Review Article - Interventional Cardiology (2012) Volume 4, Issue 1
Drug-eluting stent coatings
- Corresponding Author:
- Xiaodong Ma
Department of Plastics Engineering
University of Massachusetts, Lowell, MA 01854, USA
Tel:+1 978 934 4896
Fax:+1 978 934 4056
E-mail:coltlife@gmail.com
Abstract
Keywords
drug-eluting stent, nanotechnology, restenosis, stent coating, thrombosis
Over the last decade, stent technology has been introduced and shown excellent performance in prevention of occlusion and restenosis when compared with conventional angioplasty [1,2]. In particular, drug-eluting stents (DES) have revolutionized the treatment of coronary artery disease (CAD) in recent years, by significantly reducing in-stent restenosis (ISR) and the need for target vessel revascularization (TVR). However, with the dramatic increase in the use of DES, stent thrombosis (ST) has become a major concern, especially late ST (LST) [3]. Since restenosis and thrombosis are caused by multiple factors, an ideal stent coating including drug-vehicle materials and pharmaceutical agents should not only inhibit thrombus formation, inflammatory reaction and proliferation of smooth muscle cells, but also facilitate the process of re-endothelialization. This review focuses on the development of stent coatings and their impact on restenosis and thrombosis rates.
Overview of coronary stents
CAD continues to be the most common cause of morbidity and mortality in the western world. A well known cause of CAD is atherosclerosis [4]. Although coronary artery bypass graft surgery has been proven an effective and curative approach to CAD, there has been a steady drive to develop less invasive therapies. Percutaneous coronary interventions, including percutaneous transluminal coronary artery angioplasty (PTCA) and coronary artery stenting (CAS), are examples of therapies which have changed the treatment of CAD.
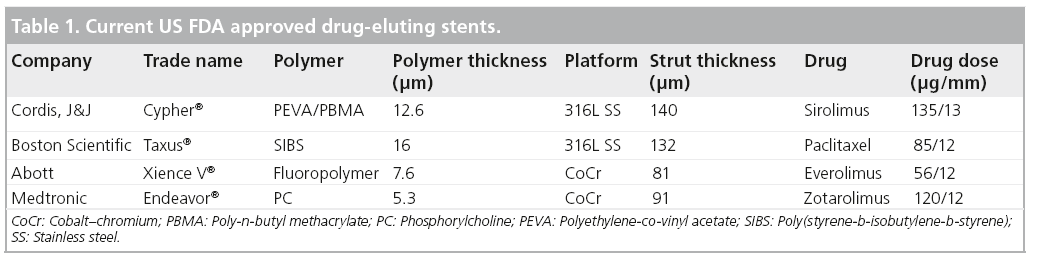
PTCA was first introduced into clinical practice by Andreas Grüntzig in the late 1970s [5]. During PTCA, an expandable balloon catheter is inserted into an artery to reach the primary atherosclerostic plaque or secondary restenotic lesion, followed by inflation of the balloon to improve vessel patency and blood flow. However, this procedure causes injury to the blood vessel, resulting in vascular elastic recoil, neointimal proliferation and negative remodeling, often leading to restenosis, which will again lead to blockage of the blood and insufficient oxygenation of cardiac tissue. Besides the occurrence of restenosis, a more severe problem is the collapse of the artery, which may occur during the PTCA procedure after the deflation of the balloon or even during the recovery period after the angioplasty. Once this occurs, emergency coronary artery bypass graft is the only option. To overcome these issues, CAS technology was proposed and developed. In CAS, a metallic stent is crimped onto a balloon catheter and then placed at the narrowed segment of the artery with inflation and deflation of the balloon. The stent functions as a scaffold to expand and support the artery wall and thus reduce restenosis after balloon angioplasty. In the late 1980s, Sigwart et al. first reported the successful insertion of bare-metal stents (BMS) into the coronary arteries of eight patients [6]. Over the years, with the development of several generations, the adoption of BMS has demonstrated an effective reduction in restenosis compared with PTCA alone [1,2,6] and significant decreases in the rates of the major adverse cardiac events and death [7,8]. Nevertheless, 20–30% of patients develop clinically evident restenosis. ISR has become a new and significant clinical problem [2,9]. To effectively reduce ISR, many investigators have been developing new solutions to inhibit cell proliferation by locally delivering antirestenotic agents to the diseased arteries where stents are implanted. A revolutionary solution was the development of DES in the early 2000s [10,11]. The DES take advantage of both reliable metallic stent platforms and the delivery of an effective antirestenotic drug. The drug is carried to the region of injury caused by stenting and delivered by a thin layer of stent coating on a standard coronary stent, thereby decreasing the rate of ISR. Table 1 shows the four US FDA approved DES.
Table 1: Current US FDA approved drug-eluting stents.
Current problems in DES : ISR & LST
Alhough DES have represented a major breakthrough in the reduction of the incidence of ISR [12–15], restenosis rates are still unacceptably high in high-risk patients with small vessels, diabetes and long segments of diffusely diseased arteries (30–60% in BMS and 6–18% in DES) [16–18]. Although the detailed mechanism of ISR is still under investigation, neointimal hyperplasia is thought to be the main cause. A number of risk factors for ISR has been recognized, such as stent material and geometry, lesion and stent length, stent number, diabetes and female sex [19].
Depending on the time of occurrence, ST can be classified into four types: acute, subacute, late and very late. Acute ST occurs between 0 and 24 h following stent implantation, subacute between 24 h and 30 days, late between 30 days and 1 year, and very late after 1 year [20]. As such, very late ST is, thus far, poorly described and it seems that DES increase the risk of very late ST moderately but significantly [20]. For this reason, many authorities advise that dual antiplatelet therapy should be continued for longer than one year after DES implantation. The current guidelines, however, recommend this therapy for at least one year. LST is a rare, but severe, complication (0–2% incidence) and a recent clinical study has shown it will result in fatal myocardial infarction (MI) in 45% of patients [21]. Therefore, LST has been a great concern recently, while acute/subacute thrombosis can be managed with the use of dual antiplatelet therapy. The precise mechanism of LST is still unknown, but it is generally believed that the combination of delayed endothelialization due to antiproliferative therapy and persistence of the nondegradable polymer, contribute to the hypersensitivity reaction, possibly with some residual active drug that may not have been eluted [22,23].
