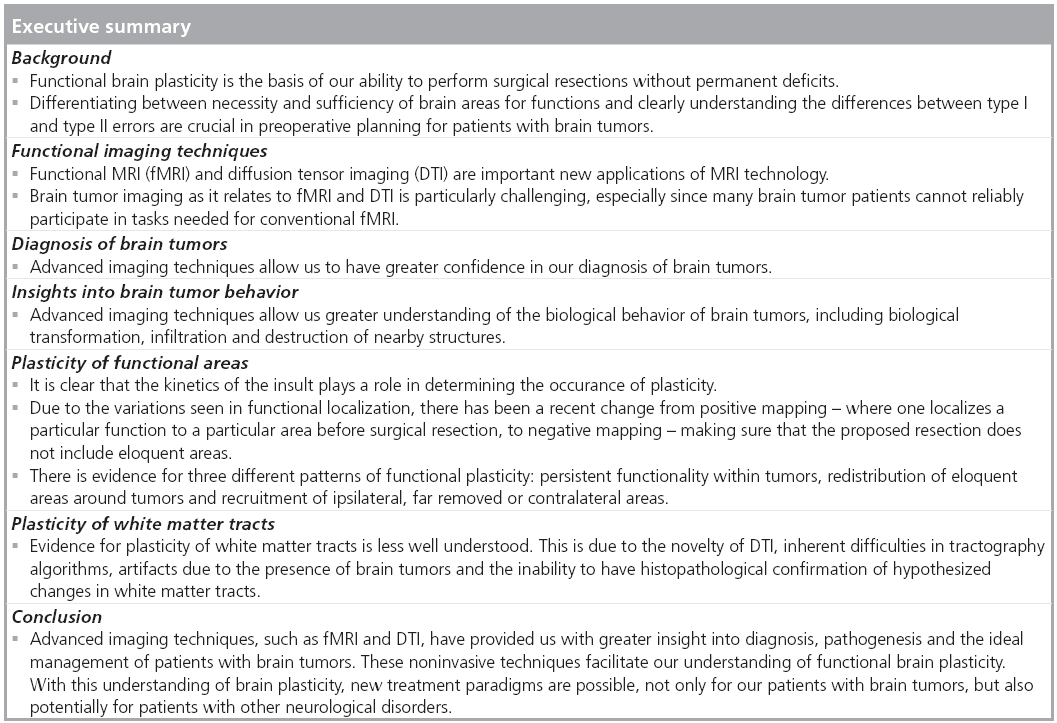
Review Article - Imaging in Medicine (2013) Volume 5, Issue 4
Effect of disease and recovery on functional anatomy in brain tumor patients: insights from functional MRI and diffusion tensor imaging
Muhammad M Abd-El-Barr1, Emam Saleh1, Raymond Y Huang2 & Alexandra J Golby*1,21Department of Neurosurgery, Brigham & Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA
2Department of Radiology, Brigham & Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA
- Corresponding Author:
- Alexandra J Golby
Department of Neurosurgery
Brigham & Women’s Hospital
Harvard Medical School, 75 Francis Street
Boston, MA 02115, USA
E-mail: agolby@bwh.harvard.edu
Abstract
Keywords
brain tumors ▪ diffusion tensor imaging ▪ functional MRI ▪ MRI ▪ plasticity ▪ surgical planning
Patients with brain tumors present a unique opportunity to understand functional brain plasticity. The fact that many patients may have large intracranial tumors with minimal neurological deficits suggests that the brain possesses methods of reorganization and plasticity that were not appreciated before the advent of advanced imaging techniques, pharmacological interventions and microsurgical techniques. Understanding the underpinnings of this reorganizational and plasticity in a general sense and on an individual level is critical in order to optimize treatment for patients with brain lesions.
A cornerstone of modern brain tumor management involves surgical resection. It has been shown that expected survival increases with the extent of resection of primary and metastatic brain tumors [1–3]. However, it has also been shown that the presence of a new neurological deficit after surgery is an independent predictor of worse outcome [4]. Thus, a central tenet of modern day brain tumor surgery involves maximum resection with minimal complications and without causing a new neurological deficit. It is this central tenet that has been the impetus of major advances in functional brain mapping in brain tumor patients, as we try to predict the functionality of areas of the brain affected by brain tumors in order to tailor resections for individual patients.
Historically, brain areas that are functionally involved in motor, sensory, language and visual functions are referred to by neurosurgeons as eloquent areas [5,6]. Determining these areas on an individual basis, particularly in patients who have mass lesions, is difficult because in addition to inherent individual differences; variable and unpredictable reorganization, invasion or displacement may occur due to the lesion [7–9]. Therefore, general knowledge about functional brain anatomy, whether derived from lesion studies, or functional brain mapping in healthy subjects can only inform surgical decisionmaking in the most general sense. In order to guide surgical decisions in patients, individual functional mapping must be developed and validated.
When trying to attribute a particular function to a certain brain area, it is important to differentiate between the necessity and sufficiency of the brain area to that function. In general, one can infer function on the basis of observation, inhibition or stimulation of a particular brain area. Observational techniques can show the involvement of various brain areas in a specific function, but cannot demonstrate alone either necessity or sufficiency [10]. Inhibitory methods, by blocking local neural activity and looking for reversible neurological deficits, can demonstrate the necessity of a particular brain area to a specific function. Stimulation methods, on the other hand, demonstrate the sufficiency of a particular area to a specific function. Two of the most common clinical methods are the Wada (or intracarotid amytal) test and direct cortical stimulation (DCS). The Wada test is an inhibitory test that tests the residual capacity of the unanesthesized hemisphere after injection of the internal carotid artery with amytal, an anesthetic. Despite its name, DCS may be used for stimulation (motor and sensory mapping) or inhibition (language mapping). In general, combining these different techniques in the pre- and intra-operative management of brain tumor patients is undertaken to ensure maximum resection while minimizing the chance of a new neurologic deficit [11,12].
Another concept that is central to functional imaging in brain tumor patients is the differences between type I and II errors and their implications. In the case of brain tumor patients, both errors can have important and sometimes devastating effects on the patient. For type I errors, ascribing a particular neurological function to a brain area, when in reality it is not involved, may cause a surgeon to erroneously limit their resection thereby leaving tumor behind that might have been resected; this can have negative survival implications [1,13]. Conversely, failure to detect that a particular area does have functional significance (false negative or type II error) can lead to a resection that causes a patient to have a neurological deficit. It has been our philosophy to err on the side of type I error, as type II error, or false negatives, can be so devastating to the patient because they could lead to the inadvertent resection of critical tissue.
Due to the fact that many brain tumor patients undergo surgical resections, this population represents a unique opportunity to study brain organization by correlating histological, genetic and neurophysiological analyses with functional imaging studies [14,15]. In this report, we review new observational techniques such as functional MRI (fMRI) and diffusion tensor imaging (DTI), and they look at how they have allowed us a greater understanding of brain connectivity, functionality and reorganization in the context of patients with brain tumors.
