF) to 14% (paroxysmal AF), after 1 year of successful ablation. For patients with an unsuccessful procedure, warfarin was continued for at least 3 months, but rarely beyond a year if the CHADS2 score was ≤1. However, warfarin was prescribed to all patients with a CHADS2 score of ≥2 for at least 3 months and 92% (paroxysmal AF) to 94% (persistent AF) of patients beyond 1 year. The reasons for cessation of warfarin were not provided. By contrast, another study reported cessation of anticoagulation in 84% of patients after 3 months of successful ablation for AF [18]. Among the patients without arrhythmic recurrences who discontinued anticoagulation, they reported a low stroke risk of 0.4% over a mean follow-up of 836 days and suggested that this is a safe approach following successful ablation. However, the study did not provide data on the CHADS2 score for stroke risk stratification.
Mechanism of thromboembolism in AF
AF is associated with stasis in the left atrium in conjunction with elevated left atrial volume and reduction in mechanical function. It has been demonstrated that left atrial appendage thrombus formation is associated with reduced contractility and the left atrial appendage emptying velocities in AF, as well as sinus rhythm [19]. Furthermore, termination of AF and restoration of sinus rhythm leads to transient mechanical dysfunction of the atria [20]. This is associated with an increased incidence of thrombus formation in the left atrium and the left atrial appendage leading to a thromboembolic phenomenon. The incidence of atrial stunning after the conversion of AF to sinus rhythm ranges from 38 to 80% [21]. This process is independent of the mode of cardioversion and has been reported with the conversion of AF or atrial flutter by transthoracic electrical cardioversion, low energy internal electrical cardioversion, pharmacological cardioversion, spontaneous conversion, conversion by overdrive pacing of atrial f lutter and radiofrequency ablation of atrial flutter [22–35]. Atrial stunning is at maximum immediately after cardioversion and progressively improves with a complete or near complete resolution within a few minutes to 4–6 weeks, depending on the duration of the preceding AF, atrial size and structural heart disease [24–26,34]. This has been, in part, the basis for recommendation of anticoagulation for a minimum of 2–3 months after an AF procedure.
Virchow’s triad describes three factors contributing to the development of thrombus: stasis, endothelial injury and a hypercoagulability [36]. There is an increasing body of evidence to support the presence of endothelial dysfunction and hypercoagulability in AF. Endothelial damage in the left atrium due to inflammation has been well described in patients with AF [37]. Hypertension, heart failure and aging are all accompanied by fibrosis and infiltration as components of left atrial remodeling [38–40]. This is associated with disruption of the collagenous extracellular matrix that forms the framework for myocyte attachment and has a potential to perpetuate not only conduction abnormalities promoting AF but also thrombus formation [41]. P-selectin and von Willebrand factor facilitate leukocyte recruitment and macrophage accumulation in inflamed endothelium. They are stored in the Weibel–Palade bodies in the endothelium and are released by inflammatory stimuli. Elevated levels of platelet P-selectin and endothelial von Willebrand factor and diminished nitric oxide production have been documented in cases of AF [42–44]. This heightened thrombotic state in AF is also found to be more severe in the left atrium [45].
Natural history of AF after catheter ablation
Calkins et al. reported the outcomes of two meta-analyses analyzing the safety and efficacy of catheter ablation of AF and antiarrhythmic drug therapy [46]. The results of 63 radiofrequency ablation studies were included in these analyses. The single-procedure success rate of ablation with no antiarrhythmic drug therapy was 57%, the multiple-procedure success rate with no antiarrhythmic drugs was 71% and the multiple-procedure success rate with antiarrhythmic drugs or with unknown antiarrhythmic drug usage was 77%. A meta-analysis of four randomized studies has reported a similar success rate of 76% at 1 year after procedure [47]. However, long-term follow-up studies have shown considerable attrition in the success rates [48]. Similarly, the efficacy of catheter ablation after persistent AF is quite humbling at a follow-up of 2 years [7]. Single-procedure, drug-free clinical success associated with case series data suggest that, with the exception of pulmonary vein isolation alone (mean 21%) and complex fractionated atrial electrogram ablation alone (mean 37%), all contemporary substrate ablation techniques for persistent/long-standing AF provide a 47% mean success rate over a period of 2 years. Hence, AF is a progressive disease with the success rate of catheter ablation diminishing over time and influenced by the type of AF.