Importance of stent coatings
As stated above, ISR remains unacceptably high in some high-risk patients and LST has been a major concern associated with current clinical applications of DES. Therefore, a number of studies have addressed these problems through modification of the stent with respect to the blood and tissue compatibility, including changing the stent material itself and modifying the outer layer of the stent surface, aggressive anticoagulant and antiplatelet therapies, or antiproliferative strategies to inhibit neointimal growth. While some materials with excellent mechanical properties have no favorable biocompatibility, other materials with good biocompatibility are not suitable for stent production. Thus, modifying or coating different materials on the stent surface is one of the effective methods to alter characteristics of the stent surface and improve the stent’s biocompatibility and hemocompatibility, and hence reduce the occurrence of thrombosis and restenosis.
Types of stent coatings
There is a variety of stent coatings with differing performance profiles that have been studied either in animal studies or clinical trials. The stent coatings can be broadly classified into three types: biocompatible coatings, drug-delivery coatings and polymer-free coatings/surfaces. Since metallic stent platforms will release ions after implantation and thus lead to inflammatory reactions, the initial stent coatings served only as an ion release barrier, with good compatibility on the stent surface. These coatings are mostly inorganic materials that usually have no capacity to carry drugs that may interfere with intima proliferation induced by artery injury with stent placement. To conquer this difficulty, biocompatible polymer coatings were developed and have been successfully coated on the stent surface as a drug carrier, to store and elute pharmaceutical agents to the lesion site. However, polymer coatings are considered one of the key factors that lead to thrombosis. Therefore, a series of strategies were employed to develop polymer-free stent coatings/surfaces. Considering the burgeoning development of nanotechnology, coating nanomaterials on the stent surface or modifying the stent surface in nanoscale is a novel and promising approach. Nanoscale is usually defined as smaller than 0.1 μm in at least one dimension, although this term is sometimes also used for materials smaller than 1 μm. Thus, the words with a nano- prefix in this paper mean that the dimension size of the material, the pore, the pattern, the particle and even the thickness is in nanoscale. Besides the three types of coatings, other coatings or strategies are also reviewed.
▪ Inorganic coatings
Gold
Preclinical studies have shown that gold-coated stents yielded fewer macroscopic and histopathologic changes in the aorta than stainless steel stents and those coated with silver, copper, Teflon® or silicone [24]. In addition, as gold is a highly radiopaque material, it can be coated on stainless steel stents to enhance fluoroscopic visibility. However, human clinical trials relating to gold coatings were disappointing, as the gold coating appeared to activate platelets and caused more neointimal hyperplasia. For example, in patients implanted with gold-coated and uncoated steel stents, 49.7 and 38.1% acquired ISR and 2.5 and 0.8% experienced thrombosis, respectively [25]. The results of another clinical trial also indicated more neointimal tissue proliferation in patients with gold-coated stents. The trial randomized 204 patients to receive uncoated (group A; n = 101) or coated (group B; n = 103) stents. The angiographic minimal luminal diameter was smaller in group B (1.47 ± 0.57 vs 1.69 ± 0.70 mm; p = 0.04), with a higher late luminal loss of 1.17 ± 0.51 versus 0.82 ± 0.56 mm (p = 0.001) [26].
Silicon carbide
Silicon carbide (SiC) is a semiconductor that was coated on metal stents for an in vitro circulation study. The results of the study have shown significantly lower platelet and leukocyte adhesion at the surface of the SiC-coated tantalum stent compared with stainless-steel stents, uncoated and heparin-coated tantalum stents [27]. However, the outcomes of human clinical trials were not satisfactory. In terms of clinical and angiographic restenosis rates, it has been reported there was no superiority of the SiC-coated stent over a stainless steel stent, after 4.7 ± 1.2 months [28]. Further improvement and evaluation of SiC coating is needed.
Iridium oxide
Hydrogen peroxide has been recognized as a strong oxidizing agent that can result in inflammatory reactions in arteries. Due to the capability of iridium oxide to promote the immediate conversion of hydrogen peroxide into water and oxygen, iridium oxide has been coated on a 316L stainless steel stent and has shown encouraging results in a clinical trial, with improved biocompatibility and fast re-endothelialization [29]. However, further evaluation in a randomized study is needed to confirm the efficacy and safety of iridium oxide-coated stent. A detailed investigation into the role of iridium oxide in the conversion of hydrogen peroxide into water and oxygen and its impact on restenosis and thrombosis is also required.
Carbon
In vitro analyses have indicated that stents coated with ‘diamond-like’ carbon were highly biocompatible and yielded less platelet activation, thrombogenicity and metal ion release, than uncoated stents [30]. Moreover, a 6-month clinical and angiographic follow-up study has shown that the carbon coating was able to dramatically reduce ST and restenosis in these relatively high-risk patients [31]. However, more recent studies have shown that carbon-coating does not provide clinical advantages in comparison with BMS [32]. Thus, the performance of carbon-coating requires further confirmation from more comprehensive and definitive studies.
Titanium-nitride-oxide
Endothelium-derived nitric oxide plays an important role in regulating endothelial function, assisting suppress platelet aggregation, cellular adhesion to the endothelium and even inhibiting smooth muscle cell proliferation [33]. It is anticipated that a nitrogen-containing oxide should have a similar benefit, although there is no evidence to support this, and it is a promising way to apply the nitrogen-containing oxide as a stent coating. The safety of the titanium-nitrideoxide- coated bioactive stent (Titan2®, Hexacath, Paris, France) has been confirmed in unselected populations [34,35]. Interestingly, bioactive stents have demonstrated even better outcomes compared with paclitaxel-eluting stents in the realworld setting of ‘high-risk’ patients and complex coronary lesions [36], as well as in patients presenting with acute MI [37]. Therefore, it is one of the few non-DES coatings with its own merit, which can compete with DES and share a part of the market.
