Research Article - Diabetes Management (2019) Volume 9, Issue 3
Dulaglutide using once-2 weeks for dialysis type 2 diabetes patients in Japan
- Corresponding Author:
- Mari Koga
Diabetic Center
Fukuoka-Wajiro Hospital
Japan
E-mail:happymarikoga@yahoo.co.jp
Abstract
Object: Dulaglitide is one of glucagon like peptide-1 receptor agonist. It is ususally used once- 1 week. The efficacy of drug is easy to remain in the patients with impaired renal function. We evaluate the efficiency of once-2 weeks dulaglitide after switching from once-1 week injection in 4 out patients of type 2 diabetes undergoing hemodialysis in Japan. Methods: After changing dulaglutide treatment from once-1 week to once-2 weeks in dialysis type 2 diabetes patients, the effect of glycemic control was evaluated by Glico albumin (GA). And mean blood glucose (mean BS), 24 h Area under the blood concentration-time curve (AUC), AUC>180 and the mean amplitude of glycemic excursions (MAGE) were evaluated by using continuous glucose monitoring (CGM). Results: GA was decrease from 20.3 ± 0.47% to 19.3 ± 0.47% in case 1, from 24.5 ± 0.79% to 22.2 ± 2.18% in case 3, and from 20.3 ± 0.47% to 19.3 ± 0.47% in case 4 after changing of dulaglutide injection from once-1 week to once-2 weeks. CGM was examined in case 4 and case 5. Mean BS, 24 h AUC and AUC>180 were improved in case 4. MAGE and AUC>180 were improved by dulaglutide injection once-1 week to once-2 weeks in case 5. Conclusion: Once-2 weeks dulaglutide treatment is useful for glycemic control in dialysis type2 diabetic patients.
Keywords
■diabetes ■glycemic ■insulin
Introduction
Recently, glucagon-like peptide 1 receptor agonist (GLP-1 receptor agonist) is available, including exenatide, liraglutide, lixisenatide and dulaglutide [1] for type 2 diabetes treatment in Japan. These drugs have shown not only to improve glucose control but improve insulin secretion, decrease glucagon release and suppress appetite by decreasing of the rate of gastric emptying or by the regulating of hypothalamus signal [2]. It also promotes weight loss in type 2 obese diabetic patients. GLP-1 receptor agonist has been suggested the effective therapy for blood glucose control of non fatty liver disease [3] and steroid therapy [4], which exist insulin resistance (IR). Biguanide and pioglitazone, which are useful treatment for insulin resistance, are difficult to use for end renal stage (ESRD) of type 2 diabetes patients even who are clear to be sufficient insulin secretion and insulin resistance.
It is no way to choose dipeptidyl peptidase 4 inhibitors (DDP-4), α-glucosidase inhibiter (Recently, glucagon-like peptide 1 receptor agonist (GLP-1 receptor agonist) is available, including exenatide, liraglutide, lixisenatide and dulaglutide [1] for type 2 diabetes treatment in Japan. These drugs have shown not only to improve glucose control but improve insulin secretion, decrease glucagon release and suppress appetite by decreasing of the rate of gastric emptying or by the regulating of hypothalamus signal [2]. It also promotes weight loss in type 2 obese diabetic patients. GLP-1 receptor agonist has been suggested the effective therapy for blood glucose control of non fatty liver disease [3] and steroid therapy [4], which exist insulin resistance (IR). Biguanide and pioglitazone, which are useful treatment for insulin resistance, are difficult to use for end renal stage (ESRD) of type 2 diabetes patients even who are clear to be sufficient insulin secretion and insulin resistance. It is no way to choose dipeptidyl peptidase 4 inhibitors (DDP-4), α-glucosidase inhibiter (α-GI), glinide and insulin therapy for maintaining good blood glucose control in such cases. The advent of GLP-1 receptor agonist is given good blood glucose control and weight loss in sufficient secretion of insulin in dialysis type 2 diabetes patients. We have experienced good glycemic control after changing from insulin to GLP-1 receptor agonist. Dulaglutide is GLP-1 receptor agonist consisting of GLP- 1(7-37) covalently linked to an Fc fragment of human IgG4 [5]. It is used for the treatment of type 2 diabetes once weekly [6,7]. The effective blood concentration of dulaglutide is continued less than 10 days at 1 time injection, and the concentration is increased after 5 times injection [8]. We tried to use dulaglutide at once-2 weeks in 4 diabetes outpatients of ESRD treated with hemodialysis.
