Research Article - Diabetes Management (2017) Volume 7, Issue 1
Effectiveness and safety of basal-bolus therapy (insulin glargine+insulin glulisine) in patients with type 1 diabetes previously uncontrolled on any insulin regimen: multinational phase-IV study
- Corresponding Author:
- Charpentier A
Department of Medicine, Sud-Francilien Hospital
Corbeil-Essonnes, France
E-mail: kerbonac@free.fr
Abstract
Introduction: Intensive insulin therapy can improve glycemic control and outcomes in patients with Type 1 diabetes as compared to conventional therapy. This study was conducted to evaluate effectiveness and safety of combination of insulin glargine once daily and insulin glulisine thrice daily in patients with type-1-diabetes mellitus previously uncontrolled on other insulin therapies. Methods: This phase-IV, international, open-label, study was conducted in adult type-1-diabetes mellitus patients with glycated hemoglobin between 8%-10%. Study period included a 2-week run-in period and a 24-week treatment period. Change in glycated hemoglobin, fasting blood glucose, 7-point blood glucose mean profile and insulin dose from baseline to week 12 and 24 were evaluated. Safety was assessed by occurrence of adverse events primarily hypoglycemia. Descriptive statistics were used for analysis. Results: From November 2012 to January 2013, 295 patients screened out of which 206 patients were treated. The mean (SE) reduction in glycated hemoglobin and fasting blood glucose from baseline to week 24 was -0.5 ± 0.1% (p<0.001) and -2.0 ± 0.4 mmol/L (p<0.001), respectively. Overall, 12% and 10% of patients achieved glycated hemoglobin <7% after 12-weeks and 24-weeks of treatment, respectively. The mean change in blood glucose level from baseline to week 12 and week 24 was significant (p<0.001) at all time-points, except before lunch and at bedtime. Serious adverse events were reported for 13 (6%) patients; most of them were severe in intensity. Serious adverse events related to insulin glargine and/or insulin glulisine were hypoglycemic seizures (three patients), hypoglycemic unconsciousness (three patients) and hypoglycemia (two patients). Conclusion: Treatment with insulin glargine and insulin glulisine improved glycemic control in patients with type-1-diabetes mellitus. The combination was well tolerated with no new safety concerns.
Abbreviations
IGlar-insulin glargine, IGlu-insulin glulisine, T1DM-type-1-diabetes mellitus, HbA1c-glycated hemoglobin, BG-blood glucose, SAEs-serious adverse events, IDF-International Diabetes Federation, DCCT-Diabetes Control and Complications Trial, ADA-American Diabetes Association, CSII-continuous subcutaneous insulin infusion, NPH-neutral protamine Hagedorn, IDet-insulin detemir, FBG-fasting blood glucose, 7-point SMBG-7-point selfmonitoring of blood glucose, AEs -adverse events, ALT-alanine aminotransferase, ASTaspartate aminotransferase, TID-thrice daily, mITT-modified intent-to-treat, PP-perprotocol, SD-standard deviation, BMI – body mass index, TEAE-treatment emergent adverse event, ESC/EASD-European Society of Cardiology/European Association for the Study of Diabetes
Keywords
type 1 diabetes mellitus, glycemic control, glargine, glulisine, hypoglycemia
Introduction
According to International Diabetes Federation (IDF) an estimated 387 million people (8.3% of adults) had Diabetes Mellitus (DM) in 2014; and by 2035, 592 million are expected to be inflicted with DM [1]. Although type 1 diabetes mellitus (T1DM) accounts for 5%-10% of diabetes cases, its incidence is increasing by 3% annually [1]. The landmark ‘Diabetes Control and Complications Trial’ (DCCT) shows that compared to conventional therapy, intensive insulin therapy effectively delays the onset and slows the progression of micro- and macrovascular complications and reduces overall mortality in patients with T1DM [2]. However, hypoglycemia and weight gain are the main limiting factors with insulin use in patients with T1DM [3].
In this context, there is a need for insulin regimens that overcome these barriers and achieve optimal glycemic control with low risk of hypoglycemia. Basal-bolus insulin therapy usually involves administration of basal insulin (with a stable 24-h serum insulin profile) and meal-time rapid-acting insulin to cover both fasting and pre-prandial glucose requirements of the patient. However, conventional intermediate and long-acting human basal insulins are limited with a pronounced peak in time-action post injection and large variability in absorption [4]. Regular insulins on the other hand show slower onset and more prolonged action than endogenous insulin secretion. Together, the combination results in high postprandial blood glucose excursions, and is often associated with 2-3 fold increase in severe hypoglycemia [3].
The new insulin analogs, including the long-acting basal analogs and the rapid-acting analogs have been developed to allow for a closer
replication of the physiological pattern of insulin secretion [5]. Subcutaneous administration of these analogs effectively controls fasting and post-prandial glucose levels to achieve target glycated hemoglobin (HbA1c) levels [6] with lower risk of hypoglycemia [7]. In T1DM patients, the American Diabetes Association (ADA) recommends continuous subcutaneous insulin infusion (CSII) therapy or the use of multiple daily insulin injections (3–4 injections of basal and prandial insulin/day), preferably with insulin analogs (especially in patients prone to hypoglycemia), where prandial insulin would match the carbohydrate intake, pre-meal blood glucose, and anticipated activity [6]. Currently available long-acting insulin analogs (glargine, detemir, degludec) provide smooth, relatively flat, 24-hour basal insulin supply, with lesser variability in action and more superior basal and fasting glucose control (thereby reducing the risk of hypoglycemia) than neutral protamine Hagedorn (NPH) insulin [8]. Rapid-acting insulin analogs (aspart, glulisine, lispro) with faster absorption profile and shorter duration of action show better post-prandial glycemic control than regular human insulin and contribute to lower nocturnal hypoglycemia [5].
