Review Article - Journal of Medicinal and Organic Chemistry (2022) Volume 5, Issue 6
Drug Design: Influence of Heterocyclic Structure as Bioisosteres
Nils Cruz*
Department of Green Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Belgium
Department of Green Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Belgium
E-mail: Cruznils@edu.ezi.com
Received: 02-Dec -2022, Manuscript No. JMOC-22-83172; Editor assigned: 05- Dec-2022, PreQC No. JMOC-22- 83172 (PQ); Reviewed: 19-Dec-2022, QC No. JMOC-22-83172; Revised: 26-Dec-2022, Manuscript No. JMOC- 22-83172 (R); Published: 30-Dec-2022 DOI: 10.37532/jmoc.2022.5(6).113- 117
Abstract
The replacement of a carboxylic acid with a surrogate structure, or bioisostere, is a classical strategy in medicinal chemistry. The general underlying principle is that by maintaining the features of the carboxylic acid critical for biological activity, but appropriately modifying the physicochemical properties, improved analogs may result. In this context, a systematic assessment of the physicochemical properties of carboxylic acid isosteres would be desirable to enable more informed decisions of potential replacements to be used for analog design. Herein we report the structure-property relationships (SPR) of 35 phenylpropionic acid derivatives, in which the carboxylic acid moiety is replaced with a series of known isosteres. The data set generated provides an assessment of the relative impact on the physicochemical properties that these replacements may have compared to the carboxylic acid analog. As such, this study presents a framework for how to rationally apply isosteric replacements of the carboxylic acid functional group.
Introduction
The replacement of an atom, or group of atoms, or even an entire scaffold of a biologically active compound with a surrogate structure that exhibits broadly similar biological properties is a fundamental strategy of medicinal chemistry, known as isosteric or bioisosteric replacement [1]. In general, for this approach to be successful, similarities must exist between at least some of the properties of the bioisostere and those of the fragment being replaced, such that the new analogs retain the biological activities of the parent compound. At the same time, however, the isosteric replacement must produce changes in the physicochemical properties or susceptibility to metabolism compared to the parent compound in order to lead to improved derivatives [2,3]. The success of any isosteric replacement is invariably context-dependent, however, and depends on the particular molecular environment of the biological target, and whether those surrogate structures will also provide the desired improvements in properties. For this reason, a screening of a series of alternative structures is almost always necessary [4]. In this situation, the availability of experimental data detailing the structure−property relationships (SPR) of the existing palette of isosteres is most desirable. However, when these data are not available, the selection/ prioritization of potential replacements is typically based on a variety of factors, such as the historical success rate of specific isosteres, calculated physicochemical properties, chemical/ medicinal chemistry intuition, as well as synthetic accessibility. The importance of the carboxylic acid functional group in drug design is illustrated by the fact that >450 marketed drugs are carboxylic acid containing molecules. However, the presence of this functional group in a drug or a drug candidate can be responsible for undesired consequences, such as limited permeability across biological membranes, metabolic instability, and potential idiosyncratic toxicities [5]. To circumvent one or more of these shortcomings, medicinal chemists typically resort to prodrug or isosteric replacement strategies. As part of our continued interest in the area of isosteric replacements of the carboxylic acid functional group, we set out to define the SPR of a number of acidic moieties that are frequently used as replacements of the carboxylic acid in drug design. In this particular context, the most important physicochemical parameters are arguably the acidity and lipophilicity, as well as the effect that the isosteric replacements may have on compound permeability. Since these three parameters are interrelated, even partial/ incomplete information can be valuable in anticipating the physiochemical outcome of an isosteric replacement [6].