▪ Polymer coatings
Polymers are large molecular compounds consisting of repeating structural units, typically connected by covalent chemical bonds. Other than the aforementioned coatings, which play a crucial role in biocompatibility improvement, polymers bear one more duty – to carry and locally release therapeutic agents to the injured area of arteries.
Nonbiodegradable polymers
In the current US market, both the first and second generations of DES are coated with nonbiodegradable polymers to control the drug-eluting profile (Table 1). In the first generation of DES, the stent coating of the Cypher®, a sirolimus-eluting stent (Cordis, NJ, USA) is a polymer blend of poly(ethylene-co-vinyl acetate) and poly(n-butylmethacrylate) loaded with sirolimus, while the coating of the Taxus®, a paclitaxel-eluting stent (Boston Scientific, MA, USA) is poly(styrene-b-isobutylene-b-styrene) loaded with paclitaxel. The clinical outcomes have shown both DES can significantly reduce restenosis compared with BMS [12–15]. However, an increase in the rate of MI and mortality was reported in patients 18 months to 3 years after the implantation of Cypher and Taxus [38–41]. In the second generation of DES, stent coatings of the Xience V®, an everolimus-eluting stent (Abott Vascular, CA, USA) and Endeavor®, a zotarolimus-eluting stent (Medtronic Vascular, CA, USA), are fluoropolymer and phosphorylcholine, respectively. Compared with the BMS, TVR was dramatically reduced by both Endeavor and Xience V stents [42,43]. It was reported in the SPIRIT III study, that Xience V stents can reduce angiographic late loss without an increase in ST, compared with Taxus stents [44] and also showed superiority in the COMPARE trial, with a lower rate of ST and fewer MIs and TVR [45]. However, Endeavor stents resulted in a higher incidence of restenosis than Cypher stents during the ENDEAVOR III trial [46]. Never the less, both Endeavor and Taxus stents demonstrated an equivalent target lesion revascularization (TLR) rate in a later trial [47]. In addition, it is worth noting that there are no cases of very late ST reported in early ENDEAVOR trials over 4 years, indicating the Endeavor stent is safe long-term [48]. However, the conclusion should be further confirmed by a follow-up study of greater duration, with a larger number of patients. PROTECT is one example of a randomized, open-label trial comparing the long-term safety of Endeavor and Cypher. The trial has enrolled 8800 patients, representative of those seen in routine clinical practice undergoing elective, unplanned, or emergency procedures in native coronary arteries in 196 centers in 36 countries. This large, international, randomized, controlled trial will provide important information on comparative rates of ST between two different DES systems and safety as assessed by patient-relevant long-term clinical outcomes [49].
Biodegradable polymers
Although some nonbiodegradable polymercoated DES claimed to be safe long-term, there remains caution regarding the inflammatory response [50]. Thus, biodegradable polymers are being considered and investigated to store and deliver drugs. The most commonly used polymers now are poly(lactic acid) (PLA), poly(glycolic acid) and their copolymer, poly(lactic-co-glycolic acid) (PLGA), which can be fully degraded and metabolized by the body [51,52]. A multitude of biodegradable polymer- coated stents are currently in clinical trials. For example, the Sparrow™ NiTi stent system (Surmodics Inc., Eden Prairie, MN, USA) employs SynBiosys™ biodegradable polymer PLGA to elute sirolimus [53]; the CE-approved Biomatrix® stent (Biosensors International, Singapore), which was licensed to Terumo Corporation (Tokyo, Japan) with a new brand name (Nobori®) in May 2007, releases a sirolimus analogue, biolimus A9, from PLA coated on 316L stainless steel stent platform [54]; both Excel® (JW Medical Systems, China) and Cura™ (OrbusNeich Medical, Inc., FL, USA) are PLA and sirolimus-coated stainless steel stents [55,56]; Conor Medstent™ stent (Conor Medsystems, CA, USA) uses PLGA while Infinnium™ stent (Sahajanand Medical Technologies, India) utilizes PLA to elute paclitaxel [57–59]. In spite of many promising preliminary results, the development of biodegradable polymers in DES is still a challenge. On the one hand, the degradation of polymers is affected by a variety of factors [60] such as pH, polymer size, molecular weight, crystallinity and so forth, making the profile of drug release more difficult to control. On the other hand, the accumulated acidic products from polymer degradation may lead to significant inflammatory response of the vessel wall. To address this issue, Ma et al. incorporated amorphous calcium phosphate nanoparticles (NPs) into PLGA, in order to neutralize the acidic environment and thus eliminate the inflammatory reaction [61], but this assumption is still under investigation.
▪ Polymer-free coatings/surfaces
Microporous surfaces
Yukon™ (Translumina, Hechingen, Germany) is a typical example of a microporous stent that elutes sirolimus. The micropore, functioning as a drug reservoir and thus removing the requirement of polymer, is created on the surface of 316L stainless steel stent by mechanical treatment or modification [62,63]. Determined by a perthometer, the microporous stent surface has an average roughness of 1.96 ± 0.21 μm. The sirolimus is spray coated on this porous surface using specific equipment that allows the entire drug loading process to be completed within 10 min [62]. Recent clinical data have demonstrated that the Yukon stent has a comparable performance with that of Taxus at 9- to 12-month follow-up [64,65]. However, compared with Cypher at 3-month follow-up, the Yukon stent has significantly greater neointimal thickening and stent strut coverage, which was confirmed by a randomized optical coherence tomography study [66]. It is probably due to the Yukon stent’s microporous surface topography and rapid sirolimus release profile. However, definitive work is needed to determine the long-term safety and efficacy of the Yukon stent.