Amongst long-acting insulin analogs, intensive treatment with insulin glargine (IGlar) is found to be superior to intermediate-acting NPH in T1DM patients, with significant reduction in HbA1c and frequency of hypoglycemic events [9]. While long-acting insulin analogs, IGlar and insulin detemir (IDet) show similar pharmacokinetic and pharmacodynamic effects during the first 12 h of administration, IGlar shows superior effects extending up to 24 h [10]. Additionally, once daily (OD) IGlar achieves similar glycemic control and comparable risk of hypoglycemia to that of twice daily IDet, each in combination with pre-meal insulin aspart [11]. Further, patients with diabetes, inadequately controlled by premixed insulin, switching from premixed insulin to IGlar based regimen experience significant improvement in glycemic control, supporting the use of basal-bolus glargine-based regimen in these patients [12]. Studies on combination of IGlar OD and multiple mealtime rapid-acting insulins, as part of basal-bolus therapy in T1DM patients demonstrate improved overall glycemic control and reduced nocturnal hypoglycemia [13]. However the appropriate rapid-acting insulin analog for combination with IGlar that provides effective glycemic control and low risk of hypoglycemia in T1DM patients is not established [14]. In few open-label studies, insulin glulisine (IGlu) and insulin lispro, both in combination with IGlar show similar reduction in HbA1c but with a lower total daily dose of IGlu [15-17]. Further, replacement of bolus insulin with IGlu in T1DM patients uncontrolled on intensive therapy with (basal) IGlar+(bolus) aspart/lispro/ regular human insulin, demonstrates improved glycemic control for 24 weeks [14]. However studies that explore the effectiveness and safety of multiple injections of IGlu and once daily IGlar are scarce.
Therefore this study was primarily conducted to evaluate the effectiveness of a combination of a single dose of IGlar and three doses of IGlu in terms of change in HbA1c level in patients with T1DM. The secondary objectives were to determine the change in HbA1c from baseline to week 12; the percentage of patients with HbA1c<7% at week 12 and week 24; the fasting blood glucose (FBG) and the 7-point selfmonitoring of blood glucose (7-point SMBG) at baseline, week 12 and week 24; the daily dose for both IGlu and IGlar at baseline, week 12 and week 24; and the incidence of symptomatic hypoglycemia and other adverse events (AEs).
Methodology
▪ Study design
This international, multicenter, open-label, non-comparative, phase-IV study in T1DM patients was conducted at 36 sites across 10 countries (Algeria, Argentina, Brazil, Columbia, Iran, Kuwait, Mexico, Saudi Arabia, South Africa, Tunisia) from November 2012 to January 2013. The study was conducted in compliance with Good Clinical Practices, the principles of Declaration of Helsinki, and in accordance with the local regulations of institutional review board /institutional ethical committee. Written, signed informed consent was obtained from each patient enrolled in the study (NCT01204593).
▪ Study population
Adult patients (18-60 years) with T1DM treated with any type of insulin regimen (other than CSII or pump and/or IGlar) for at least 1 year and having HbA1c levels between 8% to 10% assessed over the preceding 6 months were included in the study. Patients with alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) >3 times the upper limit of normal and serum creatinine >135 μmol/L in men and >110 μmol/L in women; history of hypersensitivity to IGlar and/or IGlu; brittle diabetes; impaired renal and hepatic function; episodes of diabetes ketoacidosis within 6 months prior to study entry; and pregnancy and lactation were excluded from the study.
▪ Data collection and dosing schedule
The study duration per patient was 26 weeks, including a 2-week run in-period followed by a 24-week treatment period during which the patients received IGlar and IGlu subcutaneously. For each patient after screening visit (week 2), three on-site visits at week 0 (baseline visit), week 12 and week 24 and three phone calls at week 2, week 4 and week 8 were scheduled to monitor treatment compliance. At the start of the study treatment period (week 0), eligible patients were switched from their current anti-diabetic treatment to IGlar (Lantus®, OD at bedtime) and IGlu (Apidra®, thrice daily [TID] preferably before meals) subcutaneously. The doses of IGlar and IGlu were individually titrated once a week to achieve FBG levels (between 80 and 120 mg/ dL) and 2-hour postprandial blood glucose levels (<180 mg/dL), respectively. During the study, the participants maintained a patient diary which included entries on daily blood glucose (BG) measurements, insulin doses and time of injection, BG values from each time point of 7-point SMBG profile, and information on symptomatic hypoglycemia.
▪ Statistical analysis
All the enrolled patients treated with at least one dose of study medications were defined as safety population. All patients who had satisfied the inclusion and exclusion criteria and who received at least one dose of study medication, with one HbA1c measurement at week 0 and at least one post-baseline HbA1c measurement were defined under modified intent-to-treat (mITT); while all patients under mITT population with one HbA1c measurement at week 0 and at week 24, and no important deviations were defined as perprotocol (PP) population.
The sample size was determined with the SAS 9.1 (SAS Institute, Cary, NC, USA) and confirmed with the NQuery software. For a paired t-test with a 0.05 one-sided significance level that had 80% power to reject the hypothesis H0 (HbA1c at 26weeks-HbA1c at baseline ≤0%), assuming a mean decrease of 0.40% in HbA1c and a standard deviation (SD) of differences of 2.0%, 156 patients were needed. The total sample size required was 195 patients, taking in to account a drop-out rate of 20%. Therefore a rounded sample size of 200 patients was proposed for this study.
Data were summarized using number of observations available and missing data, mean, SD, median, 25% and 75% percentiles, minimum and maximum for continuous parameters and counts and percentages for categorical parameters. If relevant, 2-sided 95% CI of the proportion was added using the Wilson score method for categorical data [18]. Descriptive statistics of effectiveness and safety parameters by visit were provided for per-protocol (PP) population while sensitivity analyses were conducted in the mITT population.