Carboxylic Acid Bioisosteres
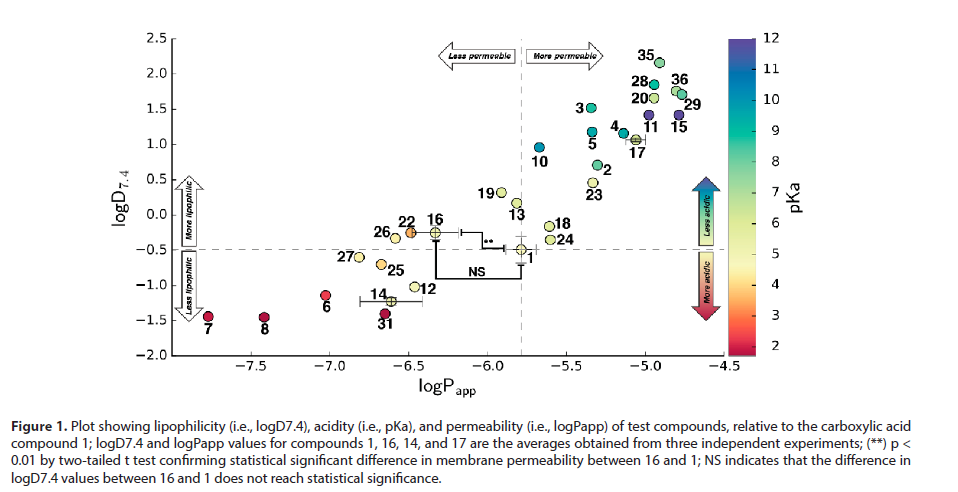
In this overview, a selection of carboxylic acid bioisosteres that have the greatest probability to improve drug-like properties of a lead compound will be discussed, as a complement to and extension of the seminal overview provided by Ballatore et al. is selection is based on several pharmacokinetic properties of the bioisostere relative to the corresponding compound that contains carboxylic acid functionality [7]. These properties include acidity, lipophilicity, and permeability derived from SPR studies and are displayed in terms of pKa value, the distribution coincident between n-octanol and aqueous buffer at pH 7.4 or the octanol-water partition coincident, and the permeability coincident, respectively. These properties depend however on the neighboring group [8]. Therefore, a given bioisostere should only be compared to the carboxylic acid that bears the same scald. Lipophilicity and acidity influence many key ADME properties as well as toxicity (Figure 1). High lipophilicity results in great permeability of the drug through cell membranes and, consequently, results in higher potency.
Figure 1: Plot showing lipophilicity (i.e., logD7.4), acidity (i.e., pKa), and permeability (i.e., logPapp) of test compounds, relative to the carboxylic acid compound 1; logD7.4 and logPapp values for compounds 1, 16, 14, and 17 are the averages obtained from three independent experiments; (**) p < 0.01 by two-tailed t test confirming statistical significant difference in membrane permeability between 16 and 1; NS indicates that the difference in logD7.4 values between 16 and 1 does not reach statistical significance.
However, the ionization of the molecule also influences the permeability of the drug as neutral molecules diffuse more easily through the lipophilic membranes [9]. Therefore, the lipophilicity is quantified at physiological pH as logD7.4. Nevertheless, if the drug is too lipophilic, the compound has an increased likelihood to bind unwanted targets, resulting in toxicity as well as poor solubility and high metabolic clearance. Thus, a balance must be found in terms of lipophilicity. Besides improved lipophilicity, the selection criteria for bioisosteres are improved permeability and/or reduced toxicity as compared to carboxylic acids. Hydroxamic acids, known for their metal-chelating properties, have led to some successes as carboxylic acid bioisosteres. However, although predicted to have higher lipophilicity and permeability (Table 1), hydroxamic acids are said to have low bioavailability and to suffer from toxicity due to glucuronidation and mutagenicity. Drugs containing thiazolidinedione (TZD) and oxazolidinedione (OZD) rings are reported to induce idiosyncratic adverse reactions as well, including liver toxicity [10]. Indeed, the first marketed thiazolidinedione drug, troglitazone, was withdrawn from the market. The thiazolidinedione ring is hypothesized to undergo an oxidative ringopening reaction by cytochrome P450 that results in the formation of electrophilic metabolites.