The success rates for catheter ablation for AF are dependent on several parameters ranging from type of AF, presence of comorbid factors such as hypertension, diabetes, heart failure, obesity and obstructive sleep apnea, the definition of procedural success, and the duration of follow-up. In order to standardize the reporting of results, the Heart Rhythm Society has laid down certain guidelines [8,9,16]. This consensus statement on AF recommends that success should be defined as freedom from symptomatic and asymptomatic AF or atrial flutter lasting longer than 30 s at 12 months after the index procedure. A 3-month blanking period is also recommended as transient atrial arrhythmias in the postablation period are common due to pericardial inflammation. However, the definition of success is arbitrary and does not take into account symptomatic improvement or stroke risk.
Postablation monitoring for recurrence of AF
The ambulatory monitoring for recurrence of AF postablation has been variable in different clinical trials diverging from varying degrees of intermittent to continuous monitoring [49–51]. The studies reporting the safety of cessation of oral anticoagulants after successful catheter ablation have usually employed variable intensities of monitoring, ranging from continuous trans-telephonic monitoring during the initial period to annual Holter monitoring as adjuncts to clinical visits and 12 lead ECGs [4,18,52–54].
In paroxysmal AF, prolonged noncontinuous recording may facilitate AF detection. It has been estimated that 7-day Holter ECG recording or daily and symptom-activated event recordings may document the arrhythmia in 70% of AF patients, and that their negative predictive value for the absence of AF is between 30 and 50% [13,50,51]. In stroke survivors, a step-wise addition of five daily short-term ECGs, one 24-h Holter ECG, and another 7-day Holter ECG will each increase the detection rate of AF to a similar extent [8,55]. It has been demonstrated that the sensitivity of 24-h Holter monitoring is dependent on the frequency of monitoring and at quarterly intervals, the sensitivity when compared with implantable loop recorder is unsatisfactory at only 0.60 [56].
Multiple studies have demonstrated that asymptomatic AF commonly occurs in patients following catheter ablation [14,57]. The methods employed for monitoring recurrence have important implications if a decision for stopping oral anticoagulation is based on demonstrating the absence of AF as a measure of success of the catheter ablation. Furthermore, the duration of AF that would provoke thrombus formation is not well established. Evidence from continuous monitoring suggests that episodes as short as 6 min increase the risk of stroke [15]. A study analyzing data from device interrogations suggests that an AF duration of >5.5 h is associated with a twofold risk of stroke compared with those who had shorter episodes of AF (2.4 vs 1.1%) [58]. Another retrospective device-based study has demonstrated significantly increased risk with 24-h AF episodes [59]. The duration of AF episode when combined with stroke risk scores improved risk stratification for stroke [15,59–61]. However, the data at present are inadequate to provide guidelines on the duration under which AF may not significantly increase risk of thrombus formation.
Thromboembolism after cessation of oral anticoagulants following successful catheter ablation for AF
Despite the advances in catheter ablation for AF in the last decade, there is a lack of robust data on the impact of this strategy on the risk of stroke and mortality. However, there are recent registry data to suggest that stroke risk after catheter ablation of AF is favorable and comparable with a population without AF [6]. Similarly, another multicentric registry-based study suggests that restoration of sinus rhythm by catheter ablation of AF is associated with a lower risk of stroke and death compared with patients treated medically [5].
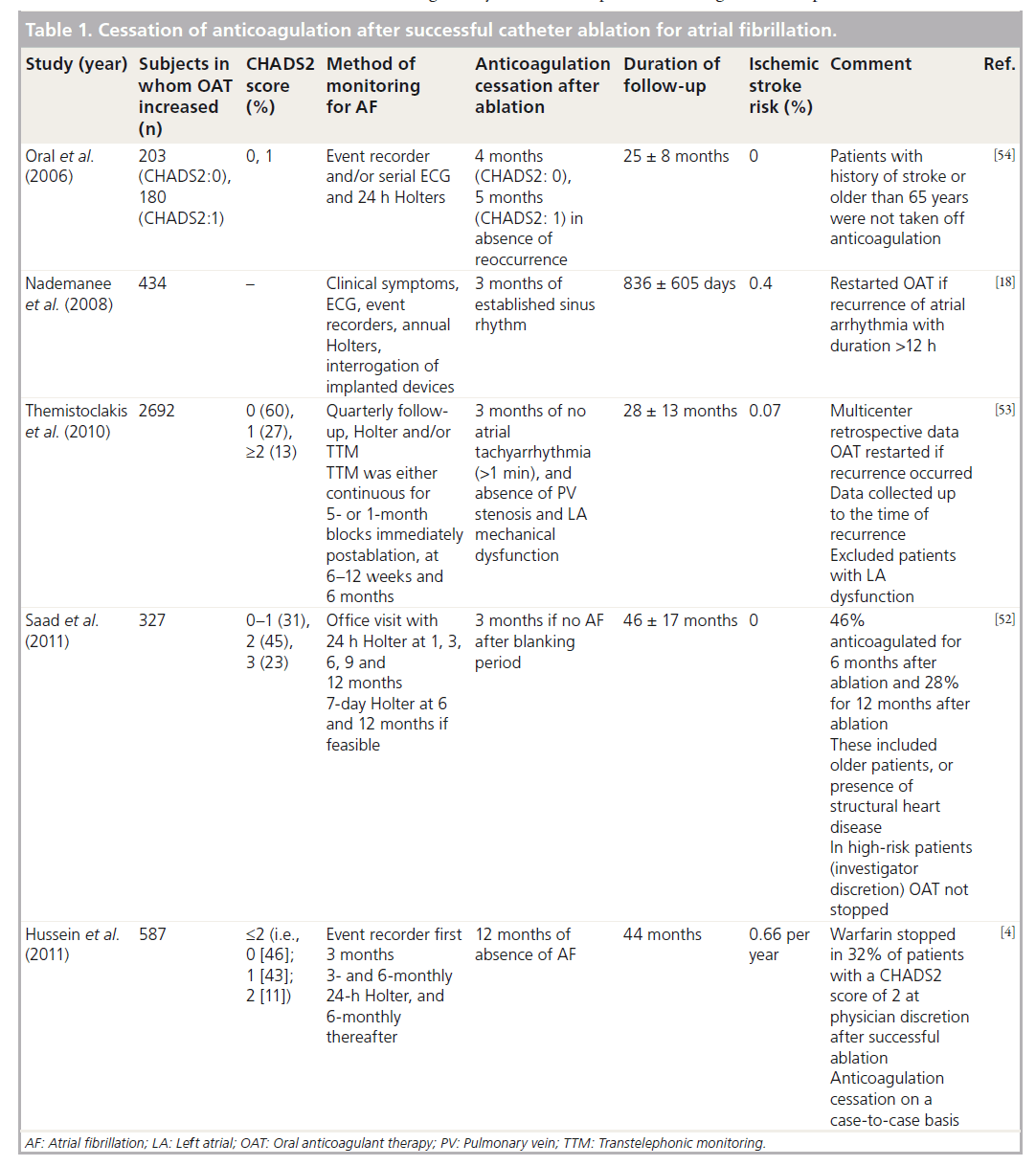
There are large case series that report low thromboembolic risk in patients undergoing successful catheter ablation for AF (Table 1) [4,18,52–54]. However, the patient cohort in these case series has been a low-to-intermediate risk with a relatively small period of follow-up after cessation of anticoagulation. Oral et al. reported no stroke following cessation of warfarin during a 2-year follow-up of low-to-intermediate-risk patients with successful ablation for AF [54]. Anticoagulation was discontinued 3–6 months after ablation. However, the authors acknowledged that the patients who had previous history of stroke or were over 65 years of age often received anticoagulation despite successful outcome of catheter ablation. Thermistoclakis et al., in a multicentric retrospective analysis, reported a 0.07% risk of ischemic stroke after cessation of oral anticoagulant therapy after successful ablation [53]. Similarly, other investigators have reported low stroke rates following cessation of oral anticoagulants after successful catheter ablation for AF (Table 1). However, these studies suffer from retrospective design and investigator bias. A select population underwent cessation of anticoagulation and those perceived at higher risk by the treating physician were continued on warfarin. Patients with previous history of stroke, left atrial dysfunction and older age were not considered for anticoagulation cessation. Furthermore, the period of follow-up was not very long, given that a proportion of patients received anticoagulation for several months after the ablation. It is not clear how anticoagulation was managed, whether the patients underwent repeat ablation and how this period was accounted for in the follow-up duration in one study [52]. In addition, anticoagulation was restarted and data were analyzed up to the time of recurrence in another study [53], suggesting that patients who stop anticoagulation should be closely monitored for recurrence of AF. Furthermore, aspirin was continued indefinitely after cessation of oral anticoagulant therapy.
Conclusion & future perspective
AF is a progressive disease. Late recurrences have been described during long-term follow-up of catheter ablation for AF. Furthermore, the stroke risk is dynamic with accretion of additional stroke risk factors over time.