Results
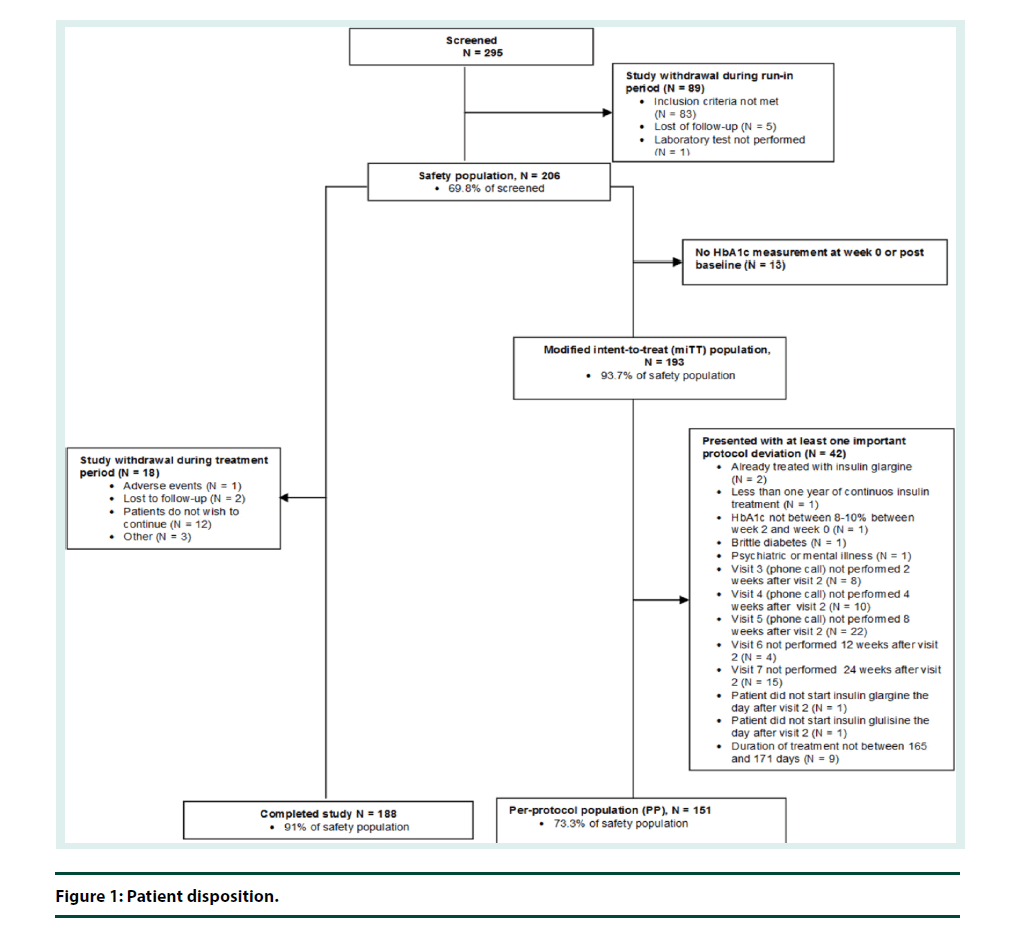
▪ Patient disposition
Out of the 295 patients screened, 69.8% (N=206) of patients were included in the safety population. Eighty nine (30.2%) were excluded for various reasons during run-in period. In the safety population, 91.3% (N=188) of patients completed the study, 93.7% (N=193) were included in the mITT population, and 73.3% (N=151) were included in the PP population (Figure 1).
▪ Patient demographics
Baseline characteristics were comparable for the safety, mITT and PP population. Patient demographic characteristics for the safety and mITT population are presented in Table 1. Majority of the patients were men (125/206; 60.7%) with mean ± SD age of 31.7 ± 10.1 years and a mean body-mass index (BMI) of 24.4 ± 4.0 kg/m2. The median (range) time since diagnosis of T1DM was 11 (1.0; 42.0) years, and time since initiation of insulin therapy was 11 (8.5 months; 42.2 years) years. Median (range) duration of current insulin therapy was 3 (<1 month; 42 years) years. The common associated diseases in T1DM patients were dyslipidemia (17.0%; 35/206), hypertension (12.1%; 25/206), and hypothyroidism (6.3%; 13/206) patients. The most frequent late diabetic complication was retinopathy (12.6%; 26/206) followed by neuropathy (5.3%; 11/206).
| Baseline Characteristics | Safety population (N=206) |
mITT population (N=193) |
||
|---|---|---|---|---|
| Mean (SD) | Median (range) | Mean (SD) | Median (range) | |
| Age (years) | 31.7 (10.1) | 30.5 (17.0; 63.0) | 31.7 (10.0) | 30.0 (17.0; 63.0) |
| Male, n (%) | 125 (60.7) | - | 116 (60.1) | - |
| Height (cm) | 167.9 (9.4) | 169.0 (136.0; 188.0) | 168.0 (9.6) | 169.0 (136.0; 188.0) |
| Weight (kg) | 68.9 (12.7) | 67.1 (34.0; 108.5) | 69.0 (12.9) | 67.1 (34.0; 108.5) |
| BMI calculated (kg/m2) | 24.4 (4.0) | 24.0 (14.5; 41.0) | 24.4 (4.0) | 24.1 (14.5; 41.0) |
| Laboratory Data | ||||
| HbA1c (%) | 9.0 (0.6) | 8.9 (7.3; 10.0) | 9.0 (0.6) | 8.9 (7.3; 10.0) |
| FBG (mmol/L) | 10.2 (5.3) | 9.3 (0.1; 23.9) | 10.3 (5.4) | 9.4 (0.1; 23.9) |
| Daily mean of 7-point Blood Glucose (mmol/L) | 10.1 (2.7) | 9.6 (5.7; 20.5) | 10.1 (2.7) | 9.6 (5.7; 20.5) |
| AST (U/L) | 21.1 (8.7) | 18.5 (10.0; 67.0) | 21.1 (8.8) | 18.0 (10.0; 67.0) |
| ALT (U/L) | 22.4 (13.7) | 18.0 (6.0; 97.0) | 22.3 (13.6) | 18.00 (6.0; 97.0) |
| Serum Creatinine (μmol/L) | 74.5 (16.4) | 73.4 (36.2; 141.4) | 74.9 (16.7) | 74.0 (36.2; 141.4) |
| Diabetes history | ||||
| Duration of diabetes at screening (years) | 12.7 (8.7) | 11.0 (1.0; 42.0) | 12.7 (8.8) | 11.0 (1.0; 42.0) |
| Time since insulin therapy at screening (months) | 151.0 (104.7) | 127.5 (8.5; 506.0) | 150.3 (105.6) | 127.6 (8.5; 506.0) |
| Previous treatment history | ||||
| Patient treated with basal insulin, n (%) | 186 (90.3) | - | 174 (90.2) | - |
| Daily dose (U) | 38.4 (17.2) | 35.5 (8.0; 108.0) | 38.7 (17.2) | 36.0 (8.0; 108.0) |
| Patient treated with prandial insulin, n (%) | 170 (82.5) | 159 (82.4) | ||
| Daily dose (U) | 24.0 (16.3) | 20.0 (2.0; 108.0) | 23.5 (15.5) | 20.0 (2.0; 108.0) |
| Patient treated with premix insulin, n (%) | 20 (9.7) | - | 19 (9.8) | - |
| Daily dose (U) | 53.2 (12.6) | 51.0 (38.0; 78.0) | 53.1 (13.0) | 50.0 (38.0; 78.0) |
Abbreviations: mITT: modified intent to treat; SD: Standard Deviation; BMI: Body mass index; HbA1c: glycated hemoglobin; FBG: Fasting blood glucose; AST: Aspartate Transaminase; ALT: Alanine Transaminase
Table 1. Patient demographics characteristics.
Previous insulin therapies included basal insulin (mainly NPH insulin) in 90.3% (186/206) patients, prandial insulin (mainly regular insulin) in 82.5% (170/206) patients, and premix insulin in 9.7% (20/206) patients. Among combination insulin therapies, majority (80.1%; 165/206) of the patients were on basal+prandial insulin. The most frequently reported concomitant medications during the treatment period included medications for the cardiovascular system (57/206; 27.7%), alimentary tract and metabolism (54/206; 26.2%) and nervous system (43/206; 20.9%).
▪ Treatment dosage
In the safety population, median (range) duration of exposure to IGlar and IGlu was 24 weeks (<1 week; 31 weeks). Median daily dose of IGlar was 28 U at first administration and at week 2; 26 U at week 4, week 8 and week 12; and 27.5 U at week 24. The median daily dose per kg was 0.4 U/kg at first administration, week 12 and week 24. Median daily dose of IGlu was 20 U at first administration, 21 U at week 2, 23 U at week 4, 24 U at week 8, week 12 and week 24. The median daily dose per kg was 0.3 U/kg at first administration and 0.4 U/kg at week 12 and week 24 (Supplementary Table 1).
▪ Effectiveness evaluation
Effectiveness parameters were assessed in the PP population and the results are summarized in Table 2. There was a significant reduction in the HbA1c levels from 9.0% ± 0.6% at baseline to 8.5% ± 1.3% at week 24 (Supplementary Figure 1). The adjusted mean (SE) change of HbA1c was -0.5% ± 0.1% from baseline to week 24 (95% CI: -0.7%;-0.2%, p<0.001). The reduction of HbA1c was also significant at week 12; from 9.0% ± 0.6% at baseline to 8.3% ± 1.2% at week 12 (p<0.001). In the PP population, HbA1c value <7% was achieved in 18 (12%) patients (95% CI: 7.8%; 18.3%) at week 12 and 14 (10%) patients (95% CI: 5.7%; 15.3%) at week 24. Similar results were obtained in the mITT population and in the sensitivity mITT population.
| N | Mean (SD) | Adjusted Mean (SE) change from baseline | Upper limit of 2-sided 95% CI | p-value | |
|---|---|---|---|---|---|
| HbA1c value (%), | |||||
| Baseline | 151 | 9.0 (0.6) | - | - | - |
| Week 12 | 149 | 8.3 (1.2) | -0.7 (0.1) | -0.5 | <0.001 |
| Week 24 | 148 | 8.5 (1.3) | -0.5 (0.1) | -0.2 | <0.001 |
| FBG (mmol/L) | |||||
| Baseline | 151 | 10.3 (5.6) | - | - | - |
| Week 12 | 148 | 8.3 (4.4) | -2.0 (0.4) | -1.3 | <0.001 |
| Week 24 | 148 | 8.3 (4.4) | -2.0 (0.4) | -1.3 | <0.001 |
| Daily mean BG from 7-point profile (mmol/L) | |||||
| Baseline | 143 | 10.1 (2.8) | - | - | - |
| Week 12 | 143 | 9.0 (2.3) | -1.2 (0.2) | -0.9 | <0.001 |
| Week 24 | 136 | 8.7 (1.8) | -1.4 (0.2) | -1.1 | <0.001 |
Table 2. Change in HbA1c, FBG and daily mean BG from baseline to week 12 and 24PP population (N=151).
The FBG levels decreased from 10.3 ± 5.6 mmol/L at baseline to 8.3 ± 4.4 mmol/L at week 12 and week 24 (Supplementary Figure 2). The adjusted mean (SE) change in FBG was -2.0 ± 0.4 mmol/L from baseline to week 12 (95% CI: -3.1; -1.0, p<0.001) and -2.0 ± 0.4 mmol/L from baseline to week 24 (95% CI: -3.1;-0.9, p<0.001) (Table 2). Daily mean BG from 7-point profile decreased from 10.2 ± 2.8 mmol/L at baseline to 9.0 ± 2.3 mmol/L at week 12 and to 8.7 ± 1.8 mmol/L at week 24. The adjusted mean (SE) change in daily mean BG was -1.2 ± 0.2 mmol/L from baseline to week 12 (95% CI: -1.8; -0.7, p<0.001) and -1.4 ± 0.2 mmol/L from baseline to week 24 (95% CI: -2.0; -0.9, p<0.001) (Table 2).
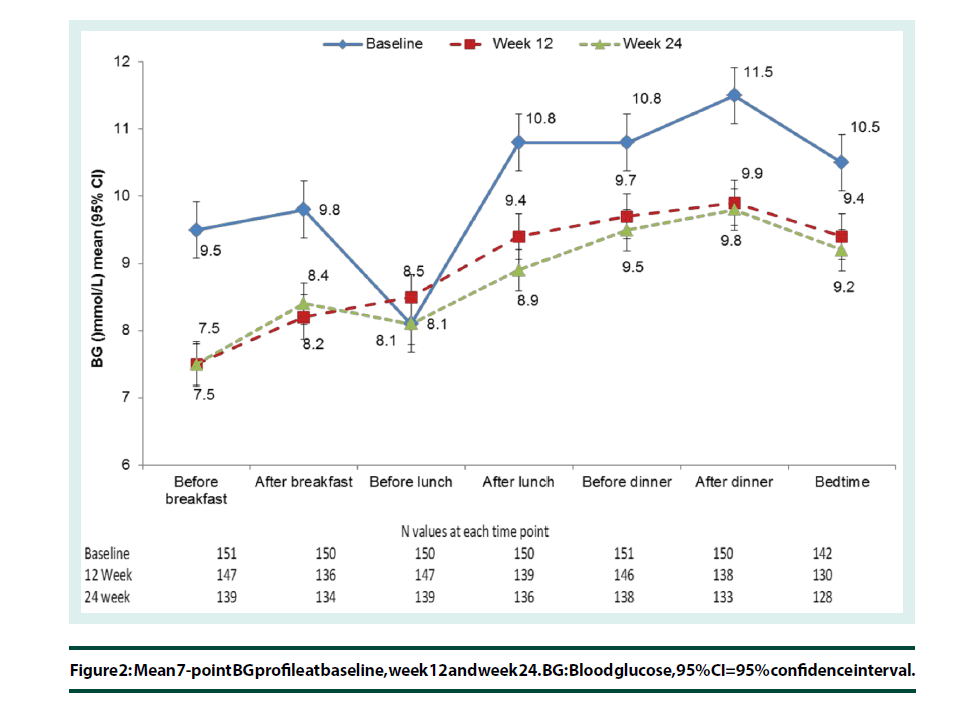
In the PP population, the 7-point BG mean profile decreased at all time-points between baseline and week 12 except before lunch, when the mean BG increased by 0.4 ± 4.3 mmol/L from baseline. A mean decrease in BG (between -1.9 and -1.1 mmol/L) was observed at all other time points. The mean change in BG level from baseline to week 12 and week 24 was statistically significant at all time-points, except before lunch and at bedtime (p=0.271 and p=0.083, respectively) at week 12 and before lunch (p=0.963) at week 24 (Figure 2).
A secondary analysis of the effectiveness parameters done on the basis of the center (country) showed significant differences between countries for all parameters except for daily mean BG from 7-point profile. This could be due to small sample size in some countries (data not provided). Effectiveness endpoint results in the mITT and sensitive mITT population were found to be similar to the PP population (data not provided).
▪ Safety evaluation
Table 3 represents the data of observed hypoglycemia confirmed by BG value before and during the treatment period. The total hypoglycemic episodes within the month before the screening were 86 (41.7%). Higher proportion of patients during the treatment than before treatment reported at least one episode of symptomatic hypoglycemia (68.4% vs. 45.1%), severe symptomatic hypoglycemia (12.2% vs. 2.4%), nocturnal symptomatic episodes (35.3% vs.14.6%), and severe nocturnal symptomatic hypoglycemia (5.4% vs.1.0%) in the safety population (Table 3).
| Patients with at least one episode of | Before treatment | During treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | <70 mg/dL (3.9 mol/L) |
<50 mg/dL (2.8 mmol/L) |
<36 mg/dL (2.0 mol/L) |
Overall | <70 mg/dL (3.9 mol/L) |
<50 mg/dL (2.8 mmol/L) |
<36 mg/dL (2.0 mol/L) |
|
| Symptomatic hypoglycemia | 93 (45.1) | 91 (44.6) | 32 (16.0) | 3 (1.5) | 141 (68.4) | 135 (66.8) | 93 (46.5) | 20 (10.4) |
| MD | 0 | 2 | 6 | 8 | 0 | 4 | 6 | 14 |
| Severe symptomatic hypoglycemia |
5 (2.4) | 5 (2.4) | 4 (1.9) | 2 (1.0) | 25 (12.2) | 23 (11.3) | 18 (8.9) | 10 (5.0) |
| MD | 0 | 0 | 0 | 0 | 1 | 3 | 4 | 4 |
| Nocturnal symptomatic hypoglycemia | 30 (14.6) | 27 (13.4) | 7(3.5) | 1(0.5) | 72 (35.3) | 70 (34.7) | 34 (17.0) | 6 (3.0) |
| MD | 1 | 4 | 5 | 5 | 2 | 4 | 6 | 8 |
| Severe nocturnal symptomatic hypoglycemia | 2(1.0) | 2 (1.0) | 2(1.0) | 1 (0.5) | 11 (5.4) | 9 (4.5) | 7 (3.4) | 4 (2.0) |
| MD | 0 | 0 | 0 | 0 | 2 | 4 | 3 | 3 |
Asymptomatic hypoglycemia: measured blood glucose (BG) level ≤70 mg/dL (3.9 mmol/L) not associated with clinical symptoms.; Symptomatic hypoglycemia: an event with clinical symptoms that were considered to result from hypoglycemia (BG measurement ≤70 mg/dL [3.9 mmol/L]); Severe symptomatic hypoglycemia: an event with clinical symptoms that were considered to result from hypoglycemia in which the patient required the assistance of another person because the patient could not treat her/himself due to acute neurological impairment directly resulting from the hypoglycemia (assistance by another person when the patient could have treated her/himself is not considered as requiring assistance) and one of the following criteria: an event associated with a measured BG level <36 mg/dL (2 mmol/L), or an event associated with prompt recovery after oral CHO, intravenous glucose or glucagon administration.
Table 3. Symptomatic hypoglycemia before and during treatment period in the safety population (N=206).
During the treatment period, 64 patients (31%) experienced at least one treatment emergent adverse event (TEAE) mainly due to infections (15.5%), investigations (5.8%), and nervous system disorders (5.3%) (Table 4). The most frequently reported non-serious TEAEs were: influenza (17/206; 8.3%), weight increase (5/206; 2.4%), weight decrease (5/206; 2.4%), sinusitis (4/206; 1.9%), and headache (4/206; 1.9%).
| Safety population (N=206) | |
|---|---|
| Any TEAE | 64 (31.1) |
| Any TEAE possibly related to insulin glargine | 11 (5.3) |
| Any TEAE possibly related to insulin glulisine | 10 (4.9) |
| Any TEAE leading to death | 0 |
| Any TEAE leading to permanent discontinuation of treatment | 1 (0.5) |
| TEAEs by SOC (frequency ≥5%) | |
| Infections and infestations | 32 (15.5) |
| Investigations | 12 (5.8) |
| Nervous system disorders | 11 (5.3) |
| Most common non-serious TEAEs (frequency>1%) | |
| Any class | 38 (18.4) |
| Infections and infestations | |
| Influenza | 17 (8.3) |
| Sinusitis | 4 (1.9) |
| Pharyngitis | 3 (1.5) |
| Upper respiratory tract infection | 3 (1.5) |
| Investigations | |
| Weight increased | 5 (2.4) |
| Weight decreased | 5 (2.4) |
| Nervous system disorders | |
| Headache | 4 (1.9) |
| Gastrointestinal disorders | |
| Gastritis | 3 (1.5) |
| Respiratory, thoracic and mediastinal disorders | |
| Rhinitis allergic | 3 (1.5) |
TEAEs: Treatment emergent adverse events; SOC: System organ classification; SAEs: Serious adverse events
Table 4. TEAEs in the safety population.
Serious adverse events (SAEs) were reported for 13 (6%) patients; most of them were severe in intensity. SAEs related to IGlar and/or IGlu were hypoglycemic seizures (three patients), hypoglycemic unconsciousness (three patients) and hypoglycemia (two patients). One SAE (hypoglycemic seizure) led to permanent discontinuation of the study treatment. No deaths were reported during the study. Overall, 11 patients (5%) experienced TEAEs possibly related to IGlar. In addition to the SAEs described above, non-serious AEs possibly related to IGlar were headache, inadequate control of diabetes mellitus, weight fluctuation (one patient each) and weight increase (two patients). TEAEs possibly related to IGlu were reported for 10 patients. Majorly TEAEs for both drugs were similar with one patient with weight increase and one patient with inadequate control of diabetes mellitus more in the IGlar group.
Overall, serum creatinine, blood pressure and heart rate remained stable during the study. The weight and BMI showed a slight increase with a mean ± SD change of 0.7 ± 3.0 kg and 0.3 ± 1.1 kg/m², respectively. The change in weight during the treatment period was reported as TEAEs for 10 patients: five patients each had a significant (>5%) increase and decrease in the weight. The weight increase was considered to be related to the study treatment in two patients.
Discussion
This 26-week international, open label, non-comparative study investigating the effectiveness and safety of basal IGlar (OD) and pre-prandial IGlu (TID) in uncontrolled T1DM patients demonstrates significant reduction in HbA1c, FBG, daily mean BG and most of the BG value of 7-point SMBG profile (excluding BG level before lunch) after 12 and 24 weeks of treatment. Overall, 12% and 10% of the patients achieved HbA1c level <7% after 12 and 24 weeks of treatment, respectively. During the study period, the median daily dose of IGlar changed minimally, while that of IGlu increased by 15%. The combination was well tolerated in T1DM patients with no new safety concerns during the study.
In our study population, median daily dose of previous basal insulin therapy was 35.5 U. On switching, IGlar was initiated at a median daily dose of 28 U. This decrease in basal dose is in accordance with Lantus® prescribing information, which recommends a 20%-30% reduction in IGlar dose in patients switching from twice daily NPH insulin to once daily regimen with IGlar to reduce the risk of nocturnal and early morning hypoglycemia [18]. Similar findings were observed in previous studies supporting basal dose reduction in patients changing from NPH to IGlar [19,20]. The relatively flat profile of action of IGlar that extends over a 24-hour period compared to distinct peak of action of NPH may also explain this dose reduction during switching [21].
Achieving good glycemic control early in the course of diabetes delays the onset and slows the progression of diabetes related complications. Evidence from DCCT clearly indicates that tight glycemic control greatly reduces the development and progression of microvascular complications in patients with T1DM [2] Accordingly several guidelines including the ADA and European Society of Cardiology/European Association for the Study of Diabetes (ESC/EASD) recommend intensive glycemic control with basal-bolus regimen for the management of T1DM [6,22]. The results of the current study are consistent with previously reported studies on T1DM patients treated with IGlar-based regimens, where it has been associated with better glycemic control than other traditional basal insulins with a significantly lower rate of overall and nocturnal hypoglycemia [20,23-26]. In randomized clinical trials, IGlar appears to improve glycemic control in terms of HbA1c and FBG reduction compared to NPH insulin, with added advantage of lower risk of hypoglycemia and weight gain [19,20,23,27-30] Similarly in cross-over trials comparing IGlar to NPH, IGlar was associated with a mean decrease in HbA1c ranging from -0.5% [13] to -0.7% [31]. When compared to detemir, IGlar provides similar glycemic control with a mean decrease in HbA1c of -0.5% after 26 weeks [11] which is maintained up to one year [28,32].
In the current study, sub-optimal glycemic control was evident from higher study-end HbA1c and FBG values compared to recommended targets for good glycemic control. Despite significant decrease in HbA1c from baseline, the mean ± SD HbA1c after 24 weeks of treatment was 8.5% ± 1.3%, which is well above the target specified by international guidelines (<7%). Similarly, though a significant (p<0.001) reduction in FBG was achieved as early as 12 weeks, the study-end FBG levels (8.3 ± 4.4 mmol/L) were considerably higher than desired targets to be achieved (4.5 to 6.7 mmol/L). Appropriate insulin dose titration is crucial in achieving good glycemic control in T1DM patients [33,34]. However in the current study, a flat median daily dose of IGlar was observed despite higher FBG values suggesting sub-optimal dose titration which may explain the reason for inadequate glycemic control. It can be speculated that the observed high baseline hypoglycemia values in the study population might have influenced the investigator’s decision in terms of intensive titration of IGlar for tight glucose control. Further, majority of patients in the current study presented with high baseline HbA1c (9.0% ± 0.6%) and long duration of diabetes (11 years). Evidence suggests that T1DM patients with disease duration ≥5 years are 3 times more vulnerable to poor control than those with short duration (OR, 3.0; P=0.000) [35]. Considering the relative contributions of FBG and postprandial BG to overall hyperglycemia, FBG is the predominant contributor in patients with high HbA1c levels. Therefore, sub-optimal insulin dose titrations together with high baseline HbA1c levels and long duration of diabetes observed in the current study may have contributed to poor glycemic control in this patient population. The mean BG value before lunch in 7-point profile observed in the current study was already low at baseline and therefore a reduction from baseline to week 24 was lower at this time point. Similar trend was reported in studies involving patients treated with basal-bolus regimens [11,28-30,32].
Frequent and severe hypoglycemia often hinders the goal of tight glucose control in T1DM patients, with intensively treated patients at a three-fold higher risk of hypoglycemia [6]. In this study, 68.4% of the patients experienced at least one symptomatic episode of hypoglycemia during the treatment period, which was higher than that observed before initiation of study treatment (45.1%). One possible reason for this difference in the pre and post intervention incidence of hypoglycemia may be explained by retrospective collection of data prior to intervention followed by prospective data collection during the study period. This might have led to under reporting of hypoglycemic episodes before the intervention was initiated. While the pharmacokinetic profiles of IGlar and IGlu are beneficial in reducing the risk of hypoglycemia, strategies such as regular SMBG, diabetes self-management, and patient education can be employed for its prevention [36].
Weight gain as a result of insulin therapy or intensification of insulin therapy is commonly seen in T1DM. In the present study, the change in weight reported as non-serious TEAE was noted for five patients who had a significant (more than 5%) weight increase and for another five patients who had a significant weight decrease. The mean (± SD) increase in weight (0.7 ± 3.0 kg) observed during this study is in line with findings of previous studies including similar target population, where mean increase of 0.1 kg to 1.0 kg was reported [11,19,27,28,32,37]. In fact evidence suggests that IGlar is associated with less weight gain than NPH insulin in patients with T1DM [19,25]. In a study involving 196 T1DM patients, the mean weight gain from baseline was significantly higher with NPH insulin (1.4 ± 1.8 kg, p=0.004) than with IGlar (no significant weight gain, p=0.4). Similar results were observed in a 16-week trial where weight gain was greater with NPH insulin than with IGlar (0.54 kg vs. –0.12 kg respectively, p=0.034) [25].
Limitations and conclusions
Some limitations of the current study are to be considered when interpreting its results. Firstly, this was an open label, non-comparative study conducted in a limited number of patients. There was a significant country effect indicating differences in FBG and HbA1c between countries; hence, the results are to be interpreted with caution due to small sample size in some countries. Furthermore, inherent attributes of the study, like the heterogeneity of the population, varied socio-economic levels, and impact of adjustment of prandial insulin bolus and carbohydrate intake could also have contributed to the observed titration difficulties and relative frequency of hypoglycemia.
Thus, in conclusion, treatment with IGlar (OD) and IGlu (TID) basal-bolus therapy improved glycemic control in T1DM patients uncontrolled on other insulin regimens. The combination was well tolerated with no specific safety concerns raised during the study. Although, the improvements noted in the current study are of clinical interest, we believe that further studies are warranted to study appropriate dose titration algorithms for insulin (basal) to elucidate the ability of the regimen in achieving optimum glycemic control in T1DM patients.
Acknowledgements
The SUBSTITUTE study was supported by Sanofi. The authors thank all the SUBSTITUTE study investigators for their contribution. The authors also acknowledge writing and editing assistance in developing this manuscript by Jeevan Scientific Technology Limited (Hyderabad, India), funded by Sanofi, and Anahita Gouri and Dr. Alina Gomes from Sanofi (India).
Conflict of interest
A. Boudiba has a scientific collaboration with Sanofi, he however has no conflict of interest with the writing of this paper. M. Al-Arouj is a member of expert committee in Sanofi, MSD, and Novartis. JM. Chantelot is employed by Sanofi. G. Charpentier was the scientific expert for the SUBSTITUTE study and received fees from Sanofi. K.A. Al-Rubeaan and L.I. Robertson have no conflict of interest to declare.
Funding
The study was sponsored by Sanofi.
References
- http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf
- The Diabetes Control and Complications Trials (DCCT), Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 329, 977–986 (1993).
- Cryer PE. Hypoglycemia risk reduction in type 1 diabetes. Exp. Clin. Endocrinol. Diabet. 109(Suppl 2), S412–S423 (2001).
- Barnett AH, Owens DR. Insulin analogues. Lancet. 349, 47–51 (1997).
- Hartman I. Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin. Med. Res. 6, 54–67 (2008).
- American Diabetes Association (ADA). Standards of medical care in diabetes–2014. Diabetes Care. 37(Suppl 1), S14–S80 (2014).
- Ulrich H, Snyder B, Garg SK. Combining insulins for optimal blood glucose control in type I and 2 diabetes: focus on insulin glulisine. Vasc. Health. Risk. Manag. 3, 245–254 (2007).
- Dardano A, Bianchi C, Del Prato S et al. Insulin degludec/insulin aspart combination for the treatment of type 1 and type 2 diabetes. Vasc. Health. Risk. Manag. 10, 465–475 (2014).
- Porcellati F, Rossetti P, Pampanelli S et al. Better long-term glycaemic control with the basal insulin glargine as compared with NPH in patients with Type 1 diabetes mellitus given meal-time lispro insulin. Diabet. Med. 21, 1213–1220 (2004).
- Porcellati F, Rossetti P, Busciantella NR et al. Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: a double-blind, randomized, crossover study. Diabetes. Care. 30, 2447–2452 (2007).
- Pieber TR, Treichel HC, Hompesch B et al. Comparison of insulin detemir and insulin glargine in subjects with Type 1 diabetes using intensive insulin therapy. Diabet. Med. 24, 635–642 (2007).
- Sharplin P, Gordon J, Peters JR et al. Switching from premixed insulin to glargine-based insulin regimen improves glycaemic control in patients with type 1 or type 2 diabetes: a retrospective primary-care-based analysis. Cardiovasc. Diabetol. 8, 9 (2009).
- Ashwell SG, Amiel SA, Bilous RW et al. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with Type 1 diabetes. Diabet. Med. 23, 285–292 (2006).
- Yanagisawa K, Ashihara J, Obara S et al. Switching to multiple daily injection therapy with glulisine improves glycaemic control, vascular damage and treatment satisfaction in basal insulin glargine-injected diabetic patients. Diabetes. Metab. Res. Rev. 30, 693–700 (2014).
- Dreyer M, Prager R, Robinson A et al. Efficacy and safety of insulin glulisine in patients with type 1 diabetes. Horm. Metab. Res. 37, 702–707 (2005).
- Kawamori R, Kadowaki T, Ishii H, Iwasaki M, Iwamoto Y. Efficacy and safety of insulin glulisine in Japanese patients with type 1 diabetes mellitus. Diabetes. Obes. Metab. 11, 891–899 (2009).
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17, 857–872 (1998).
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000284/WC500036082.pdf
- Garg SK, Paul JM, Karsten JI et al. Reduced severe hypoglycemia with insulin glargine in intensively treated adults with type 1 diabetes. Diabetes. Technol. Ther. 6, 589–595 (2004).
- Ratner RE, Hirsch IB, Neifing JL et al. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes. Care. 23, 639–643 (2000).
- Heinemann L, Linkeschova R, Rave K et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes. Care. 23, 644–649 (2000).
- Paneni F. 2013 ESC/EASD guidelines on the management of diabetes and cardiovascular disease: established knowledge and evidence gaps. Diab. Vasc. Dis. Res. 11, 5-10 (2014).
- Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy. Intern. Med. J. 35, 536–542 (2005).
- Johansen OE, Vanberg PJ, Kilhovd BK et al. Changing basal insulin from NPH to detemir or glargine in patients with type 1 diabetes and a history of severe hypoglycemia. Vasc. Health. Risk. Manag. 5, 121–128 (2009).
- Raskin P, Klaff L, Bergenstal R, Hallé JP et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes. Care. 23, 1666–1671 (2000).
- Garg S, Moser E, Dain MP et al. Clinical experience with insulin glargine in type 1 diabetes. Diabetes. Technol. Ther. 12, 835–846 (2010).
- Gomis R, Storms F, Conget I et al. Improving metabolic control in sub-optimally controlled subjects with Type 1 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes. Res. Clin. Pract. 77, 84–91 (2007).
- Grimaldi A, Vialettes B, Blayo A et al. Comparison of dinner with bedtime administration of insulin glargine in type 1 diabetic patients treated with basal-bolus regimen. Diabetes. Metab. 33, 121–128 (2007).
- Hamann A, Matthaei S, Rosak C et al. A randomized clinical trial comparing breakfast, dinner, or bedtime administration of insulin glargine in patients with type 1 diabetes. Diabetes. Care. 26, 1738–1744 (2003).
- Bolli GB, Songini M, Trovati M et al. Lower fasting blood glucose, glucose variability and nocturnal hypoglycaemia with glargine vs NPH basal insulin in subjects with Type 1 diabetes. Nutr. Metab. Cardiovasc. Dis. 19, 571–579 (2009).
- Chatterjee S, Jarvis-Kay J, Rengarajan T et al. Glargine versus NPH insulin: efficacy in comparison with insulin aspart in a basal bolus regimen in type 1 diabetes--the glargine and aspart study (GLASS) a randomised cross-over study. Diabetes. Res. Clin. Pract. 77, 215–222 (2007).
- Heller S, Koenen C, Bode B. Comparison of insulin detemir and insulin glargine in a basal bolus regimen, with insulin aspart as the mealtime insulin, in patients with type 1 diabetes: a 52-week, multinational, randomized, open-label, parallel-group, treat-to-target non inferiority trial. Clin. Ther. 31, 2086–2097 (2009).
- Hillman N, Herranz L, Grande C et al. What is the relative contribution of blood glucose levels at different time points of the day to HbA1c in Type 1 diabetes? Diabet. Med. 21, 468–470 (2004).
- Hillman N, Herranz L, Grande C et al. Is HbA(1c) influenced more strongly by preprandial or postprandial glycemia in type 1 diabetes? Diabetes. Care. 25, 1100–1101 (2002).
- Craig ME, Handelsman P, Donaghue KC et al. Predictors of glycemic control and hypoglycemia in children and adolescents with type 1 diabetes from NSW and the ATC. Med. J. Aust.177, 228–229 (2002).
- Kalra S. A person-centred approach to insulin initiation and intensification. J. Indian. Med. Assoc. 111, 743–745 (2013).
- Brown RJ, Wijewickrama RC, Harlan DM et al. Uncoupling Intensive Insulin Therapy from Weight Gain and Hypoglycemia in Type 1 Diabetes. Diabets. Technol. Therapeut. 13, 457–460 (2011).