Tetrazoles
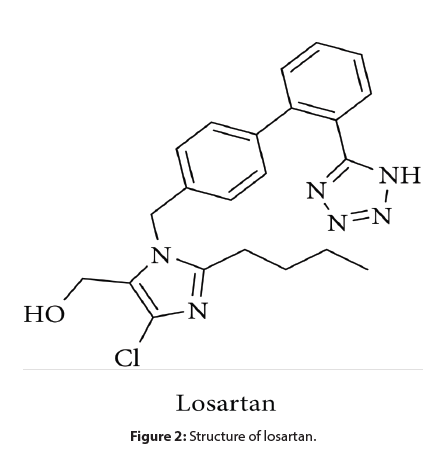
The 5-substituted 1H-tetrazole is the best known and the most frequently used bioisostere of a carboxylic acid functionality. This moiety is found in more than 20 FDAapproved drugs that exhibit various anti-bacterial, antiasthmatic, anti-cancer, anti-fungal, anti-hypertensive, antimalarial, anti-tubercular, or anti-viral properties. The most successful example of a bioisosteric replacement of the carboxylic acid moiety concerns the anti-hypertensive drug losartan (Figure 2), an antagonist of the angiotensin II type 1 receptor (AT1). More recent examples in preclinical studies are inhibitors of inflammatory kinases for the treatment of obesity and protein tyrosine phosphatase 1B inhibitors and G-protein receptor 40 (GPR40) inhibitors for the treatment of diabetes, where the introduction of a tetrazole improved the inhibitory activity of the lead compound. Generally, tetrazoles are considered to be more lipophilic than carboxylic acids while having similar acidity, as evidenced by SPR studies (Table 1) and researched by Hansch and Leo, who have shown that anionic tetrazoles are almost 10 times more lipophilic than corresponding carboxylates. However, according to SPR studies (Table 1), the increased lipophilicity of tetrazoles does not lead to enhanced permeability of the moiety in contrast to the carboxylic acid. This difference may be related, at least in part, to the fact that compounds bearing tetrazoles can establish considerably stronger hydrogen bond (HB) interactions compared to the corresponding compounds containing carboxylic acids. Stronger HB results in higher desolvation energies, and therefore, tetrazoles could exhibit lower permeability. On the other hand, the stronger HB of the tetrazole moiety may improve the binding affinity of the compound for its receptor.
Other Aromatic Heterocycles as Bioisosteres
As previously discussed, although tetrazoles are more hydrophobic than carboxylic acids, some drugs containing tetrazoles, such as candesartan, still suffer from poor membrane permeability and low bioavailability. This is because their acidity is similar to carboxylic acids. By replacement of this tetrazole moiety with other, slightly less acidic heterocycles such as 5-oxo-1,2,4-oxadiazole and 5-oxo- 1,2,4-thiadiazole, the oral bioavailability of the AT1 inhibitor is increased. This is also predicted by SPR studies (Table 1). In addition, other aromatic heterocycles such as 2-thioxo- 1,3,4-oxadiazole, 1,2,4-triazole (pKa ‑ 10.0), and imidazole have shown their effectiveness as carboxylic bioisosteres in the search of novel inhibitors of β-secretase, a therapeutic target in Alzheimer’s disease [11]. This suggests that the delocalized π-orbitals or the proton of the amine on the heterocycles is important for inhibitory activity. They also share their planarity and two hydrophobic regions above and below the plane with the carboxylic acid moiety.