Functional imaging techniques
■Functional MRI
MRI uses physical properties of unpaired atomic nuclei in a magnetic field to create contrast in soft tissues, such as the brain [16]. It has found wide application in the diagnosis of brain tumors [17], infection [17,18] and epilepsy [19]. As well as structural information, there has been an increasing interest in understanding the functional topography of the brain. Neurovascular coupling is the main physiological basis of fMRI, which posits that with increased neuronal activity, there is a local rise in blood oxygen consumption and cerebral blood perfusion [20,21]. Because the supply of oxyhemoglobin normally outstrips the demand, there is an increase in the ratio of oxyhemoglobin to deoxyhemoglobin [22]. This change in blood oxygenation forms the basis of the blood oxygenation level-dependent (BOLD) imaging modality, which is used synonymously with fMRI [23,24].
There is some controversy in the literature regarding the exact neural mechanisms underlying the BOLD signal, with some positing that the change in the BOLD signal is proportional to neuronal firing rate [25], or local field potentials [24]. There is mounting evidence that astrocytes, which play important roles in neuronal metabolism and cerebral blood flow, may play important roles in modulating the BOLD signal [26]. Nonetheless, there has been an extensive overlapping (<1 cm) of the functional maps uncovered by fMRI compared with other modalities such as PET, transcranial magnetic stimulation and DCS [10,27]. But since fMRI is based on blood flow, it is subject to artifactual changes when there is altered neural hemodynamic coupling, which is common in patients with brain tumors and other cerebral lesions [28,29]. A related limitation of fMRI is its low temporal resolution due to the delay introduced by the hemodynamic response function [30].
fMRI has been used extensively to understand the functional topography of the brain of healthy individuals and patients with many neurologic diseases in the mapping of sensory, motor and language cortical regions, as well as in a growing number of studies into the underpinnings of a myriad of human behaviors. Two of the main paradigm designs for fMRI experiments are block design and event-related design. For block design paradigms, subjects are asked to do a specific task interleaved with a control condition in time blocks of approximately 20 s. In event-related design, single behavioral events can be modeled in a complex model of the task timing [30,31]. In both block and event designs, the BOLD signal during the task and control conditions is compared with a predicted response based on the task timing. Unfortunately, this signal is often small, on the order of 0.5–5%, meaning that many trials have to be averaged to get a reasonable signal-to-noise ratio [32]. Blocked design may have larger statistical power and a relatively larger increase in the BOLD signal [33], while event design may allow for a greater appreciation of individual differences and may be less sensitive to head motion [34]. We have shown that in healthy subjects and brain tumor patients, an event-related design paradigm resulted in more robust activation of putative language areas [35]. However, eventrelated design is also much harder to implement than block design paradigms, and thus does not lend itself readily to clinical use.
One of the main drawbacks of fMRI in a clinical setting is that any task-based paradigm requires active participation of subjects. This participation requires understanding of the desired task instructions and the ability to accurately perform these tasks with precise timing. Many of the patients who are referred for neurosurgical consultation do not have intact comprehension or have focal deficits that limit their ability to accurately perform the tasks of conventional fMRI. It is because of this inability to actively participate in these tasks that ‘restingstate’ fMRI has found traction and interest in the neurosurgical community [36,37]. The basic premise of resting state fMRI is that the control epochs of the block and event design paradigms are not in fact ‘zero’, but rather this baseline state demonstrates important connectivity between brain regions and functional information may be extracted from these data. This was first noted by Raichle and colleagues in that areas of the brain that had been previously shown to have very high metabolic demands were paradoxically found to have the greatest amount of deactivation during externally imposed cognitive tasks [38]. This led to the definition of a ‘default mode network’ within the brain.
This resting-state fMRI technique was originally described by Biswal et al. [39], where it was found that there are low-frequency (<0.1 Hz) oscillations that are correlated in the BOLD signal in areas that are functionally related [40]. Importantly, there is a close correspondence between the areas of activation during ‘active’ MRI and those found to be related using restingstate fMRI [41]. Preliminary evidence suggests that this modality may prove to be important in mapping brain functionality in those patients who are unable to participate in the conventional task-based paradigms [36,37]. We have shown the feasibility of using this type of paradigm for mapping language networks in a series of healthy subjects and we are pursuing the possibility of using this paradigm for surgical planning.
As described in the introduction, observational methods such as fMRI do not prove necessity or sufficiency of a particular area for function, rather fMRI is only able to demonstrate a particular area’s involvement for such functions. Thus, there has been an interest in comparing fMRI data to other more direct methods of ascribing function to particular brain areas. DCS, where current is applied to certain areas of the brain has been considered to be the ‘gold standard’ in assessing eloquence of brain areas [15]. In a metaanalysis comparing fMRI and DCS it was found that it was difficult to directly compare these two methods as many of the tasks used in fMRI were not used during DCS. In those studies that were able to calculate sensitivity and specificity, these were found to be very variable, from 59 to 100% and 0 to 97%, respectively. This suggests that clinical fMRI, as it stands now, is not ready to supplant DCS in surgical resection mapping in brain tumor patients. Nevertheless, as a noninvasive method, which can be used across a wide variety of patients and also may be repeated over time, fMRI is emerging as a key tool for the study of individual and group brain functional anatomy and plasticity.
■Diffusion tensor imaging
Whereas MRI and fMRI describe cortical areas and give us clues to their functionality, these two modalities give us minimal information about the white matter (WM) subcortical pathways that connect these areas and may be encountered during the approach to, within, or surrounding brain tumors. DTI is an imaging modality that has gained increasing attention in mapping WM pathways in the brain. Based on the fact that the diffusion of water in axonal tracts is direction- dependent or anisotropic and greatest in a direction parallel to fiber tracts, one is able to construct a tensor (matrix) describing the magnitude and directionality of water movement for every voxel by applying magnetic field gradients in multiple directions [42]. DTI is an extension of more classical diffusion-weighted imaging, which measures the diffusivity of water in three directions, rather than in the six or more noncollinear directions that DTI is capable of [43]. Currently, the spatial resolution of DTI is on the order of a voxel size of 2 × 2 × 2 mm3, but this is improving [44]. An important parameter that can be derived from DTI is fractional anisotropy (FA), which is a measure of the degree of directionality of water diffusion and is expressed as a numerical value between 0 (least directional) and 1 (most directional) [20].
By estimating the maximum diffusivity of water in a particular voxel, one is able to construct 3D maps of the most probable WM pathways, a concept called tractography [45]. The two main approaches to the construction of these fiber pathways are deterministic and probabilistic tractography [20]. For deterministic fiber tracking, the principal direction of the diffusion tensor is tracked from voxel to voxel starting from seed voxel or a region of interest (ROI; such as a brain tumor), until the FA falls below a preset threshold [46]. For the probabilistic approach, one constructs the tracts by sampling a Gaussian probability density function at each voxel, whose covariance matrix is defined by the tensor at that voxel [47]. In the deterministic method, the next point reached is not known a priori, while in the probabilistic method, the connectivity between two areas can be tested by evaluating the probability that the two areas are connected [48]. Another parameter that has now been introduced is the related fiber density index, which is a measure of the mean number of fiber paths passing though a particular pixel or ROI [49].
DTI is presently the only method that is able to demonstrate WM anatomy in vivo. Thus DTI has earned a place as an important tool for imaging patients with brain tumors. Nevertheless, DTI is limited by many issues, including artifacts due to tumors, edema, inhomogeneities in the magnetic field and head movements. Eddy currents, which occur due to the use of heavy gradient sequences in DTI must be corrected by mathematical algorithms [50]. As with any nonsingular mathematical problem, the possibility of more than one ‘correct’ answer can have important consequences in assessing the accuracy of the tracts that are hypothesized to occur in any one voxel [51]. One particular problem that is clinically relevant, is trying to find efficient algorithms to help with the problem that within a particular voxel, there may be more than one crossing or intersecting fiber tract [52]. Again, both probabilistic and deterministic models have been used to solve this problem. We have used a two-tensor deterministic model, called the eXtended Streamline Tractography to show that this model is an efficient and reliable method to help resolve this problem [52].
DTI has been used to image many of the major WM tracts, including the cingulum, arcuate fasiculus [53,54] corticospinal pathways and optic radiations [55–57]. Four patterns of differences in anisotropy have been described in disease states: altered position and direction but normal signal, which corresponds to tract displacement; decreased anisotropy but normal direction and location, thought to correspond to vasogenic edema; decreased anisotropy signal with disrupted direction maps, thought to correspond to infiltration; and loss of anisotropic signal corresponding to tract obliteration or destruction [57]. Although some histopathological validation studies [58] have been performed in recent years in animals, there is still controversy about the correlation of histopathological changes and the changes seen in anisotropy [59].
Edema, an elevation in water content that is commonly seen in both brain tumors and stroke, changes diffusion anisotropy at a particular voxel [48,60]. Edema can be categorized as vasogenic, cytotoxic or cellular. Although edema does not change the properties of axons or myelin, it does change the weighted cellular contribution in a particular voxel, thus changing the calculated FA [60]. Vasogenic edema, which is a common reaction to inflammation (as seen in brain tumors or subacute stroke), is characterized by disruption of the blood–brain barrier and hence leakage of normally excluded intravascular proteins into the extravascular space causes a reduction in the FA most likely due to the fact that the WM tracts become less organized and less dense [61]. Cytotoxic edema, which is seen in acute stroke, is due to the damage to the cell membranes of neurons and glia, causing the intracellular space to increase, at the expense of the extracellular space. The DTI findings with cytotoxic edema are not uniform, with some groups reporting an increase in the FA [62], while other report a decrease [61]. Importantly, although DTI can give us ideas of the structural integrity of WM tracts, it does not provide information on the functional integrity of these tracts [63].
Diagnosis of brain tumors
A common clinical problem in patients with mass lesions of the brain, is making an accurate diagnosis on the basis of imaging. The differential diagnosis is wide and clinical care is impacted depending on the diagnosis. Advanced imaging techniques have allowed for greater accuracy in making these diagnoses. Besides aiding diagnosis, these new functional imaging paradigms have allowed us a greater understanding of the microscopic structure and mechanisms of tumor transformation, invasion and destruction.
The main differential diagnosis of intra-axial, supratentorial lesions in adults includes metastatic neoplasm, primary brain tumor, infection and lymphoma. A recent paper proposes a purely MRI-based algorithm to differentiate these entities, using lesion characteristics such as enhancement, spectroscopy, diffusion-weighted imaging and perfusion MRI [17]. Retrospectively evaluating images and histolopathological samples from over 100 patients, the authors were able to diagnose tumors with greater than 85% accuracy.
Advanced imaging using DTI has been investigated as a way of differentiating underlying pathology in both newly diagnosed and recurrent brain lesions. To differentiate between metastasis and glioblastoma multiforme (GBM), which are the two most common intrinsic supratentorial lesions in adult, Byrnes et al. showed that in 28 patients with histopathologically proven diagnoses, the FA in the peritumor edema was significantly less in metastases compared with GBM [64]. Similarly, the mean diffusivity was higher in the peritumoral edema surrounding metastases compared with GBM. The authors explained these findings by ascribing them to the relatively high levels of vasogenic edema surrounding metastases compared with GBM. These findings have been corroborated in other studies [65–67].
There has been some controversy about the relationship of FA and tumor cell density. In a retrospective review of patients that received stereotactic or open biopsy, Kinoshita et al. showed a positive correlation between FA and tumor cell density in the tumor core, while Stadlbauer et al. showed negative correlation, but this may be because their FA values were taken at the tumor periphery [68,69]. Another group showed that in the periphery of low-grade gliomas, there was higher FA and fiber density index compared with high-grade gliomas, which they attributed to less disruption of WM tracts by low-grade gliomas compared with high-grade gliomas [70]. This same group failed to show a statistical difference in FA values in the tumor center between low-grade and high-grade lesions. This same type of analysis is simply not possible when assessing WM tracts because tissue sampling of these WM tracts through biopsy or craniotomy are usually avoided due to the risk of neurologic deficit. In an attempt to circumvent this, Stadbauer et al. report a case series of ten patients with gliomas close to the pyramidal tracts. Five of these patients had sensorimotor deficits, which could be attributed to tumor cell invasion into the pyramidal tracts. Those patients had a lower FA ratio in the pyramidal tracts, although this difference was not significant [71]. Interestingly, they did show significant increase in uptake of 18F-FET in those patients with sensorimotor deficits using PET imaging, suggesting that this may be a feasible noninvasive test that could reliably differentiate between those patients that presumably had infiltration into their sensorimotor tracts and those that did not.
Insights into brain tumor behavior
With the advent of molecular and genetic profiling of brain tumors, it is clear that certain brain tumors, although similar in terms of histological diagnosis, may have widely different behaviors in terms of biological transformation, infiltration and destruction of nearby structures [72]. Different groups have attempted to use noninvasive techniques to gain a greater understanding of these behaviors. With this information, it is hoped that more insight can be gained into prognosis as well as amenability to different treatment paradigms.
For example, in a study of 41 patients with different grades of gliomas, Inoue et al. showed a positive correlation between FA and different grades of glioma, and an inverse relationship with mean diffusivity, although the relationship was not as significant [73]. The authors posited that the reason that high-grade gliomas have a higher FA value is owing to the fact that the tissue is more organized. This is because GBM is known to form organized structures such pseudopallisading and endothelial proliferation. However, the authors point out that the ROI in their series of high-grade gliomas were specifically chosen not to include areas of necrosis, where it has been shown to decrease FA values [74]. Partially confounding this finding is the fact that deeper WM tracts such as the splenium of the corpus callosum, a structure invaded commonly by GBM, is known to have a higher FA than more superficial WM tracts invaded by lower grade gliomas [75]. Use of histogram analysis across large areas of brain tumors appears to be promising in differentiating low-grade and high-grade gliomas by overcoming some of the subjective nature of deciding different ROIs [76,77]. Current grading of gliomas has centered on their histological differences, but it is becoming increasingly clear that molecular differences may better differentiate gliomas into clinically relevant prognostic categories [72,78]. It will be interesting to see whether using these molecular differences will uncover differences in their infiltrative and displacement characteristics that have not been able to be uncovered using the traditional histological classification.
Currently, human DTI studies are limited by a low signal-to-noise ratio and relatively low spatial resolution, making direct histological comparison of neurological structure and DTI measurements almost impossible. However, with the advent of microcoils with high magnetic fields, groups have been able to perform DTI in animals at much higher resolutions, making these correlations possible [58].
A potentially important application of diffusion- weighted imaging is in analyzing whether new enhancement in areas close to a resection cavity represents recurrence or pseudoprogression – a very important clinical distinction [79,80]. There is some evidence that immediate diffusion- weighted imaging after surgical resection may identify areas that will later show contrast enhancement, but in fact represent areas that will later progress to encephalomalacia and not correspond to tumor recurrence [81]. Similarly, it may be possible to identify areas that do correspond to tumor recurrence and focus further treatment, whether surgical or radiation-based to those areas. Other groups have attempted to assign prognostic factors to patients based on postsurgical classical and diffusion-weighted imaging [82].
Plasticity of functional areas
By studying patients that had strokes, Broca, Wernicke and Geschwind helped define the structural basis of language function [83]. These areas were found to anatomically correspond to the inferior frontal gyrus, the superior temporal gyrus and inferior parietal gyrus [84]. As these patients had permanent language deficits as a result of these lesions, the idea of specifically localized brain function began to be developed. In particular, lesion studies over the ensuing years suggested that brain plasticity in certain brain areas was very limited and once an eloquent area was lost, the associated function would also be permanently lost.
With the advent of fMRI, came the ability to study these areas in normal patients without lesions. Amongst people unaffected by neurological diseases, there appears to be general rules of localization of language function, but with some variability [85,86]. This also seems to hold true for motor and somatosensory function [87,88].
The pioneering work of George Ojemann and associates using electrocortical stimulation (ECS) revealed that patients with brain tumors had a much higher rate of variability in the localization of these important brain areas [89]. Later, Sanai et al. used this information to propose a new paradigm of brain tumor surgery in eloquent cortex. They proposed that instead of trying to find putative language areas by ECS (positive mapping), it would be safe to limit the craniotomy to the tumor region and by making sure that resections were kept 1 cm away from eloquent areas found by ECS (negative mapping), neurological outcome would not be sacrificed, yet maximum tumor resection could be obtained [7]. Using this method on 250 patients, only four (1.6%) had a persistent language deficit at 6 months postoperatively. This method of negative mapping for tumor resection around eloquent areas has found traction in the neurosurgical community. From these results, one may infer that due to the presence of these tumors, eloquent areas ‘moved’ to other areas. This reorganization of functional cortex calls into question the old dogma of the irreversibility of brain plasticity in adult patients.
It is because of this variability/plasticity of functional areas that fMRI has found an important role in the presurgical surgical planning of neurosurgical patients [10,20,63]. By preoperatively mapping eloquent functions such as motor and language, neurosurgeons can make informed decisions about surgical trajectories, maximum resection volumes and can make reasonable predictions of postoperative deficits.
Evidence for this plasticity is strengthened when studying patients that harbor low-grade (WHO grade II) gliomas. These patients often present at a younger age than the patients harboring higher grade lesions [90]. They also usually do not present with obvious neurological deficits [91], but rather with seizures or subtle cognitive deficits [92]. Another very interesting observation is that many of these tumors are located in the insula and the supplemental motor area (SMA) [93,94]. The reasons for this could be owing to the functional and histological similarities between these cortical areas – both are involved in planning and both represent transitional histological architecture between agranular and granular cortex [93,95–97]. As early as 1999, there was evidence that functional plasticity played a role in why these patients did not present with major neurological deficits, although they harbored tumors within, or close to primary motor cortex. In a study of 11 such patients, Fandino et al. showed that in patients with slow growing tumors such as WHO grade II gliomas, there was evidence that the motor cortex contralateral to the lesion ‘took over’ motor function for the ipsilateral hand [98]. On the other hand, those patients that had GBM had more typical contralateral cortical control of motor function, suggesting that the rapid growth of these tumors does not provide sufficient time for plasticity to develop.
Another piece of evidence suggesting that the kinetics of the insult on the brain is important in modulating plasticity is considering that metaanalyses of patients harboring low-grade gliomas suggested that over 90% were able to return to work within 1 year of surgery, while only 50% of young patients with strokes returned to work [99,100]. In a comparison of patients with acute, ischemic strokes and chronic, long standing lowgrade gliomas, Desmurget et al. relate that most reorganization in acute stroke occur close to the lesion site, while the reorganization in low-grade glioma often occurs distant to site of insult [101]. In another paper supporting the importance of the time course of the insult on reorganization patterns observed, Carpentier et al., evaluated 44 patients with various lesions involving motor regions. They showed that longstanding lesions, such as cortical malformations, were more likely to cause remote reorganization patterns compared with ‘acute’ lesions, such as brain tumors (the majority being high-grade gliomas) [102].
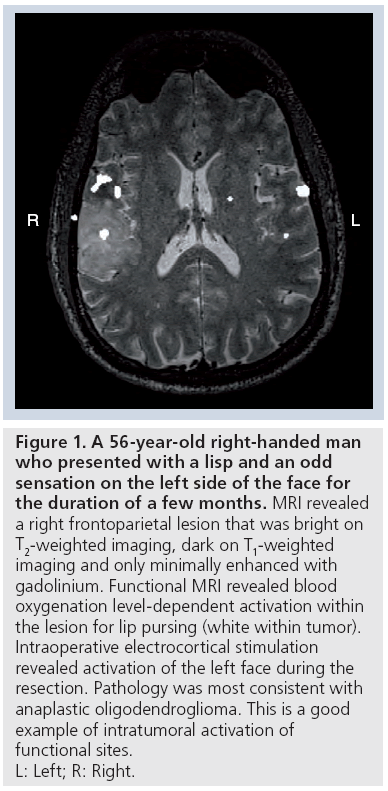
As such, three main reorganization patterns have been recognized [97,101]. In the first, due to the infiltrative nature of the tumor, functionality still persists within the tumor [9,103]. With this, there is very little chance of achieving a good resection without causing new neurological complications. This type of intratumoral activation has been estimated to occur in approximately 40% of patients with low-grade gliomas (Figure 1) [104]. Consistent with this, it has been shown that in patients where BOLD activation of SMA occurred despite infiltration of tumor, surgical resection of tumor caused transient ‘SMA syndrome’ in at least 50% of patients [95].
Figure 1: A 56-year-old right-handed man
who presented with a lisp and an odd
sensation on the left side of the face for
the duration of a few months. MRI revealed
a right frontoparietal lesion that was bright on
T2-weighted imaging, dark on T1-weighted
imaging and only minimally enhanced with
gadolinium. Functional MRI revealed blood
oxygenation level-dependent activation within
the lesion for lip pursing (white within tumor).
Intraoperative electrocortical stimulation
revealed activation of the left face during the
resection. Pathology was most consistent with
anaplastic oligodendroglioma. This is a good
example of intratumoral activation of
functional sites.
L: Left; R: Right.
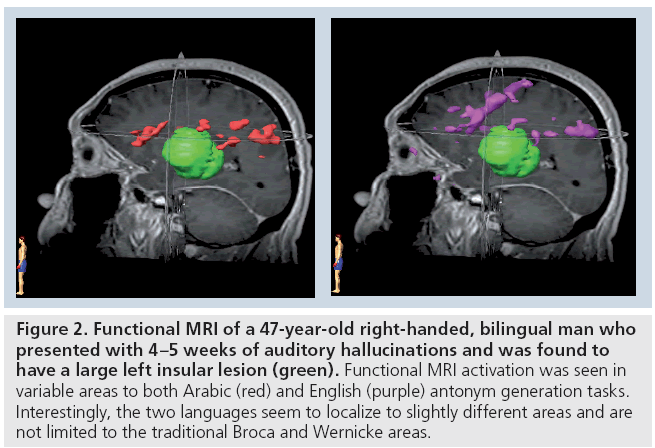
In the second pattern of reorganization, there is redistribution of eloquent areas around the tumor, allowing for a greater resection with a lower chance of deficit, most of which are transient [8]. It is important to note that this is not simply a result of anatomical deformation due to the presence of tumor [105,106]. For example, in patients with tumors in Broca’s area that were not aphasic, it was shown that these patients had activations of left inferior frontal cortex close to the tumor (Figure 2) [106].
Figure 2: Functional MRI of a 47-year-old right-handed, bilingual man who presented with 4–5 weeks of auditory hallucinations and was found to have a large left insular lesion (green). Functional MRI activation was seen in variable areas to both Arabic (red) and English (purple) antonym generation tasks. Interestingly, the two languages seem to localize to slightly different areas and are not limited to the traditional Broca and Wernicke areas.
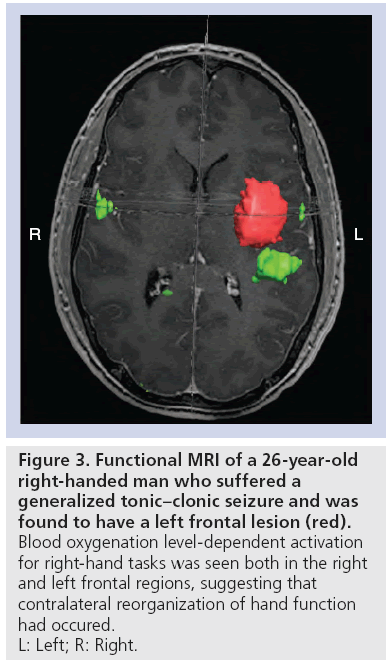
The third type of reorganization involves recruitment of cortical areas ipsilateral to the tumor, but far removed and/or the contralateral homologous cortex [107–109]. This is seen in patients with gliomas in the motor system, with subsequent activation of ‘secondary motor areas’, such as SMA and the superior parietal lobe [8]. Similarly, in patients with low-grade gliomas invading language areas, activation of remote areas not necessarily associated with language functions such as left frontolateral regions, including Brodmann areas 46, 47 and the left putamen have been observed [106,110]. Although the mechanisms may be different, activation of the contralateral hemisphere has been shown in both motor and language functions (Figure 3) [107,111,112].
Figure 3: Functional MRI of a 26-year-old
right-handed man who suffered a
generalized tonic–clonic seizure and was
found to have a left frontal lesion (red).
Blood oxygenation level-dependent activation
for right-hand tasks was seen both in the right
and left frontal regions, suggesting that
contralateral reorganization of hand function
had occured.
L: Left; R: Right.
This third type of reorganization allows for a greater extent of resection, shown to benefit to the patient for both high- and low-grade gliomas [1,90,113]. Importantly, these three reorganization patterns are not mutually exclusive and many patients show varying degrees of these reorganization patterns. Moreover, there appears to be a hierarchical architecture to the different mechanisms of reorganization, with areas close to the lesion being recruited first before ipsilateral, far removed areas, and lastly, contralateral areas [110]. Similarly, these types of reorganization are not uniform and it is clear that a patient-centered approach of studying individual reorganization patterns must be employed [90,97].
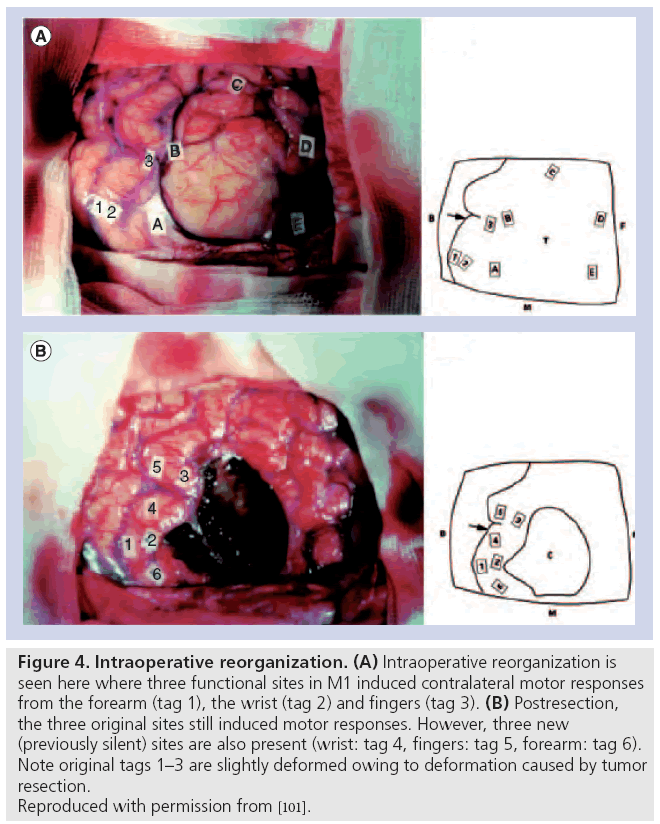
Besides the evidence of preoperative reorganization discussed above, there is important evidence of intraoperative and postoperative patterns of reorganizations after surgical resections of tumor. Intraoperatively, there have been reports of an acute ‘unmasking’ of redundant areas of motor control immediately after resection of gliomas in frontal areas [114,115]. This is seen with stimulation of contralateral motor responses in areas that were previously silent (Figure 4). The unmasking occurs with a time scale of 15–60 min [115]. The proposed mechanism of this acute reorganization involves an increase in cortical excitability, which causes an unmasking of redundant functionality [115]. These findings are reminiscent of the more extensively studied visual cortex and related ocular dominance columns, where early in development there is a redundancy in encoding of these columns, but with experience these topographical maps are trimmed [116].
Figure 4: Intraoperative reorganization. (A) Intraoperative reorganization is seen here where three functional sites in M1 induced contralateral motor responses from the forearm (tag 1), the wrist (tag 2) and fingers (tag 3). (B) Postresection, the three original sites still induced motor responses. However, three new (previously silent) sites are also present (wrist: tag 4, fingers: tag 5, forearm: tag 6). Note original tags 1–3 are slightly deformed owing to deformation caused by tumor resection. Reproduced with permission from [101].
There is also considerable evidence of chronic reorganization, where instead of unmasking of redundant areas of control, there is evidence of contralateral compensation. In the case of tumors located in the insula, it seems that nearby frontal and temporal operculae may take over important language specialization. Taking this into account may affect surgical resection planning. Similarly for tumors located in SMA, total resections are possible, which usually result in a transient ‘SMA syndrome’, which consists of spectrum of symptoms from mutism to decreased spontaneous contralateral movements and an eventual return to neurological baseline in approximately 6 weeks [95]. The return to function seems to be related to the ability of the contralateral SMA to be recruited to help in speech and motor function [117].
Regardless of the time line of reorganization, it may be that certain areas of the brain are necessary and cannot be resected without causing permanent neurological deficit. A retrospective analysis of the brain locations where resections of low-grade gliomas were stopped due to stimulation of eloquent functions using direct cortical and subcortical stimulation, Ius et al. showed that certain areas of the brain were ‘not resectable’. These areas include both cortical and subcortical structures that the authors propose as the ‘minimal common brain’, those regions that do not possess redundant pathways for immediate plasticity [118]. These areas are described in a statistical atlas that could be used to predict areas of residual tumor.
Plasticity of WM tracts
The majority of studies exploring reorganization due to brain tumors have concentrated on functional reorganization using fMRI and ECS. However, there has been a new found interest in WM changes both preoperatively and postoperatively. We have used DTI tractography to help guide surgical planning, similar to the use of fMRI.
One of the inherent difficulties of using DTI imaging to form the basis of tractography is the nonsingular nature of these algorithms. In a head-to-head test of multiple commercially available software algorithms, quite variable results were found in the anatomical accuracy of the fibers predicted in healthy volunteers, as well as the amount of incorrectly placed fibers [51]. Part of this variability is due to software and mathematical algorithms discussed above, but some of the difficulty arises from the interpretation of decreased FA. This could be owing to increased edema due to tumors or infiltration of WM tracts by the tumor itself. In a small retrospective study of 21 patients with metastases, meningiomas, GBMs or low-grade astrocytomas, Yen et al. found that by comparing to contralateral DTI imaging, metastases were more likely to cause edema and disruption of WM tracts, while extra-axial tumors caused displacement of WM tracts. GBM and low-grade gliomas seemed to cause both infiltration and disruption of WM tracts [119].
In the case of strokes, both animal and human studies have shown a timeline of changes in WM tracts after the acute insult. Acutely, there appears to be a loss of FA due to demyelination or axonal loss [120]. Chronically, there seems to be normalization of this decrease in FA, with the extent of this normalization being predictive of clinical improvement [121]. Similarly, for patients suffering from epilepsy, changes in WM tracts have been observed [122]. Interestingly, changes in the arcuate fasiculus, which is the WM tract that joins Broca’s and Wernicke’s areas have been seen both on the right and left side, perhaps signaling the changes in the laterality of these functional areas. More investigation into the WM changes associated with the reorganization of the functional areas as described is needed.
In an attempt to observe postoperative changes in WM tracts, Lazar et al. showed that patients with an improvement in their motor function after surgery had a concomitant improvement in the position and appearance of the corticospinal tract [123]. In contrast to the numerous studies showing reorganization of functional cortical areas both due to tumors and after resections, there is minimal investigation into the WM reorganization that may or may not occur in the face of functional reorganization.
Given this overwhelming evidence of plasticity, especially as it relates to functional areas, a new paradigm in surgical resection involves staged resection of low-grade gliomas. Here, maximum resection of tumor is performed, with care not to resect tumor that infiltrates eloquent areas. After some time (usually 4–5 years), there is both fMRI and ECS evidence that these eloquent area may move, making larger resections possible [124]. This reorganization may be ‘helped’ with intensive rehabilitation, as seen in stroke. A provocative case report suggests that this reorganization process could be shortened considerably through the use of high-frequency electrical stimulation delivered with a chronically implanted subdural grid [125]. Noninvasive measures such as transcranial magnetic stimulation, which has been shown to modulate functional plasticity, may prove a useful technique to help with this functional reorganization. It would allow for greater resections and minimizing neurological deficits.
Conclusion
Advanced imaging techniques such as fMRI and DTI have allowed us greater insight into the functional brain organization of patients with brain tumors. Moreover, studies in these patients have allowed us a unique porthole to investigate such important concepts of brain functionality and plasticity. It is from such studies that new paradigms of management, such as staged resections of low-grade gliomas emerge, as well as insights into different management approaches to patients with other lesions of the brain.
Future perspective
Increasingly, technological and scientific advances will allow brain tumor therapy to be individually tailored to each patient’s special needs. This individualization will be based on biologic characteristics of these brain tumors including their relationship to critical brain structures. Advanced imaging techniques, such as fMRI and DTI, will continue to play a major role in diagnosis, shaping resection planning, the understanding of biological transformation and brain plasticity, and minimizing complications for our patients. Similarly, using insights from our brain tumor patients, new treatment paradigms will emerge for patients with other neurological disorders.
Financial & competing interests disclosure
Funding sources include the National Institute of Neurological Disorder and Stroke, The National Cancer for Research Resources, The National Institute of Biomedical Imaging and Bioengineering of the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Lacroix M, Abi-Said D, Fourney DR et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 95(2), 190–198 (2001).
- Sanai N, Berger MS. Extent of resection influences outcomes for patients with gliomas. Rev. Neurol. (Paris) 167(10), 648–654 (2011).
- Kalkanis SN, Kondziolka D, Gaspar LE et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 96(1), 33–43 (2010).
- Mcgirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 65(3), 463–469; discussion 469–470 (2009).
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J. Neurosurg. 65(4), 476–483 (1986).
- Sawaya R, Hammoud M, Schoppa D et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42(5), 1044–1055; discussion 1055–1046 (1998).
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 358(1), 18–27 (2008).
- Wunderlich G, Knorr U, Herzog H, Kiwit JC, Freund HJ, Seitz RJ. Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery 42(1), 18–26; discussion 26–17 (1998).
- Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR. Functional cortex and subcortical white matter located within gliomas. Neurosurgery 38(4), 678–684; discussion 684–675 (1996).
- Tharin S, Golby A. Functional brain mapping and its applications to neurosurgery. Neurosurgery 60(4 Suppl. 2), 185–201; discussion 201–182 (2007).
- Sanai N, Berger MS. Mapping the horizon: techniques to optimize tumor resection before and during surgery. Clin. Neurosurg. 55, 14–19 (2008).
- Duffau H. The challenge to remove diffuse low-grade gliomas while preserving brain functions. Acta Neurochir. (Wien) 154(4), 569–574 (2012).
- Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics 6(3), 478–486 (2009).
- Picht T, Krieg SM, Sollmann N et al. A Comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery 72(5), 808–819 (2013).
- Ojemann GA. Effect of cortical and subcortical stimulation on human language and verbal memory. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 66, 101–115 (1988).
- Gadian DG. NMR and its Applications to Living Systems (2nd Edition). Oxford University Press, Oxford, UK (1995).
- Al-Okaili RN, Krejza J, Woo JH et al. Intraaxial brain masses: MR imaging-based diagnostic strategy – initial experience. Radiology 243(2), 539–550 (2007).
- Foerster BR, Thurnher MM, Malani PN, Petrou M, Carets-Zumelzu F, Sundgren PC. Intracranial infections: clinical and imaging characteristics. Acta Radiol. 48(8), 875–893 (2007).
- Malmgren K, Thom M. Hippocampal sclerosis – origins and imaging. Epilepsia 53(Suppl. 4), 19–33 (2012).
- Dimou S, Battisti RA, Hermens DF, Lagopoulos J. A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg. Rev. 36(2), 205–214; discussion 214 (2013).
- Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc. Brain Metab. Rev. 7(3), 240–276 (1995).
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl Acad. Sci. USA 83(4), 1140–1144 (1986).
- Ogawa S, Tank DW, Menon R et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl Acad. Sci. USA 89(13), 5951–5955 (1992).
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412(6843), 150–157 (2001).
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat. Neurosci. 3(7), 716–723 (2000).
- Figley CR, Stroman PW. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur. J. Neurosci. 33(4), 577–588 (2011).
- Krings T, Schreckenberger M, Rohde V et al. Metabolic and electrophysiological validation of functional MRI. J. Neurol. Neurosurg. Psychiatry 71(6), 762–771 (2001).
- Sakatani K, Murata Y, Fujiwara N et al. Comparison of blood-oxygen-level-dependent functional magnetic resonance imaging and near-infrared spectroscopy recording during functional brain activation in patients with stroke and brain tumors. J. Biomed. Opt. 12(6), 062110 (2007).
- Holodny AI, Schulder M, Liu WC, Maldjian JA, Kalnin AJ. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. AJNR Am. J. Neuroradiol. 20(4), 609–612 (1999).
- Haller S, Bartsch AJ. Pitfalls in FMRI. Eur. Radiol. 19(11), 2689–2706 (2009).
- Gore JC. Principles and practice of functional MRI of the human brain. J. Clin. Invest. 112(1), 4–9 (2003).
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn. Reson. Med. 25(2), 390–397 (1992).
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage 10(4), 385–396 (1999).
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum. Brain Mapp. 7(2), 106–114 (1999).
- Tie Y, Suarez RO, Whalen S, Radmanesh A, Norton IH, Golby AJ. Comparison of blocked and event-related fMRI designs for presurgical language mapping. Neuroimage 47(Suppl. 2), T107–T115 (2009).
- Breshears JD, Gaona CM, Roland JL et al. Mapping sensorimotor cortex with slow cortical potential resting-state networks while awake and under anesthesia. Neurosurgery 71(2), 305–316 (2012).
- Tie Y, Rigolo L, Norton IH et al. Defining language networks from resting-state fMRI for surgical planning-a feasibility study. Hum. Brain Mapp. doi:10.1002/hbm.22231. (2013) (Epub ahead of print).
- Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl Acad. Sci. USA 98(2), 676–682 (2001).
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34(4), 537–541 (1995).
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8(9), 700–711 (2007).
- Smith SM, Fox PT, Miller KL et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106(31), 13040–13045 (2009).
- Moseley ME, Cohen Y, Kucharczyk J et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 176(2), 439–445 (1990).
- Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 239(3), 632–649 (2006).
- Hunsche S, Moseley ME, Stoeter P, Hedehus M. Diffusion-tensor MR imaging at 1.5 and 3.0 T: initial observations. Radiology 221(2), 550–556 (2001).
- Basser PJ, Mattiello J, Lebihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66(1), 259–267 (1994).
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 44(4), 625–632 (2000).
- Holodny AI, Watts R, Korneinko VN et al. Diffusion tensor tractography of the motor white matter tracts in man: current controversies and future directions. Ann. NY Acad. Sci. 1064, 88–97 (2005).
- Bick AS, Mayer A, Levin N. From research to clinical practice: implementation of functional magnetic imaging and white matter tractography in the clinical environment. J. Neurol. Sci. 312(1–2), 158–165 (2012).
- Roberts TP, Liu F, Kassner A, Mori S, Guha A. Fiber density index correlates with reduced fractional anisotropy in white matter of patients with glioblastoma. AJNR Am. J. Neuroradiol. 26(9), 2183–2186 (2005).
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn. Reson. Med. 51(1), 103–114 (2004).
- Feigl GC, Hiergeist W, Fellner C et al. MRI diffusion tensor tractography: evaluation of anatomical accuracy of different fiber tracking software packages. World Neurosurg. doi:10.1016/j.wneu.2013.01.004. (2013) (Epub ahead of print).
- Qazi AA, Radmanesh A, O’donnell L et al. Resolving crossings in the corticospinal tract by two-tensor streamline tractography: method and clinical assessment using fMRI. Neuroimage 47(Suppl. 2), T98–T106 (2009).
- Kamada K, Todo T, Masutani Y et al. Visualization of the frontotemporal language fibers by tractography combined with functional magnetic resonance imaging and magnetoencephalography. J. Neurosurg. 106(1), 90–98 (2007).
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann. Neurol. 57(1), 8–16 (2005).
- Chen X, Weigel D, Ganslandt O, Buchfelder M, Nimsky C. Prediction of visual field deficits by diffusion tensor imaging in temporal lobe epilepsy surgery. Neuroimage 45(2), 286–297 (2009).
- Kamada K, Todo T, Morita A et al. Functional monitoring for visual pathway using real-time visual evoked potentials and optic-radiation tractography. Neurosurgery 57(Suppl. 1), 121–127; discussion 121–127 (2005).
- Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am.
- J. Neuroradiol. 25(3), 356–369 (2004). 58 Flint JJ, Hansen B, Fey M et al. Cellular-level diffusion tensor microscopy and fiber tracking in mammalian nervous tissue with direct histological correlation. Neuroimage 52(2), 556–561 (2010).
- Cortez-Conradis D, Favila R, Isaac-Olive K, Martinez-Lopez M, Rios C, Roldan-Valadez E. Diagnostic performance of regional DTI-derived tensor metrics in glioblastoma multiforme: simultaneous evaluation of p, q, L, Cl, Cp, Cs, RA, RD, AD, mean diffusivity and fractional anisotropy. Eur. Radiol. 23(4), 1112–1121 (2013).
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 34(1), 51–61 (2008).
- Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury – a review. NMR Biomed. 15(7–8), 561–569 (2002).
- Carano RA, Li F, Irie K et al. Multispectral analysis of the temporal evolution of cerebral ischemia in the rat brain. J. Magn. Reson. Imaging 12(6), 842–858 (2000).
- Spena G, Nava A, Cassini F et al. Preoperative and intraoperative brain mapping for the resection of eloquent-area tumors. A prospective analysis of methodology, correlation, and usefulness based on clinical outcomes. Acta Neurochir. (Wien) 152(11), 1835–1846 (2010).
- Byrnes TJ, Barrick TR, Bell BA, Clark CA. Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed. 24(1), 54–60 (2011).
- Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 232(1), 221–228 (2004).
- Chiang IC, Kuo YT, Lu CY et al. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46(8), 619–627 (2004).
- Wang S, Kim S, Chawla S et al. Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage 44(3), 653–660 (2009).
- Stadlbauer A, Ganslandt O, Buslei R et al. Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 240(3), 803–810 (2006).
- Kinoshita M, Hashimoto N, Goto T et al. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 43(1), 29–35 (2008).
- Chen Y, Shi Y, Song Z. Differences in the architecture of low-grade and high-grade gliomas evaluated using fiber density index and fractional anisotropy. J. Clin. Neurosci. 17(7), 824–829 (2010).
- Stadlbauer A, Polking E, Prante O et al. Detection of tumour invasion into the pyramidal tract in glioma patients with sensorimotor deficits by correlation of (18) F-fluoroethyl-L: tyrosine PET and magnetic resonance diffusion tensor imaging. Acta Neurochir. (Wien) 151(9), 1061–1069 (2009).
- Verhaak RG, Hoadley KA, Purdom E et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1), 98–110 (2010).
- Inoue T, Ogasawara K, Beppu T, Ogawa A, Kabasawa H. Diffusion tensor imaging for preoperative evaluation of tumor grade in gliomas. Clin. Neurol. Neurosurg. 107(3), 174–180 (2005).
- Sinha S, Bastin ME, Whittle IR, Wardlaw JM. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am. J. Neuroradiol. 23(4), 520–527 (2002).
- Shimony JS, Mckinstry RC, Akbudak E et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology 212(3), 770–784 (1999).
- Jakab A, Molnar P, Emri M, Berenyi E. Glioma grade assessment by using histogram analysis of diffusion tensor imaging-derived maps. Neuroradiology 53(7), 483–491 (2011).
- Kang Y, Choi SH, Kim YJ et al. Gliomas: histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging – correlation with tumor grade. Radiology 261(3), 882–890 (2011).
- Theeler BJ, Yung WK, Fuller GN, De Groot JF. Moving toward molecular classification of diffuse gliomas in adults. Neurology 79(18), 1917–1926 (2012).
- Hygino Da Cruz LC Jr, Vieira IG, Domingues RC. Diffusion MR imaging: an important tool in the assessment of brain tumors. Neuroimaging Clin. N. Am. 21(1), 27–49, vii (2011).
- Lee WJ, Choi SH, Park CK et al. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad. Radiol. 19(11), 1353–1361 (2012).
- Smith JS, Cha S, Mayo MC et al. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J. Neurosurg. 103(3), 428–438 (2005).
- Nakamura H, Murakami R, Hirai T, Kitajima M, Yamashita Y. Can MRI-derived factors predict the survival in glioblastoma patients treated with postoperative chemoradiation therapy? Acta Radiologica 54(2), 214–220 (2013).
- Geschwind N. The organization of language and the brain. Science 170(3961), 940–944 (1970).
- Sedat J, Duvernoy H. Anatomical study of the temporal lobe. Correlations with nuclear magnetic resonance. J. Neuroradiol. 17(1), 26–49 (1990).
- Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proc. Natl Acad. Sci. USA 108(39), 16428–16433 (2011).
- Herholz K, Thiel A, Wienhard K et al. Individual functional anatomy of verb generation. Neuroimage 3(3 Pt 1), 185–194 (1996).
- Marshall I, Simonotto E, Deary IJ et al. Repeatability of motor and working-memory tasks in healthy older volunteers: assessment at functional MR imaging. Radiology 233(3), 868–877 (2004).
- Rombouts SA, Barkhof F, Hoogenraad FG, Sprenger M, Scheltens P. Within-subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. Magn. Reson. Imaging 16(2), 105–113 (1998).
- Ojemann GA. Cortical organization of language. J. Neurosci. 11(8), 2281–2287 (1991).
- Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J. Neurosurg. 115(5), 948–965 (2011).
- Walker DG, Kaye AH. Low grade glial neoplasms. J. Clin. Neurosci. 10(1), 1–13 (2003).
- Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 3(3), 159–168 (2004).
- Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer 100(12), 2622–2626 (2004).
- Sanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J. Neurosurg. 112(1), 1–9 (2010).
- Krainik A, Lehericy S, Duffau H et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 57(5), 871–878 (2001).
- Dronkers NF. A new brain region for coordinating speech articulation. Nature 384(6605), 159–161 (1996).
- Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity – a review. J. Neurooncol. 79(1), 77–115 (2006).
- Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J. Neurosurg. 91(2), 238–250 (1999).
- Varona JF. Long-term prognosis of ischemic stroke in young adults. Stroke Res. Treat. 2011, 879817 (2010).
- Duffau H, Capelle L, Denvil D et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J. Neurosurg. 98(4), 764–778 (2003).
- Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 130(Pt 4), 898–914 (2007).
- Carpentier AC, Constable RT, Schlosser MJ et al. Patterns of functional magnetic resonance imaging activation in association with structural lesions in the rolandic region: a classification system. J. Neurosurg. 94(6), 946–954 (2001).
- Duffau H, Capelle L, Denvil D et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J. Neurol. Neurosurg. Psychiatry 74(7), 901–907 (2003).
- 104 Schiffbauer H, Ferrari P, Rowley HA, Berger MS, Roberts TP. Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery 49(6), 1313–1320; discussion 1320–1311 (2001).
- Righini A, De Divitiis O, Prinster A et al. Functional MRI. primary motor cortex localization in patients with brain tumors. J. Comput. Assist. Tomogr. 20(5), 702–708 (1996).
- Meyer PT, Sturz L, Schreckenberger M et al. Preoperative mapping of cortical language areas in adult brain tumour patients using PET and individual non-normalised SPM analyses. Eur. J. Nucl. Med. Mol. Imaging 30(7), 951–960 (2003).
- Baciu M, Le Bas JF, Segebarth C, Benabid AL. Presurgical fMRI evaluation of cerebral reorganization and motor deficit in patients with tumors and vascular malformations. Eur. J. Radiol. 46(2), 139–146 (2003).
- Caramia MD, Telera S, Palmieri MG et al. Ipsilateral motor activation in patients with cerebral gliomas. Neurology 51(1), 196–202 (1998).
- Shinoura N, Suzuki Y, Yamada R, Kodama T, Takahashi M, Yagi K. Restored activation of primary motor area from motor reorganization and improved motor function after brain tumor resection. AJNR Am. J. Neuroradiol. 27(6), 1275–1282 (2006).
- Thiel A, Herholz K, Koyuncu A et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann. Neurol. 50(5), 620–629 (2001).
- Holodny AI, Schulder M, Ybasco A, Liu WC. Translocation of Broca’s area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J. Comput. Assist. Tomogr. 26(6), 941–943 (2002).
- Petrovich NM, Holodny AI, Brennan CW, Gutin PH. Isolated translocation of Wernicke’s area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. AJNR Am. J. Neuroradiol. 25(1), 130–133 (2004).
- Sanai N, Polley MY, Mcdermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 115(1), 3–8 (2011).
- Duffau H, Sichez JP, Lehericy S. Intraoperative unmasking of brain redundant motor sites during resection of a precentral angioma: evidence using direct cortical stimulation. Ann. Neurol. 47(1), 132–135 (2000).
- Duffau H. Acute functional reorganisation of the human motor cortex during resection of central lesions: a study using intraoperative brain mapping. J. Neurol. Neurosurg. Psychiatry 70(4), 506–513 (2001).
- Clothiaux EE, Bear MF, Cooper LN. Synaptic plasticity in visual cortex: comparison of theory with experiment. J. Neurophysiol. 66(5), 1785–1804 (1991).
- Krainik A, Duffau H, Capelle L et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology 62(8), 1323–1332 (2004).
- Ius T, Angelini E, Thiebaut De Schotten M, Mandonnet E, Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a ‘minimal common brain’. Neuroimage 56(3), 992–1000 (2011).
- Yen PS, Teo BT, Chiu CH, Chen SC, Chiu TL, Su CF. White Matter tract involvement in brain tumors: a diffusion tensor imaging analysis. Surg. Neurol. 72(5), 464–469; discussion 469 (2009).
- Liu Y, D’arceuil HE, Westmoreland S et al. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke 38(1), 138–145 (2007).
- Dijkhuizen RM, Van Der Marel K, Otte WM et al. Functional MRI and diffusion tensor imaging of brain reorganization after experimental stroke. Transl. Stroke Res. 3(1), 36–43 (2012).
- Govindan RM, Makki MI, Sundaram SK, Juhasz C, Chugani HT. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res. 80(1), 30–41 (2008).
- Lazar M, Alexander AL, Thottakara PJ, Badie B, Field AS. White matter reorganization after surgical resection of brain tumors and vascular malformations. AJNR Am. J. Neuroradiol. 27(6), 1258–1271 (2006).
- Robles SG, Gatignol P, Lehericy S, Duffau H. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J. Neurosurg. 109(4), 615–624 (2008).
- Barcia JA, Sanz A, Balugo P et al. High-frequency cortical subdural stimulation enhanced plasticity in surgery of a tumor in Broca’s area. Neuroreport 23(5), 304–309 (2012).