Cessation of anticoagulation is a very attractive concept after successful ablation of AF. However, to approach this, certain questions require clarification, most importantly: what is the minimum duration of AF episodes or cumulative AF that can provoke thrombus formation, and how does it change across different stroke risk categories? What is the risk of stroke in sinus rhythm in the presence of high CHADS2 score and CHA2DS2–VASc scores? Which is the best tool to monitor the recurrence of AF of the above duration with reasonable confidence? These answers could potentially guide us in assessing the stroke risk and, accordingly, the risk–benefit of oral anticoagulants in this scenario. Furthermore, the introduction of novel oral anticoagulants with superior risk–benefit ratios has added another variable to the equation. In addition, left atrial appendage closure may have a role in stroke risk reduction in selected cases.
Given the absence of randomized data, it is prudent to continue anticoagulation after catheter ablation in patients at high risk of stroke. In our opinion, cessation of anticoagulation following catheter ablation should only be considered in the presence of documented durable absence of recurrent atrial arrhythmias in patients at low-to-intermediate stroke risk. The patients should be made aware of the current consensus guidelines and allowed to make an informed decision on an individual basis. Upon cessation of oral anticoagulant therapy, these patients should be carefully monitored and anticoagulation re-instituted promptly in the event of recurrence of atrial tachyarrhythmias.
Executive summary
Background
▪ The data from observational studies suggests that successful ablation for atrial fibrillation (AF) may modify its natural progression and reduce the risk of stroke.
▪ The rationale behind stopping anticoagulation after successful catheter ablation for AF is that the maintenance of sinus rhythm after successful ablation may reduce the risk of stroke and alter the risk/benefit ratio for the use of oral anticoagulants in AF.
Mechanism of thromboembolism in AF
▪ In addition to stasis, there is emerging evidence that endothelial injury and hypercoagulability play a role in thrombus formation in AF.
Natural progression of AF after catheter ablation
▪ The success rate of ablation for AF after multiple procedures is high at 1 year; however, there is considerable attrition over time.
Postablation monitoring for recurrence of AF
▪ The postablation monitoring varies from intermittent to continuous monitoring, with sensitivity increasing with intensity of monitoring.
Thromboembolism after cessation of oral anticoagulants following successful catheter ablation for AF
▪ Several observational single- and multi-center studies have reported reduced risk of stroke, despite cessation of oral anticoagulants, following successful catheter ablation for AF in patients with low-to-intermediate risk of stroke.
▪ However, these observational studies suffer from bias with cessation of anticoagulation offered to patients deemed at lower risk.