| Isostere | R | pKa | logD7.4 | LogP | logPapp |
|---|---|---|---|---|---|
| Carboxylic acid | R1 | 4.64 | −0.49 ± 0.19 | ND | −5.79 ± 0.10 |
| R2 | 4.76 | −1.65c | ND | ND | |
| R3 | 3.96 | −1.5 | ND | ND | |
| R4 | 4.13 ± 0.01 | ND | 1.98 ± 0.04 | ND | |
| R5 | 4 | ND | ND | ND | |
| R6 | 6.52 | ND | 1.09 | ND | |
| Hydroxamic acid | R1 | 8.18 | 0.71 | ND | −5.30 |
| Sulfonic acid | R1 | <2 | −1.45 | ND | −7.42 |
| Sulfinic acid | R1 | 2.1 | −1.30 | ND | ND |
| Phosphonic acid (X = OH) | R1 | 2.34 | −1.14 | ND | −7.03 |
| Phosphinic acid (X = H) | R1 | 1.98 | −1.44 | ND | −7.77 |
| Sulfonamides | R1 | 10.04 | 0.96 | ND | −5.67 |
| R1 | >12 | 1.42 | ND | −4.98 | |
| Acyl sulfonamides | R1 | 4.94 | −1.02 | ND | −6.46 |
| R1 | 4.49 ± 0.04 | −0.09 | 2.67 ± 0.01 | −5.79 | |
| Sulfonylurea | R1 | 5.04 | −1.23 ± 0.06 | ND | −6.61 ± 0.20 |
| Acyl sulfonimidamide | R3 | 7.38 | 1.2 | ND | ND |
| Cyclic sulfonimidamide | R5 | 6 | 1.6 | ND | −4.18 |
| Trifluoromethyl carbinol | R2 | ND | 1.03c | ND | ND |
| Trifluoromethyl ketone | R2 | ND | 0.25c | ND | ND |
| Tetrazole | R1 | 5.09 | −0.25 ± 0.10 | ND | −6.33 ± 0.15 |
| R2 | ND | −0.37c | ND | ND | |
| R3 | 4.2 | −1.0 | ND | ND | |
| R4 | 4.73 ± 0.03 | ND | 1.07 ± 0.01 | ND | |
| R5 | 4 | 1.3 | ND | −5.14 | |
| Tetrazolone | R6 | 6.36 | ND | 0.79 | ND |
| 5-Oxo-1,2,4-oxadiazole (X = O, Y = O) | R1 | 5.73 | 0.32 | ND | −5.91 |
| R4 | 5.12 ± 0.04 | ND | 1.14 ± 0.04 | ND | |
| 5-Oxo-1,2,4-thiadiazole (X = S, Y = O) | R1 | 6.5 | 1.66 | ND | −4.94 |
| R4 | 5.99 ± 0.09 | ND | 2.22 ± 0.08 | ND | |
| 5-Thio-1,2,4-oxadiazole (X = O, Y = S) | R1 | 3.58 | −0.25 | ND | −6.49 |
| R4 | 3.19 ± 0.02 | ND | 2.39 ± 0.02 | ND | |
| Tetronic acid (X = O) | R2 | ND | −0.06c | ND | ND |
| Tetramic acid (X = H) | R1 | 6.08 | −0.35 | ND | −5.60 |
| Cyclopentane-1,3-diones | R1 | 4.01 | −0.70 | ND | −6.67 |
| Cyclopentane-1,2-diones | R1 | 8.88 | 1.85 | ND | −4.94 |
| Squaric acid | R1 | <2 | −0.84 | ND | ND |
| 3-Hydroxyisoxazole (X = O) | R1 | 5.36 | 0.46 | ND | −5.33 |
| 3-Hydroxyisothiazole (X = S) | R2 | ND | 1.25c | ND | ND |
| 2-4-Oxazolidinedione | |||||
| (X = O) | R1 | 5.86 | −0.16 | ND | −5.61 |
| 2,4-Thiazolidinedione | |||||
| (X = S) | R1 | 6.19 | 1.07 ± 0.03 | ND | −5.06 ± 0.06 |
| Oxetan-3-ol (X = O) | R1 | >12 | 2.07 | ND | −5.08 |
| Thietan-3-ol (X = S) | R1 | >12 | 2.99 | ND | −4.88 |
| Thietan-1-oxide-3-ol (X = SO) | R1 | >12 | 1.22 | ND | −5.21 |
| Thietan-1,1,-dioxide-3-ol (X = SO2) | R1 | 9.31 | 1.24 | ND | −4.91 |
Sulfonamides and Derivatives
Sulfonamides are known to be carboxylic acid bioisosteres since the development in the 1930s and 1940s of the antibacterial agents sulfadiazine and sulfanilamide, the latter being the active form of prontosil [12]. These drugs show activity because of the similarities of the sulfonamide moiety with the carboxylic acid of the natural substrate para-aminobenzoic acid (PABA) of the enzyme tetrahydropteroate synthetase that they inhibit. The sulfonamide analog of losartan (Figure 2) has shown good activity, which also proves that the sulfonamide moiety is a potent carboxylic acid surrogate. Although being weakly acidic and nonplanar in contrast to carboxylic acids, sulfonamides exhibit similar geometry of the hydrogen bond environment and a comparable average electron density to carboxylic acids. Unlike the sulfonamide moiety, its derivatives such as acyl sulfonamides and sulfonylureas display pKa values that fall within the range of carboxylic acids [13].