Material and Method
We studied 4 outpatients of type2 DM in ESRD treat with dialysis at Fukuoka- Wajiro-hospital in Japan (TABLE 1). They were undergoing of dialysis 3 times in a week. Sex is 2 males and 2 females, age 52~79 y, BMI 18.5~32.1 kg/m2, and the duration of Diabetes mellitus (DM) is 7~29 years. The duration of dialysis was 5~11 y. 3 of 4 patients had proliferative diabetic retinopathy and multiple symmetrical & autonomic diabetic neuropathy. 1 of 4 patients did not have retinopathy and neuropathy, and was medicated by steroid therapy for glomerular nephritis. They were cared by insulin therapy before using GLP-1 receptor agonist, and had sufficient insulin secretion. Liraglutide was started from 0.3 mg/day and was gradually increased 0.6 mg/day to 0.9 mg/day with using insulin. Insulin was gradually decreased and was stopped finally. 0.9 mg liraglutide was changed to once-week of dulaglutide treatment. Informed consent was obtained from the patients orally before the changing from once-1 week to once- 2 weeks injection of dulaglutide. We evaluated glucose control by GA. 2 of 4 patients also underwented 24 hours CGM with iPro2 (Medtronic, Northridge, CA). Glycemic control was estimated maximal or minimal blood BS, the mean BS, MAGE, AUC>180 mg/dl (AUC>180) and <70 mg/dl(AUC<70).
Result
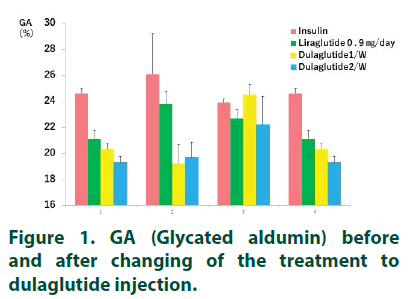
GA was decrease from 20.3 ± 0.47% to 19.3 ± 0.47% in case 1, from 24.5 ± 0.79% to 22.2 ± 2.18% in case 3, and from 20.3 ± 0.47% to 19.3 ± 0.47% in case 4 after changing of dulaglutide injection from once-1 week to once-2 weeks (TABLE 1). Case 2 was slightly increased after changing dulaglutide therapy. GA was also improved in all cases by changing of the therapy from insulin therapy to liraglutide and from liraglutide to dulaglutide once-2 weeks’ injection (TABLE 2 and FIGURE 1). CGM was examined in Case 1 and Case 2. Mean BS, 24 h AUC and AUC>180 were improved in Case 1 (TABLE 3). In Case 2, 24 h AUC was increased from 3807 ± 573.2 mg/dl.h to 4392 ± 337.2 mg/ dl.h, but MAGE and AUC>180 were improved after changing dulaglutide therapy.
Discussion
We proved the improvement of glucose metabolism after modifying dulaglutide once-1 week to once-2 weeks in patients with dialysis type 2 DM. HbA1c and the pharmacokinetics parameters of CGM analysis were reduced by changing the therapy. Plasma concentration of dulaglutide in ESRD is showed same as normal renal function [8]. Plasma dulaglutide and insulin concentration are also increased after five time’s injection. The insulin secretion was sufficient in 4 patients, but they were treated by insulin therapy before treating with GLP-1 receptor agonist. IR is increased at lower GFR levels [9]. IR is thought to accelerate by insulin therapy for in insulin sufficient statement. High insulin concentration is shown to accelerate arteriosclerosis. IR, which is caused by oxidative stress, chronic inflammation, metabolic acidosis, Vitamin D deficiency, decreased erythropoietin, exist in renal failure patients [10,11]. Fasting plasma insulin concentration, the area under the curve for plasma insulin concentration and total insulin delivery are significantly correlated with insulin sensitivity [12]. IR and hyperglucagonemia are caused bad glucose control. Fasting plasma glucagon concentration was increased in the patients with chronic renal failure [13,14]. There was shown the positive correlation of serum creatinine with plasma glucagon concentration. Alogliptin, is one of dipeptidyl peptidase-4 (DDP-4), improves steroid-induced hyperglycemia by decrease of glucagon levels through an increase in plasma GLP-1 levels [15]. GLP-1 was also showed to inhibit glucagon in fasting state and postprandilal state of type1 DM [16]. Dulaglutide is superior effect for glycemic control compared with sitagliptin which is one of DDP-4 [17]. Glucagon receptor knockout mice prevented hyperglycemia after β -cell destruction by streptozotocin [18]. Inhibition of glucagon was key factor for regulation of glucose control, and GLP-1 receptor agonist was effective for glucose control by suppress of glucagon in ESRD of type 2 DM. GLP-1 receptor agonist is also suggested to decrease cardiovascular death, cardiovascular event, nonfatal stroke and the progression of diabetic kidney disease in SUSTAIN [19] and LEADER [20]. HbA1c was more decreased and hypoglycemia was reduced in the patients treated with GLP-1 receptor agonist compared with insulin therapy [21-23]. Unconscious hypoglycemia is easy to occur in the patients of ESRD by the progressing of diabetic autonomic neuropathy. Strict glucose lowering control is increased the risk of cardiovascular disease and total mortality in Accord study [24]. Cyrus showed that hypoglycemia induced chest pain and ECG abnormality [25]. Cardiovascular event by severe hypoglycemia might be increased mortality. Hypoglycemia also induced the thickness of carotid artery [26], the damage of peripheral neuron [27], renal impairement [28] and the risk of dementia [29,30]. Body weight loss was appeared in the patients treated with GLP-1 receptor agonist, and weight gain was showed in the patients treated with insulin30). Insulin resistance, induced by obesity, accelerate atherosclerosis. Diabetic complications are progress in the patients of ESRD, but glucoselowering treatment of ESRD is limited. GLP- 1 receptor agonist may be useful treatment to inhibit small & large vessel complication in ESRD compared with insulin therapy. 0.75 mg dulaglutide was showed to be superior than 0.9 mg liraglutide in HbA1c reduction [31,32]. Dulaglutide was showed to be more efficacy than insulin glargine, metformin, exenatide and metformin in HbA1c reduction and BW loss in spite of low rates of hypoglycemia [33]. Daily insulin or daily GLP-1 receptor agonist injection is sometimes stressful for diabetic patients. We have clinically experienced cases to become good glucose control after switching from once daily GLP-1 receptor agonist to once weekly GLP- 1 receptor agonist. Once-weekly dulaglutide is useful treatment in glucose metabolism and in specific simplified procedures. Plasma concentration of dulaglutide in ESRD is showed same as normal renal function. Plasma dulaglutide concentration was increased after five-time injection [8]. Plasma liraglutide concentration was increased in dialysis type 2 DM patients compared with control [33].
Conclusion
Plasma dulaglutide concentration was probably increased in dialysis type 2 DM patients after few injections. Dulaglutide injection is used the same dose for a week, but the concentration of dulaglutide might be too much for dialysis type 2 DM patients. Some dialysis type 2 DM patients were experienced to complained gastrointestinal sufficient. The plasma dulaglutide concentration might be high for glycemic control, and the complaint was released after changing dulaglutide injection from once-a week to once-2 weeks. We resulted the good glycemic control could be maintained after changing dulaglutide injection from once-1 week to once-2 weeks. Our cases were few, and further study will be hoped.-GI), glinide and insulin therapy for maintaining good blood glucose control in such cases. The advent of GLP-1 receptor agonist is given good blood glucose control and weight loss in sufficient secretion of insulin in dialysis type 2 diabetes patients. We have experienced good glycemic control after changing from insulin to GLP-1 receptor agonist. Dulaglutide is GLP-1 receptor agonist consisting of GLP-1(7-37) covalently linked to an Fc fragment of human IgG4 [5]. It is used for the treatment of type 2 diabetes once weekly [6,7]. The effective blood concentration of dulaglutide is continued less than 10 days at 1 time injection, and the concentration is increased after 5 times injection [8]. We tried to use dulaglutide at once-2 weeks in 4 diabetes outpatients of ESRD treated with hemodialysis.
Material and Method
We studied 4 outpatients of type2 DM in ESRD treat with dialysis at Fukuoka- Wajiro-hospital in Japan (TABLE 1). They were undergoing of dialysis 3 times in a week. Sex is 2 males and 2 females, age 52~79 y, BMI 18.5~32.1 kg/m2, and the duration of Diabetes mellitus (DM) is 7~29 years. The duration of dialysis was 5~11 y. 3 of 4 patients had proliferative diabetic retinopathy and multiple symmetrical & autonomic diabetic neuropathy. 1 of 4 patients did not have retinopathy and neuropathy, and was medicated by steroid therapy for glomerular nephritis. They were cared by insulin therapy before using GLP-1 receptor agonist, and had sufficient insulin secretion. Liraglutide was started from 0.3 mg/day and was gradually increased 0.6 mg/day to 0.9 mg/day with using insulin. Insulin was gradually decreased and was stopped finally. 0.9 mg liraglutide was changed to once-week of dulaglutide treatment. Informed consent was obtained from the patients orally before the changing from once-1 week to once- 2 weeks injection of dulaglutide. We evaluated glucose control by GA. 2 of 4 patients also underwented 24 hours CGM with iPro2 (Medtronic, Northridge, CA). Glycemic control was estimated maximal or minimal blood BS, the mean BS, MAGE, AUC>180 mg/dl (AUC>180) and <70 mg/dl(AUC<70).
Table 1. Characteristics of patients.
| Age (y) | Sex | BMI | Duration of DM | Dialysis of DM | Retinopathy | Neuropathy | |
|---|---|---|---|---|---|---|---|
| 1 | 63 | M | 22.8 | 12 y | 10 y | Proliferative | + |
| 2 | 64 | F | 32.1 | 17 y | 6 y | Proliferative | + |
| 3 | 79 | F | 18.5 | 13 y | 11 y | Proliferative | + |
| 4 | 72 | F | 20.1 | 29 y | 7 y | Proliferative | + |
| 5 | 52 | M | 31 | 7 y | 5 M | - | + |
Result
GA was decrease from 20.3 ± 0.47% to 19.3 ± 0.47% in case 1, from 24.5 ± 0.79% to 22.2 ± 2.18% in case 3, and from 20.3 ± 0.47% to 19.3 ± 0.47% in case 4 after changing of dulaglutide injection from once-1 week to once-2 weeks (TABLE 1). Case 2 was slightly increased after changing dulaglutide therapy. GA was also improved in all cases by changing of the therapy from insulin therapy to liraglutide and from liraglutide to dulaglutide once-2 weeks’ injection (TABLE 2 and FIGURE 1). CGM was examined in Case 1 and Case 2. Mean BS, 24 h AUC and AUC>180 were improved in Case 1 (TABLE 3). In Case 2, 24 h AUC was increased from 3807 ± 573.2 mg/dl.・h to 4392 ± 337.2 mg/ dl.h, but MAGE and AUC>180 were improved after changing dulaglutide therapy.
Table 2 GA (Glycated albumin) level following insulin, liraglutide, dulaglutide once a week and dulaglutide once 2 weeks.
| Insulin | Liraglutide 0.9 ㎎/day |
Dulaglutide 1/W |
Dulaglutide 1/2 W |
|
|---|---|---|---|---|
| 1 | 24.6 ± 0.38 | 21.1 ± 0.69 | 20.3 ± 0.47 | 19.3 ± 0.47 |
| 2 | 26.1 ± 3.1 | 23.8 ± 0.98 | 19.2 ± 1.49 | 19.7 ± 1.17 |
| 3 | 23.9 ± 0.28 | 22.73 ± 0.70 | 24.5 ± 0.79 | 22.2 ± 2.18 |
| 4 | 24.6 ± 0.38 | 21.1 ± 0.69 | 20.3 ± 0.47 | 19.3 ± 0.47 |
Table 3 Pharmacokinetics parameters of CGM analysis after treatment of dulaglutide.
| Case 1 | Case 2 | |||
|---|---|---|---|---|
| 1/W | 1/2 W | 1/W | 1/2 W | |
| Maximal glucose level (㎎/dl) | 323 | 241 | 379 | 301 |
| Minimal glucose level (㎎/dl) | 40 | 44 | 70 | 88 |
| 24 h mean glucose level (㎎/dl) | 161 ± 53 | 137 ± 44 | 164 ± 60 | 183 ± 39 |
| MAGE (㎎/dl) | 104.6 ± 32.2 | 67.3 ± 24.0 | 104 ± 37.8 | 102.5 ± 32.0 |
| AUC(24 h) (㎎/dl・h) | 3831.1 ± 260.6 | 3225.4 ± 820.4 | 3807 ± 573.2 | 4392 ± 337.2 |
| AUC>180(㎎/dl・h) | 905.7 ± 523.1 | 494.3 ± 150.0 | 1224.8 ± 574.3 | 781.0 ± 309.2 |
| AUC<70(㎎/dl・h) | 0 | 0 | 525.1 | 321.8 |
MAGE: mean amplitude of glycemic excursion; AUC: area under the blood concentration-time curve mean ± SD.
Discussion
We proved the improvement of glucose metabolism after modifying dulaglutide once-1 week to once-2 weeks in patients with dialysis type 2 DM. HbA1c and the pharmacokinetics parameters of CGM analysis were reduced by changing the therapy. Plasma concentration of dulaglutide in ESRD is showed same as normal renal function [8]. Plasma dulaglutide and insulin concentration are also increased after five time’s injection. The insulin secretion was sufficient in 4 patients, but they were treated by insulin therapy before treating with GLP-1 receptor agonist. IR is increased at lower GFR levels [9]. IR is thought to accelerate by insulin therapy for in insulin sufficient statement. High insulin concentration is shown to accelerate arteriosclerosis. IR, which is caused by oxidative stress, chronic inflammation, metabolic acidosis, Vitamin D deficiency, decreased erythropoietin, exist in renal failure patients [10,11]. Fasting plasma insulin concentration, the area under the curve for plasma insulin concentration and total insulin delivery are significantly correlated with insulin sensitivity [12]. IR and hyperglucagonemia are caused bad glucose control. Fasting plasma glucagon concentration was increased in the patients with chronic renal failure [13,14]. There was shown the positive correlation of serum creatinine with plasma glucagon concentration. Alogliptin, is one of dipeptidyl peptidase-4 (DDP-4), improves steroid-induced hyperglycemia by decrease of glucagon levels through an increase in plasma GLP-1 levels [15]. GLP-1 was also showed to inhibit glucagon in fasting state and postprandilal state of type1 DM [16]. Dulaglutide is superior effect for glycemic control compared with sitagliptin which is one of DDP-4 [17]. Glucagon receptor knockout mice prevented hyperglycemia after β-cell destruction by streptozotocin [18]. Inhibition of glucagon was key factor for regulation of glucose control, and GLP-1 receptor agonist was effective for glucose control by suppress of glucagon in ESRD of type 2 DM. GLP-1 receptor agonist is also suggested to decrease cardiovascular death, cardiovascular event, nonfatal stroke and the progression of diabetic kidney disease in SUSTAIN [19] and LEADER [20]. HbA1c was more decreased and hypoglycemia was reduced in the patients treated with GLP-1 receptor agonist compared with insulin therapy [21-23]. Unconscious hypoglycemia is easy to occur in the patients of ESRD by the progressing of diabetic autonomic neuropathy. Strict glucose lowering control is increased the risk of cardiovascular disease and total mortality in Accord study [24]. Cyrus showed that hypoglycemia induced chest pain and ECG abnormality [25]. Cardiovascular event by severe hypoglycemia might be increased mortality. Hypoglycemia also induced the thickness of carotid artery [26], the damage of peripheral neuron [27], renal impairement [28] and the risk of dementia [29,30]. Body weight loss was appeared in the patients treated with GLP-1 receptor agonist, and weight gain was showed in the patients treated with insulin30). Insulin resistance, induced by obesity, accelerate atherosclerosis. Diabetic complications are progress in the patients of ESRD, but glucoselowering treatment of ESRD is limited. GLP- 1 receptor agonist may be useful treatment to inhibit small & large vessel complication in ESRD compared with insulin therapy. 0.75 mg dulaglutide was showed to be superior than 0.9 mg liraglutide in HbA1c reduction [31,32]. Dulaglutide was showed to be more efficacy than insulin glargine, metformin, exenatide and metformin in HbA1c reduction and BW loss in spite of low rates of hypoglycemia [33]. Daily insulin or daily GLP-1 receptor agonist injection is sometimes stressful for diabetic patients. We have clinically experienced cases to become good glucose control after switching from once daily GLP-1 receptor agonist to once weekly GLP- 1 receptor agonist. Once-weekly dulaglutide is useful treatment in glucose metabolism and in specific simplified procedures. Plasma concentration of dulaglutide in ESRD is showed same as normal renal function. Plasma dulaglutide concentration was increased after five-time injection [8]. Plasma liraglutide concentration was increased in dialysis type 2 DM patients compared with control [33].
Conclusion
Plasma dulaglutide concentration was probably increased in dialysis type 2 DM patients after few injections. Dulaglutide injection is used the same dose for a week, but the concentration of dulaglutide might be too much for dialysis type 2 DM patients. Some dialysis type 2 DM patients were experienced to complained gastrointestinal sufficient. The plasma dulaglutide concentration might be high for glycemic control, and the complaint was released after changing dulaglutide injection from once-a week to once-2 weeks. We resulted the good glycemic control could be maintained after changing dulaglutide injection from once-1 week to once-2 weeks. Our cases were few, and further study will be hoped.
References
- Baggio L, Drucker D. Biology of Icretins: GLP-1 and GIP. Gastroenterology. 132(6), 2131-2157 (2007).
- Baggio L, Drucker D. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J. Clin. Invest. 124(10), 4223-4226 (2014).
- Townsend S, Newsome P. Review article: new treatments in non-alcoholic fatty liver. Aliment. Pharmacol. Ther. 46(5), 494-507 (2017).
- Radhakutty A, Burt M. Critical review of the evidence underlying management of glucocorticoid-induced hyperglycemia. Eur J Endocrinol 179(4), R207-R218 (2018).
- Glaesner W, Vick A, Millican R et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes. Metab. Res. Rev. 26(4), 287-296 (2010).
- Garber A. Long-acting glucagon-like peptide 1 receptor agonists. Diabetes Care 34(2), 5279-5284 (2011).
- Tran K, Park Y, Pandya S et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am. Health. Drug. Benefits. 10(4), 178-187 (2017).
- http://www.pmda.go.jp/downfiles/ph/PDF/530471_2499416G1029_1_03.pdf.
- Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am. J. Physiol. Renal. Physiol. 311(6), F1087-1108 (2016).
- Xu H, Carrero J. Insulin resistance in chronic kidney disease. Nephrology 22(4), 31-34 (2017).
- Liao M, Sung C, Hung K et al. Insulin resistance in patients with chronic kidney disease. J. Biomed. Biotechnol. Article ID691369,12 pages (2012).
- Fliser D, Pacini G, Engelleiter R et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney. Int. 53(5), 1343-1347 (1998).
- Kuku S, Jaspan J, Emmanouel D et al. Heterogeneity of plasma glucagon. circulating components in normal subjects and patients with chronic renal failure. J. Clin. Invest. 58(3), 742-750 (1976).
- Wang X, Yang J, Chang B et al. Glucagon secretion is increased in patients with type 2 diabetic nephropathy. J.Diabetes.Complications. 30(3), 488-493 (2016).
- Ohashi N, Tsuji N, Naito Y et al. Alogliptin improves steroid-induced hyperglycemia in treatment-naïve Japanese patients with chronic kidney disease by decrease of plasma glucagon levels. Med. Sci. Monit. 20, 587-593 (2014).
- Holst J, Christensen M, Lund A et al. Regulation of glucagon secretion by incretins. Diabetes. Obes. Metab. 13(1), 89-94 (2011).
- Weinstock R, Guerci B, Umpierrez G et al. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5) : a randomized, phase III study. Diabetes. Obes. Metab. 17(9), 849-858 (2015).
- Conarello S, Jiang G, Mu J et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 50(1), 142-150 (2007).
- Marso S, Bain S, Consoli A et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375(19), 1834-1844 (2016)
- Marso S, Daniels G, Brown-Frandsen K et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4), 311-322 (2016).
- Singh S, Wright E, Kwan A et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes. Obes. Metab. 19(2), 228-238 (2017).
- Kaneko S, Oura T, Matsui A et al. Efficacy and safety of subgroup analysis stratified by baseline HbA1c in Japanese phase 3 study of dulaglutide 0.75mg compared with insulin glargine in patients with type 2 diabetes. Endocr. J. 64(12), 1165-1172 (2017).
- Araki E, Inagaki N, Tanizawa Y et al. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes : a randomized, open-label, phase III, non-inferiority study. Diabetes. Obes. Metab. 17(10), 994-1002 (2015).
- Miller M, Williamson J, Gerstein H et al. Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD trials. Diabetes. Care. 37(3), 634-643 (2014).
- Chow E, Bernjak A, Williams S et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 63(5), 1738-1747 (2014).
- Mita T, Katakami N, Shiraiwa T et al. Relationship between frequency of hypoglycemic episodes and changes in carotid atherosclerosis in insulin-treated patients with type 2 diabetes mellitus. Sci.Rep. 39965 (2017) .
- Ozaki K, Sano T, Tsuji N et al. Insulin-induced hypoglycemic peripheral motor neuropathy in spontaneously diabetic WBN/Kob rats. Comp. Med. 60(4), 282-287 (2010).
- Lee Y, Yen S, Shin S et al. Sever hypoglycemia on renal as a predictor of End-stage renal disease in type 2 diabetes; a national chohort study.Int. J. Environ. Res. Public. Health. 16(5), 681 (2019).
- Crane P, Walker R, Hubbard R et al. Glucose levels and risk of dementia. N. Engl. J. Med. 369, 540-548 (2013).
- Jendle J, Grunberger G, Blevins T et al. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes. Metab. Res. Rev. 32(8), 776-790 (2016).
- Miyagawa J, Odawara M, Takamura T et al. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes. Obes. Metab. 17(10), 974-983 (2015).
- Odawara M, Miyagawa J, Iwamoto N et al. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes : 52 weeks of treatment in a randomized phase III study. Diabetes. Obes. Metab. 18(3), 249-257 (2016).
- Idorn T, Knop F, Jorgensen M et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and end-stage renal disease: an investigator -initiated, placebo-controlled, double-blinded, parallel group, randomized trials. Diabetes. Care. 39(2), 206-213 (2016).