Microstructured surfaces
Similar to Yukon, the BioFreedom™ stent (Biosensors International Group, Ltd., CA, USA) has a selectively microstructured abluminal surface, texturized by displacing the metal using a microabrasion process on a 316L stainless steel stent platform. A large number of created crevices on this microstructured surface, allows for a high level of drug (Biolimus A9) adhesion, without an application of polymers. A recent first cohort of the BioFreedom firstin- man study in which 75 low risk patients were randomized to be treated with either a standard-dose (SD; biolimus A9 225 μg) or a low-dose (LD; biolimus A9 112 μg), BioFreedom or the TAXUS™ Liberté, demonstrated a significant reduction of in-stent late loss at 4 months, in the two BioFreedom groups (BioFreedom SD = 0.08 mm vs BioFreedom LD = 0.12 mm vs TAXUS Liberté = 0.37 mm; p < 0.0001 and p = 0.002, respectively) [67].
Slotted tubular surfaces
The Optima™ stent developed by Carbostent and Implantable Devices (CID S.r.l., Saluggia, Italy) is a slotted tubular multicellular architecture 316L stainless steel stent. The stent system provides slots on the abluminal surface as reservoirs for drug (tacrolimus) loading and an integrated carbon coating (Carbofilm™) for reducing the risk of ST [67]. Similar to Optima, the Cre8™ stent involves abluminal reservoir technology that elutes amphilimus, a combination of sirolimus plus an organic acid. It also has a bioinducer surface, which is made of carbon and is intended to optimize hemocompatibility. The NEXT study, an international prospective randomized clinical trial, showed the Cre8 to be statistically superior to TAXUS Liberté with respect to in-stent late lumen loss (0.14 mm Cre8 vs 0.34 mm Taxus; p < 0.0001) [201].
Nanoporous surfaces
Nanoporous stent surfaces are attractive due to their higher drug loading capacity with increased surface area and influence on drug releasing profiles. A nanoporous hydroxyapetite (a biocompatible crystalline derivative of calcium phosphate) coating with 0.3–1 μm thickness and a porosity of 40–60% in volume, has been developed by MIV Therapeutics, Inc. (Vancouver, BC, Canada) [68]. Impregnated with a low dose of sirolimus, this nanothinmicroporous hydroxyapatite-coated stent has shown promising results in both animal studies and an initial 1-year clinical trial [69]. A stainless steel stent coated with nanoporous aluminium oxide is another example of developing nanoporous stent surfaces. In the process of manufacturing, a thin layer of aluminum was coated on the surface of a 316L stainless steel stent and then the coated stent was anodized to convert the aluminum layer to a porous aluminum oxide. An early animal study indicated a good vascular compatibility of the stent with regard to loading and releasing tacrolimus from the porous structure [70]. However, disappointing results were reported from a subsequent animal study, with evidence of particle debris shed from the coating contributing to increased neointimal hyperplasia [71]. Due to the unsatisfactory clinical performance, further development of this concept and technology has been abandoned [72].
NPs
NPs have been extensively applied in various drug delivery systems, but few studies have reported the results of stent surfaces coated with NPs. Recently, an active coating of NPs was successfully deposited on the surfaces of metallic stents via cationic electrodeposition coating technology and both in vitro drug release kinetics and in vivo feasibility of this NP-eluting stent were characterized and evaluated [73]. The investigators concluded that the NP-eluting stent is a potential innovative platform exhibiting unique aspects in vascular compatibility and an efficient drug delivery system compared with the dip-coated polymer-eluting stent [73]. Another interesting NP-mediated drug delivery system is composed of magnetic NPs loaded with endothelial cells (ECs) and 304 grade stainless steel [74]. Instead of being coated directly on stent surface, the PLAmodified magnetic NPs loaded with ECs were targeted by a magnetic field gradient towards the stent surface after injection, which enables artificial endothelialization and repeated dosing. Although promising results were reported, further evaluation of this method in animal studies and clinical trials is required, as the idea is still at the experimental stage.
Nanopatterned surfaces
Surface chemistry and topography are two key factors for adhesion of proteins and cells, and thus a specific stent coating with periodic structure would influence the growth and proliferation of ECs. It has been reported that a higher percentage of endothelial coverage on nanoscale patterns is achieved in comparison to microscale patterns or random nanostructured surfaces [75]. Therefore, modifying the stent surface with nanopatterns is a promising approach to enhance the process of re-endothelialization. However, it is worthy of noting that submicron (>100 nm in the lateral scale) titanium surface features promoted higher EC adhesion density compared with pure nanometer (<100 nm in both the lateral and vertical scale) surface features [76].
Dual polymer-free DES
The strategy of dual polymer-free DES is to improve the antirestenotic performance of polymer- free stents by using a second antiproliferative agent that inhibits cell growth through a different cell cycle pathway, such as paclitaxel and sirolimus [77]. In the ISAR–TEST-2 study, 1007 patients were randomized to treatment with Cypher (n = 335), Endeavor (n = 339), or a dual polymer-free DES (n = 333) that eluted sirolimus and the antioxidant probucol [78,79]. The primary end point was binary angiographic restenosis at 6–8 month follow-up angiography, which in the dual-DES group (11.0%) was significantly lower than that in the Endeavor group (19.3%; p = 0.002) but comparable with that in the Cypher group (12.0%; p = 0.68). Secondary end points were angiographic in-stent late loss, TLR, death/MI and ST at 12 months. Similarly, TLR with dual-DES (6.8%) was significantly lower than Endeavor (13.6%; p = 0.001) but not different to that of Cypher (7.2%; p = 0.83). No differences were observed between stent groups in terms of death/MI or ST [78]. At 2-year follow- up, there was no differential safety profile between the three groups. Moreover, the antirestenotic efficacy of both dual-DES and Endeavor remained durable between 1 and 2 years, with dual-DES maintaining an advantage over the entire 2-year period [79]. Thus, those findings suggest dual polymer-free DES is a promising strategy for the future.
▪ Other coatings/strategies
Heparin
Due to its anticoagulant properties [80] and inhibitory effect on arterial smooth muscle cell proliferation [81,82], heparin has been widely applied to the surfaces of vascular implants. In particular, heparin coating of stents has been proven to be effective and safe [83,84]. However, other randomized clinical human trials have shown no differences regarding the rate of thrombosis and restenosis between heparincoated stents and uncoated stents. For instance, ST and restenosis were 1.9 versus 1.3% and 33.1 versus 30.3%, respectively, in a trial using heparin-coated and uncoated Jostent® (Jomed International AB, Helsinghorg, Sweden) [85]. In addition, no impact was reported on the angiographic or clinical events by comparing heparin- coated stents with BMS in the treatment of stenoses in small coronary arteries [86].
Hyaluronic acid
Hyaluronic acid (HA) is a ubiquitous, nonsulfated glycosaminoglycan and is widely distributed in various tissues of the body as a major component of extracellular matrix. The HA-coated stainless steel stents and tubes have been reported to significantly reduce the platelet thrombus formation in a baboon thrombosis model [87]. In addition, compared with stainless steel stents, HA cross-linking with N-(3-dimethylaminopropyl)-N´-ethyl carbodiimide-coated stents had an advantageous inherent antiproliferative effect regarding neointimal formation after local vessel wall injury (overstretch model) and led to a reduced inflammatory response in undiseased pig coronary arteries [88]. However, a later animal study showed different results in which airway stents coated with a cross-linked derivative of HA were evaluated in a rabbit airway model of subglottic stenosis [89]. The data suggested that this HA-derivative-coated stent may help reduce stenosis in patients without airway injury, but has no significant advantages on healing improvement in posttraumatic models [89].
Fibrin
Fibrin is a fibrous protein, produced through the cleavage of fibrinopeptides from fibrinogen by thrombin during blood clotting [90]. The efficacy and safety of a fibrin-film-covered stent has been evaluated in a porcine coronary injury model, in which fibrin acted as an excellent biocompatible and biodegradable polymeric coating when incorporated onto metal stents [91]. Since the fibrin-film provides a complete endoluminal paving with delivery of antithrombogenic or antiproliferative therapy, such a ‘hybrid stent’ offers particular benefit to the arterial injury site by covering and delivering drugs to the entire lesion surface [91]. Although the results of animal studies indicated fibrin is a promising stent coating, the efficacy and safety of a fibrin-coated stent needs to be further evaluated in human clinical trials.
ECs
Since EC damage and exposure of subendothelial matrix at the artery injury site is the basis for both thrombus and neointimal formation [92,93], it seems reasonable to deliver ECs on stents to diseased arterial sites for rapid re-endothelialization and EC proliferation, differentiation and release of growth factors, which in turn could inhibit thrombosis and neointimal hyperplasia [94]. ECs were first seeded on stents and studied in vitro by Van der Giessen et al. who concluded that autologous EC-coated stents might protect against early thrombosis [95]. The outcomes of some animal studies have also shown enhanced re-endothelialization and inhibition of neointimal hyperplasia achieved using EC seeding [96–98]. A recent novel attempt involved seeding endothelial progenitor cells (EPCs) on the stent surface. The results of in vitro experiments suggested that on-stent cell delivery of EPCs may be novel therapeutic devices for re-endothelialization and reduce the risk of LST and ISR [99]. Genous™ capture R stent and Combo™ bioengineered sirolimus-eluting stents are examples of such products, developed by OrbusNeich Medical, Inc. (FL, USA). Genous stent is covered with monoclonal, antihuman CD34 antibodies to capture EPCs on the luminal surface of 316L stainless steel R stent BMS. It has been evaluated in a number of clinical trials including HEALING FIM, HEALING II, GENIUSSTEMI and TRIAS, and appeared effective in stable patients [54]. However, a potential shortcoming of this technology is that some unwanted cells, including smooth muscle progenitor cells, may also be sequestered resulting in neointimal proliferation since CD34+ markers used to phenotype EPCs are nonspecific [100]. Besides the EPC-capture coating on the luminal surface, the Combo stent has an additional abluminal coating containing a low-dose sirolimus and a biodegradable SynBiosys polymer. The clinical evaluation of the Combo stent is underway in the REMEDEE trial [101]. Nevertheless, further development of this concept has been limited by the rapid loss of seeded ECs, the damage of ECs upon stent expansion and the difficulty of maintaining EC adherence to the artery wall due to blood flow [93,94].
Conclusion
In this review, a wide range of stent coating materials and surface modification methods were discussed. Particularly, the application of nanotechnology in stent coatings or surface modifications is an attractive field of research. Following the revolutionized development of DES for the treatment of CAD, stent coatings play a vital role, undertaking tasks ranging from enhancing stent biocompatibility to carrying drugs or other agents, to improving stent performance. Hence, the development of stent coatings including material selection and coating strategies is a worthy endeavor.
Future perspective
Since stent coating provides a promising approach for combining mechanical properties and biocompatibility of different materials, especially in collaboration with emerging nanotechnology, many attempts have been made to increase the biocompatibility and hemocompatibility of coronary stents, to deliver effective pharmaceutical agents to inhibit neointimal hyperplasia or to facilitate the growth and proliferation of ECs. In spite of promising early results reported on fully biodegradable polymeric or metallic stents, stent coating is still an indispensible direction for future research. A combination of two technologies would be an option with great promise. However, it remains a challenge for modern engineering and cardiology to design an ideal stent once and for all, with complete elimination of restenosis and thrombosis. However, with the dramatic development and growth of nanotechnology, coating nanomaterials on stent surface or modifying stent surface in nanoscale, offers us a valued and promising research direction in future stent development.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ The introduction of stent technology has dramatically changed the world of interventional cardiology. Nevertheless, in-stent restenosis has become a major clinical issue.
▪ The advent of stent coatings has significantly improved the stent performance owing to their enhanced biocompatibility and capacity for drug delivery.
▪ Although drug-eluting stents have revolutionized the treatment of coronary artery disease, late stent thrombosis has been a great concern since it may result in a very high rate of fatal myocardial infarction.
▪ While inorganic coatings merely improved stent biocompatibility, polymer coatings function as drug vehicles. However, the persistence of nondegradable polymers and the acidic products from biodegradable polymers have been considered to be major factors leading to inflammation and thrombosis.
▪ The advancement of nanotechnology may provide a promising strategy for development of the next generation of drug-eluting stents.
References
- Fischman DL, Leon MB, Baim DS et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 331(8), 496-501 (1994).
- Serruys PW, De Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 331(8), 489-495 (1994).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667-678 (2007).
- Lusis AJ. Atherosclerosis. Nature 407(6801), 233-241 (2000).
- Grüntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet 1(8058), 263 (1978).
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 316(12), 701-706 (1987).
- Kimmel SE, Localio AR, Krone RJ, Laskey WK. The effects of contemporary use of coronary stents on in-hospital mortality. J. Am. Coll. Cardiol. 37(2), 499-504 (2001).
- Kimmel SE, Localio AR, Brensinger C et al. Effects of coronary stents on cardiovascular outcomes in broad-based clinical practice. Arch. Intern. Med. 160(17), 2593-2599 (2000).
- Erbel R, Haude M, Höpp HW et al. Coronary-artery stenting compared with balloon angioplasty for restenosis after initial balloon angioplasty. N. Engl. J. Med. 339(23), 1672-1678 (1998).
- Jenkins N, Prendergast B, Thomas M. Drug eluting coronary stents. BMJ 325(7376), 1315-1316 (2002).
- Salam AM, Al Suwaidi J, Holmes DR. Drug-eluting coronary stents. Curr. Probl. Cardiol. 31(1), 8-119 (2006).
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346(23), 1773-1780 (2002).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315-1323 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymerbased, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221-231 (2004).
- Stone GW, Ellis SG, Cannon L et al. Comparison of a polymer-based paclitaxeleluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA 294(10), 1215-1223 (2005).
- Iijima R, Ikari Y, Miyazawa A, Nakajima H, Hara K. Predictors of restenosis after implantation of 2.5 mm stents in small coronary arteries. Circ. J. 68(3), 236-240 (2004).
- Schofer J, Schlüter M, Gershlick AH et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, Randomized controlled trial (E-SIRIUS). Lancet 362(9390), 1093-1099 (2003).
- Muramatsu T, Tsukahara R, Ho M et al. Clinical outcome of stent implantation in small coronary arteries using different type of coronary stents. J. Invas. Cardiol. 13(9), 634-639 (2001).
- Garza L, Aude YW, Saucedo JF. Can we prevent in-stent restenosis? Curr. Opin. Cardiol. 17(5), 518-525 (2002).
- Lemesle G, Delhaye C, Bonello L, De Labriolle A, Waksman R, Pichard A. Stent thrombosis in 2008: definition, predictors, prognosis and treatment. Arch. Cardiovasc. Dis. 101(11-12), 769-777 (2008).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126-2130 (2005).
- Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701-705 (2004).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193-202 (2006).
- Tanigawa N, Sawada S, Kobayashi M. Reaction of the aortic wall to six metallic stent materials. Acad. Radiol. 2(5), 379-384 (1995).
- Kastrati A, Schomig A, Dirschinger J et al. Increased risk of restenosis after placement of gold-coated stents results of a randomized trial comparing gold-coated with uncoated steel stents in patients with coronary artery disease. Circulation 101(21), 2478-2483 (2000).
- Vom Dahl J, Haager PK, Grube E et al. Effects of gold coating of coronary stents on neointimal proliferation following stent implantation. Am. J. Cardiol. 89(7), 801-805 (2002).
- Monnink SH, Van Boven AJ, Peels HOJ et al. Silicon-carbide coated coronary stents have low platelet and leukocyte adhesion during platelet activation. J. Investig. Med. 47(6), 304-310 (1999).
- Unverdorben M, Sattler K, Degenhardt R et al. Comparison of a silicon carbide-coated stent versus a noncoated stent in humans. J. Interv. Cardiol. 16(4), 325-333 (2003).
- Di Mario C, Grube E, Nisanci Y et al. MOONLIGHT: a controlled registry of an iridium oxide-coated stent with angiographic follow-up. Int. J. Cardiol. 95(2-3), 329-331 (2004).
- Gutensohn K, Beythien C, Bau J et al. In vitro analyses of diamond-like carbon-coated stents. Reduction of metal ion release, platelet activation and thrombogenicity. Thromb. Res. 99(6), 577-585 (2000).
- Antoniucci D, Bartorelli A, Valenti R et al. Clinical and angiographic outcome after coronary arterial stenting with the carbostent. Am. J. Cardiol. 85(7), 821-825 (2000).
- Colombo A, Airoldi F. Passive coating: the dream does not come true. J. Invas. Cardiol. 15(10), 566-567 (2003).
- Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart 85(3), 342 (2001).
- Karjalainen PP, Ylitalo AS, Juhani Airaksinen KE. Real world experience with the TITAN® stent: a 9-month follow-up report from the Titan PORI Registry. EuroIntervention 2(2), 187-191 (2006).
- Mosseri M, Tamari I, Plich M et al. Short- and long-term outcomes of the titanium-NO stent registry. Cardiovasc. Revasc. Med. 6(1), 2-6 (2005).
- Karjalainen PP, Ylitalo A, Airaksinen JKE. Titanium and nitride oxide-coated stents and paclitaxel-eluting stents for coronary revascularization in an unselected population. J. Invas. Cardiol. 18(10), 462-468 (2006).
- Karjalainen PP, Ylitalo A, Niemelä M et al. Two-year follow-up after percutaneous coronary intervention with titanium-nitrideoxide- coated stents versus paclitaxel-eluting stents in acute myocardial infarction. Ann. Med. 41(8), 599-607 (2009).
- Aziz S, Morris JL, Perry RA. Late stent thrombosis associated with coronary aneurysm formation after sirolimus-eluting stent implantation. J. Invas. Cardiol. 19(4), E96-E98 (2007).
- Camenzind E. Treatment of in-stent restenosis: back to the future? N. Engl. J. Med. 355(20), 2149-2151 (2006).
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115(11), 1440-1455 (2007).
- Pfisterer ME. The BASKET-LATE-Study. Basel stent cost-effectiveness trial: late thrombotic events trial. Herz 31(3), 259 (2006).
- Tsuchida K, Piek JJ, Neumann FJ et al. One-year results of a durable polymer everolimus-eluting stent in de novo coronary narrowings (the SPIRIT FIRST Trial). EuroIntervention 1(3), 266-272 (2005).
- Fajadet J, Wijns W, Laarman GJ et al. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation 114(8), 798-806 (2006).
- Stone GW, Midei M, Newman W et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA 299(16), 1903-1913 (2008).
- Kedhi E, Joesoef KS, Mcfadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomized trial. Lancet 375(9710), 201-209 (2010).
- Kandzari DE, Leon MB, Popma JJ et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J. Am. Coll. Cardiol. 48(12), 2440-2447 (2006).
- Slottow TLP, Steinberg DH, Waksman R. Overview of the 2007 Food and Drug Administration Circulatory System Devices Panel meeting on patent foramen ovale closure devices. Circulation 116(6), 677-682 (2007).
- Meredith IT, Ormiston J, Whitbourn R et al. Four-year clinical follow-up after implantation of the endeavor zotarolimuseluting stent: ENDEAVOR I, the first-inhuman study. Am. J. Cardiol. 100(8B), 56M-61M (2007).
- Camenzind E, Wijns W, Mauri L et al. Rationale and design of the Patient Related OuTcomes with Endeavor versus Cypher stenting Trial (PROTECT): randomized controlled trial comparing the incidence of stent thrombosis and clinical events after sirolimus or zotarolimus drug-eluting stent implantation. Am. Heart J. 158(6), 902-909 (2009).
- Finn AV, Nakazawa G, Joner M et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 27(7), 1500-1510 (2007).
- Hollinger JO. Preliminary report on the osteogenic potential of a biodegradable copolymer of polyactide (PLA) and polyglycolide (PGA). J. Biomed. Mater. Res. 17(1), 71-82 (1983).
- Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mat. 5(1), 1-16 (2003).
- Abizaid AC, De Ribamar Costa Junior J, Whitbourn RJ, Chang JC. The CardioMind coronary stent delivery system: stent delivery on a .014†guidewire platform. EuroIntervention 3(1), 154-157 (2007).
- Martin DM, Boyle FJ. Drug-eluting stents for coronary artery disease. Med. Eng. Phys. 33(2), 148-163 (2011).
- Ge J, Qian J, Wang X et al. Effectiveness and safety of the sirolimus-eluting stents coated with bioabsorbable polymer coating in human coronary arteries. Catheter. Cardiovasc. Interv. 69(2), 198-202 (2007).
- Lee CH, Lim J, Low A et al. Sirolimuseluting, bioabsorbable polymer-coated constant stent (Cura™) in acute STelevation myocardial infarction: a clinical and angiographic study (CURAMI Registry). J. Invas. Cardiol. 19(4), 182-185 (2007).
- Aoki J, Ong A, Abizaid A et al. One-year clinical outcome of various doses and pharmacokinetic release formulations of paclitaxel eluted from an erodable polymer - insight in the Paclitaxel In-Stent Controlled Elution Study (PISCES). EuroIntervention 1(2), 165-172 (2005).
- Serruys PW, Sianos G, Abizaid A et al. The effect of variable dose and release kinetics on neointimal hyperplasia using a novel paclitaxel-eluting stent platform: the paclitaxel in-stent controlled elution study (PISCES). J. Am. Coll. Cardiol. 46(2), 253-260 (2005).
- Vranckx P, Serruys P, Gambhir S et al. Biodegradable-polymer-based, paclitaxeleluting Infinnium™ stent: 9-month clinical and angiographic follow-up results from the SIMPLE II prospective multicenter registry study. EuroIntervention 2(3), 310-317 (2006).
- Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21(23), 2335-2346 (2000).
- Ma X, Oyamada S, Wu T et al. In vitro and in vivo degradation of poly(d,l-lactide-coglycolide)/ amorphous calcium phosphate copolymer coated on metal stents. J. Biomed. Mater. Res. A 96A(4), 632-638 (2011).
- Wessely R, Hausleiter J, Michaelis C et al. Inhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple and on-site stent coating. Arterioscler. Thromb. Vasc. Biol. 25(4), 748-753 (2005).
- Hausleiter J, Kastrati A, Wessely R et al. Prevention of restenosis by a novel drugeluting stent system with a dose-adjustable, polymer-free, on-site stent coating. Eur. Heart J. 26(15), 1475-1481 (2005).
- Mehilli J, Kastrati A, Wessely R et al. Randomized trial of a nonpolymer-based rapamycin-eluting stent versus a polymerbased paclitaxel-eluting stent for the reduction of late lumen loss. Circulation 113(2), 273-279 (2006).
- Ruef J, Störger H, Schwarz F, Haase J. Comparison of a polymer-free rapamycineluting stent (Yukon™) with a polymer-based paclitaxel-eluting stent (TAXUS) in real-world coronary artery lesions. Catheter. Cardiovasc. Interv. 71(3), 333-339 (2008).
- Moore P, Barlis P, Spiro J et al. A randomized optical coherence tomography study of coronary stent strut coverage and luminal protrusion with rapamycin-eluting stents. JACC Cardiovasc. Interv. 2(5), 437-444 (2009).
- Abizaid A, Costa JR. New drug-eluting stents: an overview on biodegradable and polymer-free next-generation stent systems. Circ. Cardiovasc. Interv. 3(4), 384-393 (2010).
- Rajtar A, Kaluza GL, Yang Q et al. Hydroxyapatite-coated cardiovascular stents. EuroIntervention 2(1), 113-115 (2006).
- Costa Jr JR, Abizaid A, Costa R et al. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc. Interv. 2(5), 422-427 (2009).
- Wieneke H, Dirsch O, Sawitowski T et al. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheter. Cardiovasc. Interv. 60(3), 399-407 (2003).
- Kollum M, Farb A, Schreiber R et al. Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimus-eluting stents in a porcine model of restenosis. Catheter. Cardiovasc. Interv. 64(1), 85-90 (2005).
- Tsujino I, Ako J, Honda Y, Fitzgerald PJ. Drug delivery via nano, micro and macroporous coronary stent surfaces. Expert Opin Drug Deliv. 4(3), 287-295 (2007).
- Nakano K, Egashira K, Masuda S et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology: efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC Cardiovasc. Interv. 2(4), 277-283 (2009).
- Polyak B, Fishbein I, Chorny M et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc. Natl Acad. Sci. USA 105(2), 698-703 (2008).
- Palmaz JC, Benson A, Sprague EA. Influence of surface topography on endothelialization of intravascular metallic material. J. Vasc. Interv. Radiol. 10(4), 439-444 (1999).
- Khang D, Lu J, Yao C, Haberstroh KM, Webster TJ. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 29(8), 970-983 (2008).
- Ma X, Oyamada S, Gao F et al. Paclitaxel/ sirolimus combination coated drug-eluting stent: in vitro and in vivo drug release studies. J. Pharm. Biomed. Anal. 54(4), 807-811 (2011).
- Byrne RA, Mehilli J, Iijima R et al. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial versus polymer-based drug-eluting stents. Eur. Heart J. 30(8), 923-931 (2009).
- Byrne RA, Kastrati A, Tiroch K et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stents versus polymer-based cypher and endeavor drug-eluting stents. J. Am. Coll. Cardiol. 55(23), 2536-2543 (2010).
- Hirsh J. Heparin. N. Engl. J. Med. 324(22), 1565-1574 (1991).
- Clowes AW, Karnovsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature 265(5595), 625-626 (1977).
- Guyton JR, Rosenberg RD, Clowes AW, Karnovsky MJ. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ. Res. 46(5), 625-634 (1980).
- Serruys PW, Van Hout B, Bonnier H et al. Randomized comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet 352(9129), 673-681 (1998).
- Mehran R, Aymong ED, Ashby DT et al. Safety of an aspirin-alone regimen after intracoronary stenting with a heparincoated stent: final results of the HOPE (hepacoat and an antithrombotic regimen of aspirin alone) study. Circulation 108(9), 1078-1083 (2003).
- Wohrle J, Al-Khayer E, Grotzinger U et al. Comparison of the heparin coated vs the uncoated Jostent®: no influence on restenosis or clinical outcome. Eur. Heart J. 22(19), 1808-1816 (2001).
- Haude M, Konorza TFM, Kalnins U et al. Heparin-coated stent placement for the treatment of stenoses in small coronary arteries of symptomatic patients. Circulation 107(9), 1265-1270 (2003).
- Verheye S, Markou CP, Salame MY et al. Reduced thrombus formation by hyaluronic acid coating of endovascular devices. Arterioscler. Thromb. Vasc. Biol. 20(4), 1168-1172 (2000).
- Heublein B, Evagorou EG, Rohde R et al. Polymerized degradable hyaluronan: a platform for stent coating with inherent inhibitory effects on neointimal formation in a porcine coronary model. Int. J. Artif. Organs 25(12), 1166-1173 (2002).
- Sondrup C, Liu Y, Shu XZ, Prestwich GD, Smith ME. Cross-linked hyaluronan-coated stents in the prevention of airway stenosis. Otolaryngol. Head Neck Surg. 135(1), 28-35 (2006).
- Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 112(2-3), 267-276 (2004).
- Mckenna CJ, Camrud AR, Sangiorgi G et al. Fibrin-film stenting in a porcine coronary injury model: efficacy and safety compared with uncoated stents. J. Am. Coll. Cardiol. 31(6), 1434-1438 (1998).
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J. Pathol. 190(3), 300-309 (2000).
- Kipshidze N, Dangas G, Tsapenko M et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J. Am. Coll. Cardiol. 44(4), 733-739 (2004).
- Consigny PM. Endothelial cell seeding on prosthetic surfaces. J. Long Term Eff. Med. Implants 10(1-2), 79-95 (2000).
- Van Der Giessen WJ, Serruys PW, Visser WJ, Verdouw PD, Van Schalkwijk WP, Jongkind JF. Endothelialization of intravascular stents. J. Interv. Cardiol. 1(2), 109-120 (1988).
- Thompson MM, Budd JS, Eady SL, Underwood MJ, James RF, Bell PR. The effect of transluminal endothelial seeding on myointimal hyperplasia following angioplasty. Eur. J. Vasc. Surg. 8(4), 423-434 (1994).
- Kipshidze N, Ferguson JJ, Keelan MH et al. Endoluminal reconstruction of the arterial wall with endothelial cell/glue matrix reduces restenosis in an atherosclerotic rabbit. J. Am. Coll. Cardiol. 36(4), 1396-1403 (2000).
- Conte MS, Vanmeter GA, Akst LM, Clemons T, Kashgarian M, Bender JR. Endothelial cell seeding influences lesion development following arterial injury in the cholesterol-fed rabbit. Cardiovasc. Res. 53(2), 502-511 (2002).
- Shirota T, Yasui H, Shimokawa H, Matsuda T. Fabrication of endothelial progenitor cell (EPC)-seeded intravascular stent devices and in vitro endothelialization on hybrid vascular tissue. Biomaterials 24(13), 2295-2302 (2003).
- Inoue T, Sata M, Hikichi Y et al. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: impact on restenosis. Circulation 115(5), 553-561 (2007).
- Granada JF, Inami S, Aboodi MS et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ. Cardiovasc. Interv. 3(3), 257-266 (2010).
▪ Website
201 Carbostent & Implantable Devices. www.cidvascular.com