Conclusion
The carboxylic acid is a highly versatile functionality in drug design. However, it can entail some downsides such as toxicity, poor lipophilicity, and membrane permeability. There are a plethora of possible replacements for the carboxylic acid functionality in a bioactive agent that could improve the activity, selectivity, and pharmacokinetics of the compound. Although the success of the replacements of these bioisosteres cannot be predicted, this paper gives a short overview of the most promising bioisosteres based on their ADME properties and their potential to avoid toxicity problems. Examples of classical carboxylic acid bioisosteres are the trifluoromethyl ketones and the sulfonamide derivatives, even though they do not share the planarity of the carboxylic acid. On the other hand, the aromatic heterocycles from which the tetrazole moiety is the best known and most investigated, do exhibit a planar geometry. Other heterocyclic bioisosteres could be worthy alternatives as they improve the bioavailability and permeability of the compound compared to the parent structure bearing a tetrazole moiety. By selecting these alternative heterocycles, the use of explosive and toxic azide reagents for the synthesis of tetrazoles is also avoided. Yet the search for new bioisosteres keeps advancing in the hope to find new potent drugs for use in the human body.
Data Availability
No data were used to support this study.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Parihar MS, Brewer GJ. Amyloid-β as a modulator of synaptic plasticity. J Alzheimer's Dis. 22, 741–763 (2010).
- Yuan TF, Shan C. Glial inhibition of memory in Alzheimer’s disease. Sci China Life Sci. 57, 1238–1240 (2014).
- Prince M, Bryce R, Albanese E et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dement. 9, 63–75 (2013).
- Mohandas E, Rajmohan V, Raghunath B et al. Neurobiology of Alzheimer′s disease. Indian J Psychiatry. 51, 55–61 (2009).
- Swerdlow RH. Pathogenesis of Alzheimers disease. Clin Interv Aging. 2, 347–359 (2007).
- Suzhen D, Yale D, Feng G et al. Advances in the pathogenesis of Alzheimer's disease: a re-evaluation of amyloid cascade hypothesis. Transl. Neurodegener. 1, 18 (2012).
- Rauch SM, Huen k, Miller MC et al. Changes in brain β-amyloid deposition and aquaporin 4 levels in response to altered agrin expression in mice. J Neuropathol Exp Neurol. 70, 1124–1137 (2011).
- Lopategui Cabezas I, Herrera Batista A, Pentón Rol G et al. The role of glial cells in Alzheimer disease: Potential therapeutic implications. Neurología. 29, 305–309 (2014).
- Vickers JC, Dickson TC, Adlard A et al. The cause of neuronal degeneration in Alzheimer's disease. Prog Neurobiol. 60, 139–165 (2000).
- Gandy S. Alzheimer's disease: New data highlight nonneuronal cell types and the necessity for presymptomatic prevention strategies. Biol Psychiatry. 75, 553–557 (2014).
- Finsterwald C, Magistretti PJ, Lengacher S et al. Astrocytes: New targets for the treatment of neurodegenerative diseases. Curr Pharm Des. 21, 3570–3581 (2015).
- Claycomb K, Johnson K, Winokur P et al. Astrocyte Regulation of CNS Inflammation and Remyelination. Brain Sci. 3, 1109–1127 (2013).
- Abbott NJ, Rönnbäck L, Hansson E et al. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 7, 1-4 (2012).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref