Research Article - Interventional Cardiology (2022) Volume 14, Issue 6
Dual versus tapered reference benchmarks for assessing stent expansion using optical coherence tomography
- Corresponding Author:
- Matt Sibbald
Department of Interventional Cardiology, McMaster University, 1280 Main St West, Hamilton, L8L2X2, Canada,
E-mail: sibbald@mcmaster.ca
Received date: 10-Nov-2022, Manuscript No. FMIC-22-79507; Editor assigned: 14-Nov-2022, PreQC No. FMIC-22-79507 (PQ); Reviewed date: 28-Nov-2022, QC No. FMIC-22-79507;Revised date: 05-Dec-2022, Manuscript No. FMIC-22-79507 (R);Published date: 15-Dec-2022, DOI: 10.37532/1755-5310.2022.14(6). 591
Abstract
Background: Intravascular imaging studies frequently highlight stent under expansion as a major predictor of repeat interventions. However, no consistent method to identify stent expansion exists. Two types of benchmarks are routinely available, dual and tapered reference, with unclear differences between them. We then sought to compare stent expansion using two different methods of reference benchmarking on Optical Coherence Tomography (OCT) imaging, one involving both proximal and distal stent edges (dual reference) and the other a computed estimate of reference size for each frame (tapered reference).
Methods: We retrospectively analyzed stent expansion in 249 OCTs of patients undergoing percutaneous intervention for de novo chronic coronary disease or acute coronary syndromes. We compared stent expansion using dual versus tapered benchmarks.
Results: Suboptimal expansion (<90%) occurred in 71% of cases when using dual reference and 60% when using tapered reference (χ2=143, p<0.0001). Agreement in identifying suboptimal expansion between dual and tapered modes was 88% (k=0.74, p<0.0001). Dual reference identified suboptimal expansion in 28 cases (11%) where expansion was optimal by tapered mode; whereas tapered mode identified suboptimal expansion in only 2 cases (<1%) where expansion was optimal by the dual reference approach.
Conclusion: In this real world series, identification of suboptimal expansion by OCT imaging was frequent, with high agreement between dual and tapered benchmarks. Differences of sufficient magnitude to change post dilation strategy were present in 1 out of every 10 cases.
Keywords
Coronary stenosis • Percutaneous Coronary Intervention (PCI) • Optical Coherence Tomography (OCT) • Stent expansion
Abbreviations
OCT: Optical Coherence Tomography; MSA: Minimum Stent Area; LSA: Least Expanded Stent Area
Introduction
While long term outcomes after coronary stenting have improved over time, stent related recurrent events are still problematic [1]. Intravascular imaging studies have consistently identified stent underexpansion as a major predictor of repeat intervention, with improvements in outcomes linked to better expansion achieved at the index procedure [2-5].
Despite the importance of stent expansion to clinical outcomes, there is no consistent methodology used to define adequate expansion, with one trial using the distal reference area [2] and others using both distal and proximal reference areas [5-7]. Several recent Optical Coherence Tomography (OCT) based trials have defined optimal stent expansion of the proximal half of the stent as at least 90% of the proximal reference area, and the distal half of the stent as at least 90% of the distal reference area [6-8]. However, these approaches incompletely address vessel tapering and bifurcations. Newer volumetric methods of assessing expansion, which use computational methods to create a tapered benchmark along the entire length of the stent, may address these limitations [9]. Since imaging evaluation of stent expansion is central to the optimization of PCI procedures, it is important to understand if the use of a tapered benchmark changes the detection of stent under expansion and potential patient management after imaging.
The goal of this study was to compare the frequency of optimal stent expansion and the resultant differences in anticipated need for further post-dilation using dual reference versus tapered reference benchmarks. The secondary objective was to identify any systematic differences in the location of post-dilation recommendations between dual reference and tapered reference benchmarks.
Materials and Methods
We conducted a retrospective cohort study in a large academic centre performing an average of 2800 Percutaneous Coronary Intervention (PCI) annually with 10 interventional cardiologists from 2018 through 2021.
Patient population
Patients presenting with de novo chronic coronary disease or acute coronary syndromes who underwent OCT guided PCI were included. PCI was performed as per the individual operator’s standard of care, and all cases had OCT performed post stent implantation to assess stent expansion. Use and sizing of NC post dilation balloons after stent expansion and prior to OCT imaging was at the discretion of the operator and usually guided by the pre-stent OCT imaging. Single and multivessel PCI were included.
Cases were excluded if image quality precluded expansion assessment because of blood contamination or failure to image the entire stent length.
OCT images were acquired after intracoronary nitroglycerin was administered using a frequency domain optical fiber system (Abbott, Santa Clara). OCT analysis was conducted offline using Aptivue Optis Software (Abbott, Santa Clara) by two readers independently. Disagreements were resolved through consensus. The readers were blinded to clinical, angiographic, and procedural data. The imaging core laboratory used the following definitions:
Proximal and distal references: Proximal and distal references areas were defined as the lumen area of the first frame outside of the stented segment. Reference areas were detected using automated lumen detection and confirmed manually.
Dual reference proximal and distal Minimum Stent Areas (MSA): Stented areas were bisected into equal length proximal and distal segments, and the MSA was identified within the proximal and distal segment using the software’s automated expansion module. When a stent spanned one or more bifurcations with side branch origins greater than 2.5 mm, the division between proximal and distal stent segments was manually readjusted to be at the site of the largest bifurcation. MSAs were manually confirmed.
Dual reference proximal and distal minimum stent expansion: Proximal minimum stent expansion was calculated as the proximal MSA/proximal reference area, and distal minimum stent expansion as distal MSA/distal reference area. Expansion was represented as a percentage.
Tapered expansion MSA: Software identified MSA within the entire stented segment, confirmed manually.
Tapered minimum stent expansion: Tapered minimum stent expansion was assessed as defined previously, using the software’s automated expansion module. In brief, this involves a frame-by-frame comparison of actual versus ideal vessel profile, based on an interpolated cylindrical taper from proximal to distal reference area benchmarks, adjusted for significant side branches [9]. This methodology identifies the Least expanded Stent Area (LSA), which is not always the MSA. If the interpolated cylindrical reference at the site of MSA is similarly small, the MSA may have greater expansion than a more proximal LSA location where the reference is disproportionately larger.
Calcium at MSA and LSA: Calcium was defined as sharply-demarcated plaque with low backscatter and attenuation [10]. The number of quadrants of calcium was noted at the MSA and LSA cross-sections. Where plaque characteristics were ambiguous, adjacent frames and pre-stent OCT runs were used to differentiate calcium from other plaque morphology.
Expansion was categorized into optimal and suboptimal, based on achievement of >90% of the reference [6]. Failure to meet this benchmark would result in a post dilation recommendation in recent trial protocols [6,7].
Analysis
Categorical variables were summarized with numbers and percentages and continuous variables as averages with standard deviations. Kappa statistics were used to compare categorical expansion between dual reference and tapered approaches. Statistical analysis was performed with SPSS (IBM, Redmond)
Power calculation
With a minimum relevant 10% difference in tapered and dual reference expansion, and a 50% rate of post-dilation recommendation with an anticipated agreement of 0.6 and expected precision of 0.1, a sample size of 246 runs will be required to allow an alpha error rate of 0.05.
Ethics: Approved by the Hamilton Integrated Research Ethics Board, Protocol #13502.
Results
Two hundred and forty nine runs were analyzed from 221 patients. Patient demographics are found in Table 1, with 168 (76.0%) were male, mean age 62.8 years. Over half of all patients (51.2%) had a prior myocardial infarction, and 56.9% have had a prior percutaneous intervention.
| Characteristics | |
|---|---|
| Age (y), mean ± SD | 62.9 ± 11.7 |
| Sex (male), n (%) | 169(75.8) |
| CCS, n (%) | |
| 0 | 18(8.1) |
| I | 15(6.7) |
| II | 22(9.9) |
| III | 23(10.3) |
| IV | 37(16.6) |
| NYHA, n (%) | |
| I | 74(33.2) |
| II | 10(4.5) |
| III | 5(2.2) |
| IV | 2(0.9) |
| N/A | 34(15.2) |
| History | |
| Prior myocardial infarction, n(%) | 115(51.6) |
| Prior percutaneous intervention, n (%) | 130(58.3) |
| Prior bypass surgery, n (%) | 7(3.1) |
| Prior cerebrovascular disease, n (%) | 11(4.9) |
| Prior peripheral vascular disease, n (%) | 10(4.5) |
| Creatinine (μmol/L), mean ± SD | 89.8±65.2 |
| Cardiac risk factors | |
| Hypertension, n (%) | 139 (62.3) |
| Dyslipidemia, n (%) | 142 (63.7) |
| Current Smoker, n (%) | 59 (26.4) |
| Former Smoker, n (%) | 55 (24.7) |
| Diabetes, n (%) | 72 (32.3) |
| Indication | |
| Chest Pain, n (%) | 119 (53.4) |
| NSTEMI, n (%) | 54 (24.2) |
| STEMI, n (%) | 33 (14.8) |
Table 1: Cohort demographics with prior myocardial infarction and prior percutaneous intervention.
Procedural characteristics are shown in Table 2. The majority of patients (70.4%) had one vessel stented, most commonly the LAD, with average number of stents per vessel of 1.29 with average stent diameter 3.10 mm and the average stent length was 24.0 mm. Post dilation was performed prior to OCT imaging in 41% of cases with non-compliant balloons that was less than or equal to the stent diameter in 16%, 0.25 mm larger than the stent diameter in 7% and greater than or equal to 0.5 mm larger than the stent diameter in 18%.
| Characteristics | |
|---|---|
| Radial Access, n (%) | 176(79.6) |
| Number of vessels stented per patient, n (%) | |
| 1 | 157(70.4) |
| 2 | 59(26.5) |
| 3 | 6(2.7) |
| Vessels stented, n (%) | |
| LM | 2(0.4) |
| Ramus | 2(0.8) |
| LAD | 162(66.1) |
| LCX | 29(11.7) |
| RCA | 50(20.5) |
| Bypass Graft | 1(0.4) |
| Lesion severity %, n (%) | |
| <50% | 1(0.4) |
| 50-69% | 12(5.0) |
| 70-89% | 112(46.9) |
| 90%+ | 114(47.7) |
| Predilation balloon size n (%) | |
| ≤ 2.0 mm | 71(31.8%) |
| 2-3.0 mm | 33(14.8%) |
| ≥ 3.0 mm | 37(16.6%) |
| Number of stents per patient | 1.33 ± 0.6 |
| Stent diameter | |
| 2.25 | 16(4.9) |
| 2.5 | 35(10.9) |
| 2.75 | 58(18.0) |
| 3 | 85(26.4) |
| 3.25 | 1(0.3) |
| 3.5 | 86(26.7) |
| ≥ 4.00 | 41(12.4) |
| Post-Dilation Pre-OCT Balloon Size n (%) | |
| ≤ 2.5 mm | 11(3.6%) |
| 2.5-3.5 mm | 46(15.0%) |
| ≥ 3.5 mm | 67(22%) |
| Post-Dilation Pre-OCT Balloon Size relative to stent n (%) | |
| Same size or smaller than the stent diameter | 49(16.0%) |
| 0.25 mm larger than the stent | 20(6.5%) |
| 0.50 mm larger than the stent | 33(10.8%) |
| >0.5 mm larger than the stent | 22(7.2%) |
Table 2: Procedural characteristics with average number of stents.
OCT analysis revealed an average length of stent of 31 mm ± 12 mm with an average of 1.47 ± 1.40 bifurcations are shown in Table 3. Proximal and distal reference vessel areas were 9.13 ± 3.91 and 6.68 ± 3.25 respectively. Using dual reference, the average minimum stent expansion was 78% overall, with minimum stent expansion of 82% in the proximal segment and 96% in the distal segment. In contrast, mean minimum expansion by tapered mode was 84%.
| N | Mean | Std. Deviation | |
|---|---|---|---|
| Stented segment characteristics | |||
| Length | 249 | 31 | 12 |
| Proximal reference area | 249 | 9.13 | 3.91 |
| Distal reference area | 249 | 6.68 | 3.25 |
| Proximal reference diameter | 249 | 3.39 | 0.94 |
| Distal reference diameter | 249 | 2.84 | 0.65 |
| # of bifurcations | 248 | 1 | 1 |
| Minimum Stent Areas (MSA) | |||
| Dual reference | |||
| Proximal MSA | 240 | 7.01 | 2.58 |
| Distal MSA | 240 | 6.04 | 2.41 |
| Tapered reference MSA | 240 | 5.88 | 2.36 |
| Stent segment expansion | |||
| Dual reference minimum stent expansion (mean minimum expansion) | 239 | 78 | 18 |
| Proximal Segment | 238 | 82 | 20 |
| Distal Segment | 240 | 96 | 16 |
| Tapered reference minimum stent expansion (mean minimum expansion at least expanded stent area, LSA) | 240 | 84 | 16 |
| Quadrants of calcium at MSA | |||
| Dual reference | |||
| Calcium quadrants at proximal MSA | 249 | 1.08 | 1.02 |
| Calcium quadrants at distal MSA | 249 | 0.8 | 0.94 |
| calcium quadrants at tapered LSA | 249 | 0.92 | 1.01 |
| Percentage of stent segments with suboptimal expansion (<90%) | |||
| Dual reference | 249 | 0.71 | 0.46 |
| Proximal | 248 | 0.61 | 0.49 |
| Distal | 239 | 0.31 | 0.46 |
| Tapered reference | 249 | 0.6 | 0.49 |
Table 3: OCT analysis of stented segments.
Suboptimal expansion (<90%) occurred in 71% of cases when using dual reference (61% of cases had suboptimal expansion in the proximal segment, and 31% had suboptimal expansion in the distal segment). In contrast, suboptimal expansion occurred in 60% when using tapered reference (compared with dual reference, χ2=143, p<0.0001). Agreement in identifying suboptimal expansion between dual and tapered modes was 88% (k=0.74, p<0.0001). Dual reference identified suboptimal expansion in 28 cases (11%) where expansion was optimal by tapered mode; whereas tapered mode identified suboptimal expansion in only 2 cases (<1%) where expansion was optimal by the dual reference approach.
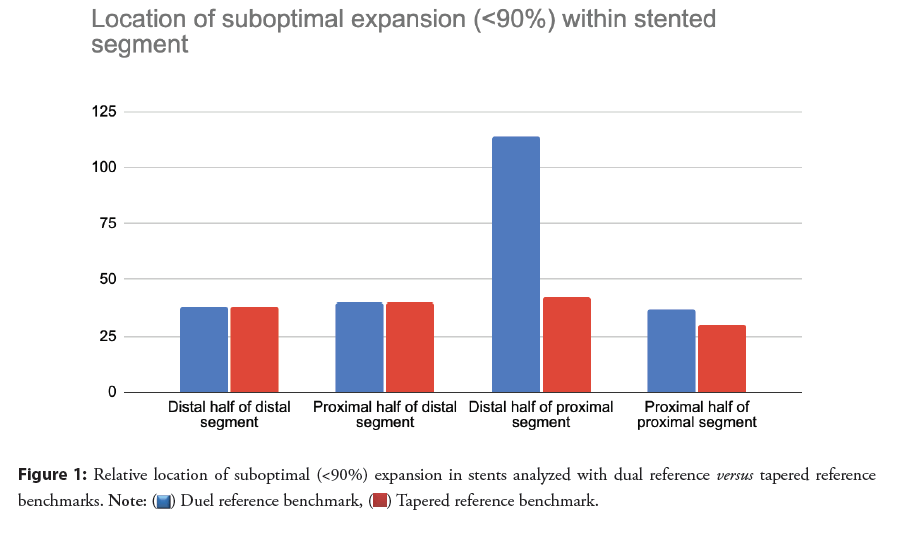
The location of suboptimal expansion within the stented region is shown in Figure 1. In dual reference mode, under expansion <90% was clustered at the distal ends of the proximal half and through the distal half of the stented area. In contrast, much less under expansion was identified with tapered mode at the end of the proximal half of the stented area. Two example cases where discrepancies existed are described in Figures 2A and 2B.
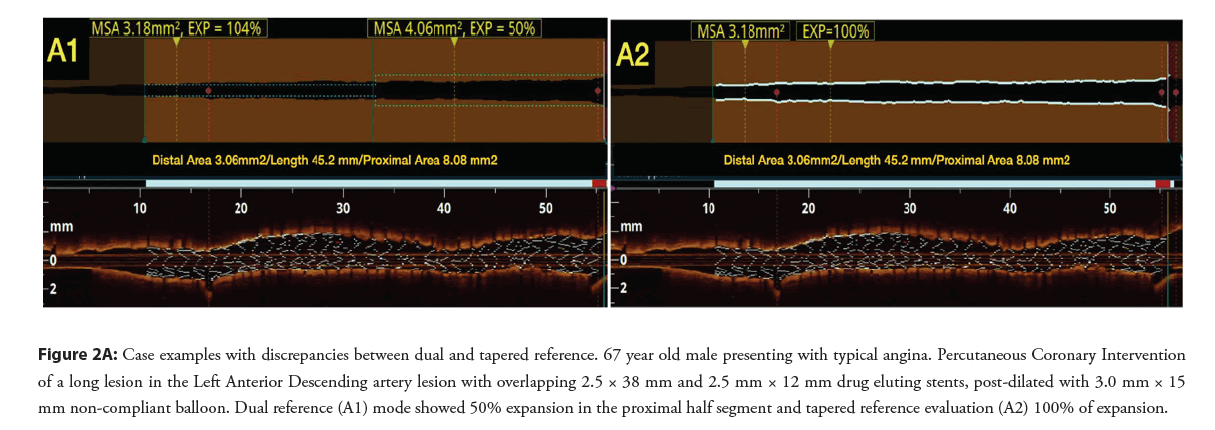
Figure 2A: Case examples with discrepancies between dual and tapered reference. 67 year old male presenting with typical angina. Percutaneous Coronary Intervention of a long lesion in the Left Anterior Descending artery lesion with overlapping 2.5 × 38 mm and 2.5 mm × 12 mm drug eluting stents, post-dilated with 3.0 mm × 15 mm non-compliant balloon. Dual reference (A1) mode showed 50% expansion in the proximal half segment and tapered reference evaluation (A2) 100% of expansion.
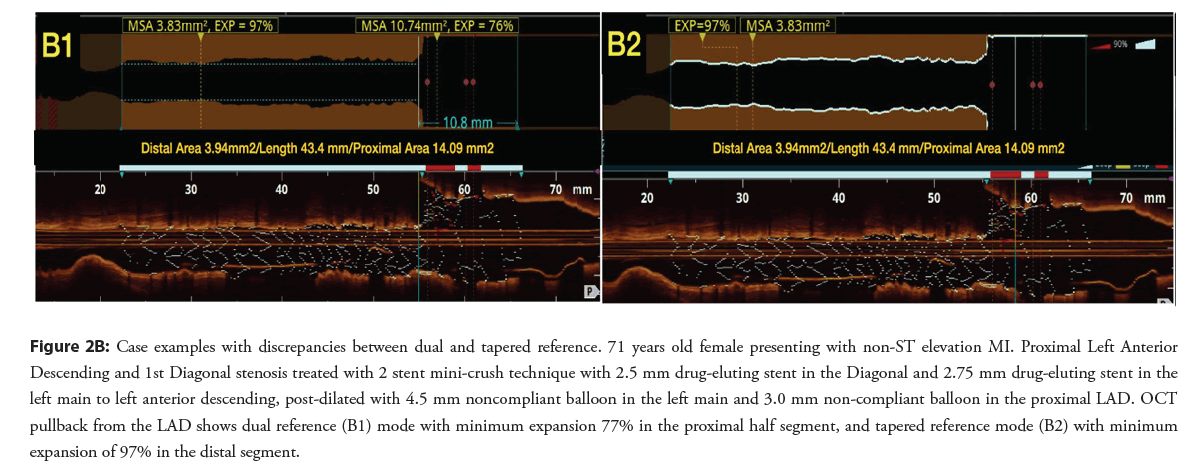
Figure 2B: Case examples with discrepancies between dual and tapered reference. 71 years old female presenting with non-ST elevation MI. Proximal Left Anterior Descending and 1st Diagonal stenosis treated with 2 stent mini-crush technique with 2.5 mm drug-eluting stent in the Diagonal and 2.75 mm drug-eluting stent in the left main to left anterior descending, post-dilated with 4.5 mm noncompliant balloon in the left main and 3.0 mm non-compliant balloon in the proximal LAD. OCT pullback from the LAD shows dual reference (B1) mode with minimum expansion 77% in the proximal half segment, and tapered reference mode (B2) with minimum expansion of 97% in the distal segment.
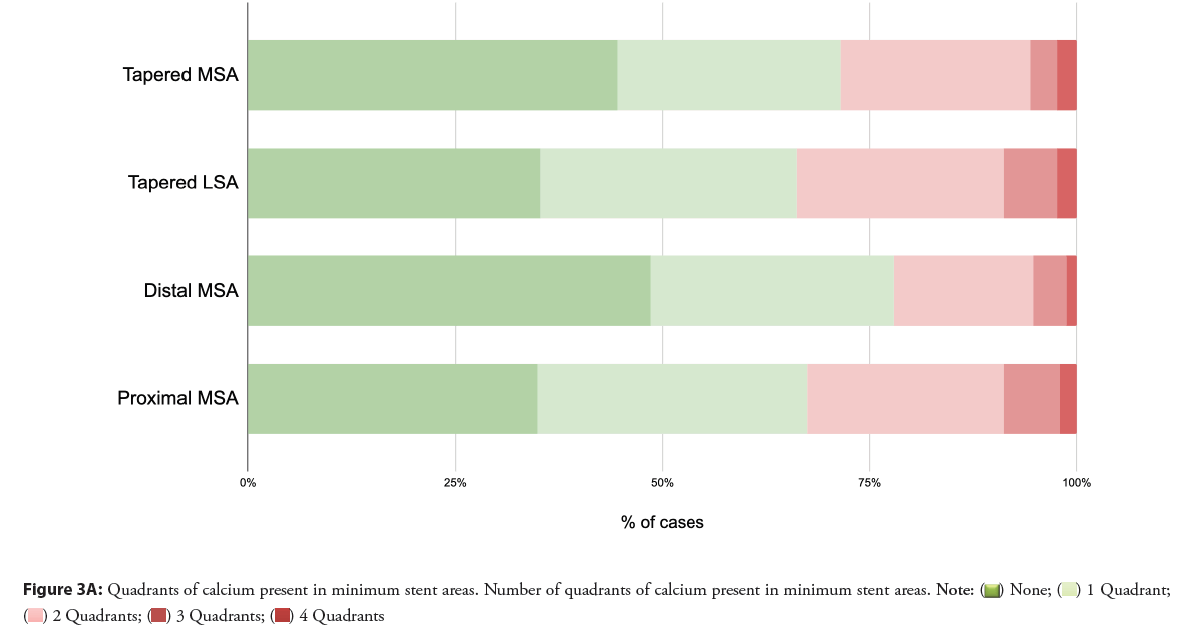
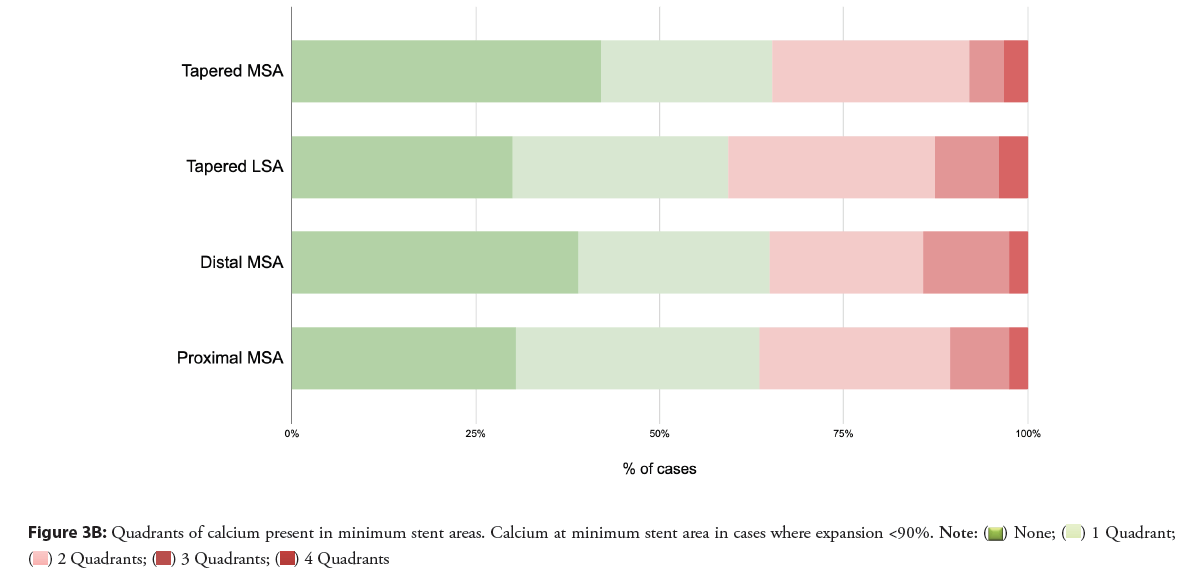
Calcium was common at minimum stent areas in both dual and tapered analyses, and spanned two or more quadrants in 22%-34% of cases are shown in Figures 3A and 3B. Where the MSA or LSA was <90%, the percentage of cases with two or more quadrants containing calcium was somewhat higher, 35%-41%.
Discussion
In this real world series, identification of suboptimal expansion by imaging assessment (<90%) was frequent, consistent with prior reports [4,11,12]. Despite best practices in a high volume center with high volume operators, thin strut third generation stent platforms, and selective post-dilation, stent under expansion remains a common finding.
This study focused on comparing dual reference and tapered modes of assessing stent expansion. In general, the agreement between these modes was high. Differences of sufficient magnitude to change post dilation strategy only were present in 1 out of every 10 cases. This is less pronounced than findings of prior work that compared a volumetric benchmark with an average of the proximal and distal reference where nearly 60% of cases differed [13]. The use of an averaged benchmark compared to a dual reference mode may account for some of these differences as the dual reference mode does not take into account vessel taper.
Of note, the differences between dual reference and tapered mode are almost entirely within the proximal half of the stented region, and clustered just proximal to the stent midline. When the under expansion benchmark is 90% of the proximal reference, a common practice is to use an External Elastic Lamina (EEL) sized non-compliant balloon which risks oversizing particularly in the setting of tapered geometry of the vessel. A strategy that uses EEL sized non-compliant balloons to aggressively dilate the distal end of the proximal half of the stent based on a dual reference paradigm [7], could increase the risk of EEL rupture and perforation. One could, therefore, hypothesize that tapered mode may have a safety advantage over dual reference expansion.
While calcium was common at areas of under expansion in this study, 35%-41% of cases of under expansion <90% had multiple quadrants of calcium, and only a small percentage of cases had 3 or more quadrants of calcium. The degree of calcification in the vast majority of these cases would not meet the threshold to use devices deliberately designed to modify calcium [14]. Alternate mechanisms accounting for under expansion may include resistance by fibrous tissue itself or resistance of the outer vessel architecture in the setting of large plaque volume and recoil. Routine use of more aggressive lesion preparation [15], longer stent and post-dilation inflation times [16], higher pressure post dilation [17] and use of lithotripsy post stenting [18] may improve expansion.
Limitations several limitations of this work are worth noting. First, this is a real world case series of OCT directed stent implantation. Case selection and technique is still left to the discretion of the operator. The preferential use of OCT in longer lesions and bifurcation lesions are most likely to highlight differences in dual reference and tapered modes as both more vessels taper would be expected in longer lesions and in lesions involving large bifurcations. Second, proprietary software was used to determine both dual reference and tapered mode benchmarks.
Conclusion
The dual reference mode only allowed two segments of the artery regardless of the number of bifurcations. A more nuanced approach might divide the artery into segments for each bifurcation, likely resulting in less discrepancy with tapered mode. Identifying under expansion using tapered reference mode leads to less identified under expansion compared with a dual reference approach, particularly at the distal end of the proximal half of the stent.
Acknowledgements
None
Funding
None
Declaration of Competing Interest
Dr. Sibbald, Pinilla, Dutra and Sheth are consultants for Abbott Vascular Inc. None of the other authors have conflicts of interest.
References
- Madhavan MV, Kirtane AJ, Redfors B, et al. Stent-related adverse events >1 year after percutaneous coronary intervention. J Am Coll Cardiol. 75(6): 90-604 (2020).
[Cross ref] [Google scholar] [PubMed]
- Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents. Circ Cardiovasc Interv. 11(11): e006243 (2018).
[Cross ref] [Google scholar] [PubMed]
- Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound–guided vs angiography-guided everolimus-eluting stent implantation: The ivus-xpl randomized clinical trial. JAMA. 314(20): 2155-2163 (2015).
[Cross ref] [Google scholar] [PubMed]
- Prati F, Romagnoli E, Burzotta F, et al. Clinical impact of OCT findings during PCI: The CLI-OPCI II study. JACC Cardiovasc Imaging. 8(11): 1297-305 (2015).
[Cross ref] [Google scholar] [PubMed]
- Tsunenari S, Shiro U, Seung-Jung P, et al. Incidence and Clinical Significance of Poststent Optical Coherence Tomography Findings. Circulation. 132(11): 1020-1029 (2015).
[Cross ref] [Google scholar] [PubMed]
- Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): A randomised controlled trial. Lancet. 388: 2618-2628 (2016).
[Cross ref] [Google scholar] [PubMed]
- Ali Z, Landmesser U, Galougahi KK, et al. Optical coherence tomography-guided coronary stent implantation compared to angiography: A multicentre randomised trial in PCI-design and rationale of ILUMIEN IV: OPTIMAL PCI. EuroIntervention. 16(13):1092-1099 (2021).
[Cross ref] [Google scholar] [PubMed]
- Meneveau N, Souteyrand G, Motreff P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-st-elevation acute coronary syndrome: Results of the multicenter, randomized doctors study (does optical coherence tomography optimize results of stenting). Circulation. 134:906-917 (2016).
[Cross ref] [Google scholar] [PubMed]
- Stent-related adverse events >1 year after percutaneous coronary intervention
- Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. 59(12):1058-1072 (2012).
[Cross ref] [Google scholar] [PubMed]
- Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: Insights from the national PESTO French registry. Eur Heart J. 37(15): 1208-1216 (2016).
[Cross ref] [Google scholar] [PubMed]
- Prati F, Kodama T, Romagnoli E, et al. Suboptimal stent deployment is associated with subacute stent thrombosis: Optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators: the CLI-THRO study. Am Heart J. 169(2): 249-256 (2015).
[Cross ref] [Google scholar] [PubMed]
- Dallan LAP, Pereira GTR, Zimin V, et al. Comparison of stent expansion using a volumetric versus the conventional method through optical coherence tomography in an all-comers population. Cardiovasc Revasc Med. 24: 48-54 (2021).
[Cross ref] [Google scholar] [PubMed]
- Fujino A, Mintz G, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 13(18):e2182-e2189 (2018).
[Cross ref] [Google scholar] [PubMed]
- Rheude T, Rai H, Richardt G, et al. Super high-pressure balloon versus scoring balloon to prepare severely calcified coronary lesions: The isar-calc randomized trial. EuroIntervention. 17(6): 481-488 (2020).
[Cross ref] [Google scholar] [PubMed]
- Saad M, Bavineni M, Uretsky BF, et al. Improved stent expansion with prolonged compared with short balloon inflation: A meta-analysis. Catheter Cardiovasc Interv. 92: 873-880 (2018).
[Cross ref] [Google scholar] [PubMed]
- Secco GG, Buettner A, Parisi R, et al. Clinical experience with very high-pressure dilatation for resistant coronary lesions. Cardiovasc Revasc Med. 20(12): 1083-1087 (2019).
[Cross ref] [Google scholar] [PubMed]
- Yeoh J, Cottens D, Cosgrove C, et al. Management of stent underexpansion using intravascular lithotripsy-Defining the utility of a novel device. Catheter Cardiovasc Interv. 97(1): 22-29 (2021).
[Cross ref] [Google scholar] [PubMed]