Conclusion & future perspective
▪ In the absence of randomized data, anticoagulation should be continued for patients with AF and high risk for stroke. In patients with intermediate risk, the decision should be individualized and patients made aware of current guidelines in the event of discontinuation of oral anticoagulants.
▪ The duration of AF that is thrombogenic, the degree of thrombus risk in sinus rhythm in the presence of stroke risk factors and a sensitive monitoring tool are important issues that require clarification.
▪ A randomized trial is essential to adequately address this important issue.
Financial & competing interests disclosure
R Pathak and R Mahajan are supported by LJ Mahar Electrophysiology Scholarships from the University of Adelaide. R Mahajan is supported by the Australian Postgraduate Award from the University of Adelaide. R Pathak is supported by Lions Medical Research Scholarship. HS Lim is supported by the Postgraduate Research Scholarship from the National Health and Medical Research Council of Australia. SR Willoughby is supported by a Career Development Fellowship by the National Health and Medical Research Council of Australia. P Sanders is supported by the National Heart Foundation of Australia. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- Wong CX, Brooks AG, Leong DP, Roberts-Thomson KC, Sanders P. The increasing burden of atrial fibrillation compared with heart failure and myocardial infarction: a 15-year study of all hospitalizations in Australia. Arch. Intern. Med. 172(9), 739–741 (2012).
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22(8), 983–988 (1991).
- Lin HJ, Wolf PA, Kelly-Hayes M et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 27(10), 1760–1764 (1996).
- Hussein AA, Saliba WI, Martin DO et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ. Arrhythm. Electrophysiol. 4(3), 271–278 (2011).
- Hunter RJ, Mccready J, Diab I et al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 98(1), 48–53 (2012).
- Bunch TJ, Crandall BG, Weiss JP et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J. Cardiovasc. Electrophysiol. 22(8), 839–845 (2011).
- Brooks AG, Stiles MK, Laborderie J et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 7(6), 835–846 (2010).
- Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 31(19), 2369–2429 (2010).
- Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace 14(10), 1385–1413 (2012).
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 146(12), 857–867 (2007).
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann. Intern. Med. 120(11), 897–902 (1994).
- Lip GY. Implications of the CHA(2)DS(2)– VASc and HAS-BLED Scores for thromboprophylaxis in atrial fibrillation. Am. J. Med. 124(2), 111–114 (2011).
- Piorkowski C, Kottkamp H, Tanner H et al. Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J. Cardiovasc. Electrophysiol. 16(12), 1286–1292 (2005).
- Hindricks G, Piorkowski C, Tanner H et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 112(3), 307–313 (2005).
- Healey JS, Connolly SJ, Gold MR et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 366(2), 120–129 (2012).
- Calkins H, Kuck KH, Cappato R et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J. Interv. Card. Electrophysiol. 33(2), 171–257 (2012).
- Mardigyan V, Verma A, Birnie D et al. Anticoagulation management pre- and post atrial fibrillation ablation: a survey of Canadian centres. Can. J. Cardiol. 29(2), 219–223 (2012).
- Nademanee K, Schwab MC, Kosar EM et al. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J. Am. Coll. Cardiol. 51(8), 843–849 (2008).
- Pollick C, Taylor D. Assessment of left atrial appendage function by transesophageal echocardiography. Implications for the development of thrombus. Circulation 84(1), 223–231 (1991).
- Fatkin D, Kuchar DL, Thorburn CW, Feneley MP. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for ‘atrial stunning’ as a mechanism of thromboembolic complications. J. Am. Coll. Cardiol. 23(2), 307–316 (1994).
- Khan IA. A trial stunning: basics and clinical considerations. Int. J. Cardiol. 92(2–3), 113–128 (2003).
- Sparks PB, Kulkarni R, Vohra JK et al. Effect of direct current shocks on left atrial mechanical function in patients with structural heart disease. J. Am. Coll. Cardiol. 31(6), 1395–1399 (1998).
- Sparks PB, Jayaprakash S, Vohra JK et al. Left atrial ‘stunning’ following radiofrequency catheter ablation of chronic atrial flutter. J. Am. Coll. Cardiol. 32(2), 468–475 (1998).
- Sparks PB, Jayaprakash S, Mond HG, Vohra JK, Grigg LE, Kalman JM. Left atrial mechanical function after brief duration atrial fibrillation. J. Am. Coll. Cardiol. 33(2), 342–349 (1999).
- Sanders P, Morton JB, Morgan JG et al. Reversal of atrial mechanical stunning after cardioversion of atrial arrhythmias: implications for the mechanisms of tachycardia-mediated atrial cardiomyopathy. Circulation 106(14), 1806–1813 (2002).
- Sanders P, Morton JB, Kistler PM, Vohra JK, Kalman JM, Sparks PB. Reversal of atrial mechanical dysfunction after cardioversion of atrial fibrillation: implications for the mechanisms of tachycardia-mediated atrial cardiomyopathy. Circulation 108(16), 1976–1984 (2003).
- Pollak A, Falk RH. Aggravation of postcardioversion atrial dysfunction by sotalol. J. Am. Coll. Cardiol. 25(3), 665–671 (1995).
- Louie EK, Liu D, Reynertson SI et al. ‘Stunning’ of the left atrium after spontaneous conversion of atrial fibrillation to sinus rhythm: demonstration by transesophageal Doppler techniques in a canine model. J. Am. Coll. Cardiol. 32(7), 2081–2086 (1998).
- Kontos MC, Paulsen WH. Impairment of left atrial appendage function after spontaneous conversion of atrial flutter. Clin. Cardiol. 21(10), 769–771 (1998).
- Irani WN, Grayburn PA, Afridi I. Prevalence of thrombus, spontaneous echo contrast, and atrial stunning in patients undergoing cardioversion of atrial flutter. A prospective study using transesophageal echocardiography. Circulation 95(4), 962–966 (1997).
- Grimm RA, Stewart WJ, Maloney JD et al. Impact of electrical cardioversion for atrial fibrillation on left atrial appendage function and spontaneous echo contrast: characterization by simultaneous transesophageal echocardiography. J. Am. Coll. Cardiol. 22(5), 1359–1366 (1993).
- Grimm RA, Stewart WJ, Arheart K, Thomas JD, Klein AL. Left atrial appendage ‘stunning’ after electrical cardioversion of atrial flutter: an attenuated response compared with atrial fibrillation as the mechanism for lower susceptibility to thromboembolic events. J. Am. Coll. Cardiol. 29(3), 582–589 (1997).
- Mattioli AV, Bonatti S, Melotti R, Mattioli G. Atrial stunning, inflammation and nutritional status after cardioversion from atrial fibrillation. Int. J. Cardiol. 129(3), 344–347 (2008).
- Manning WJ, Zimetbaum PJ. Direct current cardioversion of atrial fibrillation – the next 40 years. Mayo Clin. Proc. 77(9), 895–896 (2002).
- Mahajan R, Brooks AG, Sullivan T et al. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: implications for left atrial appendage closure. Heart 98(15), 1120–1126 (2012).
- Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br. J. Haematol. 143(2), 180–190 (2008).
- Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 96(4), 1180–1184 (1997).
- Lau DH, Mackenzie L, Kelly DJ et al. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm 7(9), 1282–1290 (2010).
- Sanders P, Morton JB, Davidson NC et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation 108(12), 1461–1468 (2003).
- Stiles MK, John B, Wong CX et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the ‘second factor’. J. Am. Coll. Cardiol. 53(14), 1182–1191 (2009).
- Marin F, Roldan V, Climent V, Garcia A, Marco P, Lip GY. Is thrombogenesis in atrial fibrillation related to matrix metalloproteinase-1 and its inhibitor, TIMP-1? Stroke 34(5), 1181–1186 (2003).
- Minamino T, Kitakaze M, Sanada S et al. Increased expression of P-selectin on platelets is a risk factor for silent cerebral infarction in patients with atrial fibrillation: role of nitric oxide. Circulation 98(17), 1721–1727 (1998).
- Fukuchi M, Watanabe J, Kumagai K et al. Increased von Willebrand factor in the endocardium as a local predisposing factor for thrombogenesis in overloaded human atrial appendage. J. Am. Coll. Cardiol. 37(5), 1436–1442 (2001).
- Cai H, Li Z, Goette A et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation 106(22), 2854–2858 (2002).
- Willoughby SR, Roberts-Thomson RL, Lim HS et al. Atrial platelet reactivity in patients with atrial fibrillation. Heart Rhythm 7(9), 1178–1183 (2010).
- Calkins H, Reynolds MR, Spector P et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ. Arrhythm. Electrophysiol. 2(4), 349–361 (2009).
- Noheria A, Kumar A, Wylie JV Jr, Josephson ME. Catheter ablation vs antiarrhythmic drug therapy for atrial fibrillation: a systematic review. Arch. Intern. Med. 168(6), 581–586 (2008).
- Weerasooriya R, Khairy P, Litalien J et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J. Am. Coll. Cardiol. 57(2), 160–166 (2011).
- Senatore G, Stabile G, Bertaglia E et al. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J. Am. Coll. Cardiol. 45(6), 873–876 (2005).
- Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 3(12), 1445–1452 (2006).
- Edgerton JR, Mahoney C, Mack MJ, Roper K, Herbert MA. Long-term monitoring after surgical ablation for atrial fibrillation: how much is enough? J. Thorac. Cardiovasc. Surg. 142(1), 162–165 (2011).
- Saad EB, D’avila A, Costa IP et al. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score ≤3: a long-term outcome study. Circ. Arrhythm. Electrophysiol. 4(5), 615–621 (2011).
- Themistoclakis S, Corrado A, Marchlinski FE et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J. Am. Coll. Cardiol. 55(8), 735–743 (2010).
- Oral H, Chugh A, Ozaydin M et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 114(8), 759–765 (2006).
- Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke 35(7), 1647–1651 (2004).
- Hanke T, Charitos EI, Stierle U et al. Twenty-four-hour Holter monitor follow-up does not provide accurate heart rhythm status after surgical atrial fibrillation ablation therapy: up to 12 months experience with a novel permanently implantable heart rhythm monitor device. Circulation 120(Suppl. 11), S177–S184 (2009).
- Oral H, Veerareddy S, Good E et al. Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 15(8), 920–924 (2004).
- Glotzer TV, Daoud EG, Wyse DG et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ. Arrhythm. Electrophysiol. 2(5), 474–480 (2009).
- Botto GL, Padeletti L, Santini M et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J. Cardiovasc. Electrophysiol. 20(3), 241–248 (2009).
- Boriani G, Santini M, Lunati M et al. Improving thromboprophylaxis using atrial fibrillation diagnostic capabilities in implantable cardioverter-defibrillators: the multicentre Italian ANGELS of AF Project. Circ. Cardiovasc. Qual. Outcomes 5(2), 182–188 (2012).
- Boriani G, Botto GL, Padeletti L et al. Improving stroke risk stratification using the CHADS2 and CHA2DS2–VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke 42(6), 1768–1770 (2011).
•• Multicentric observational study suggesting a changed risk/benefit ratio favoring cessation of oral anticoagulants following successful ablation for atrial fibrillation (AF).
•• Observational study demonstrating safe discontinuation of oral anticoagulants following successful catheter ablation in patients with risk factors other than an age of >65 years or a history of previous stroke.
• Observational study demonstrating limited sensitivity of 24-h Holter monitoring to detect AF recurrence.
•• Prospective observational study evaluating the relationship between risk of stroke and duration of AF burden based on diagnostic data from implantable devices. The authors demonstrate doubling of stroke risk with AF burden >5.5 h on any of the preceding 30 days.
•• Observational study based on device dignostics of implanted pacemakers showing a relationship between the duration of AF and CHADS2 score with stroke risk.