Research Article - Pharmaceutical Bioprocessing (2016) Volume 4, Issue 6
Effects of celecoxib or omega-3 fatty acids alone and in combination with risperidone on the behavior and brain biochemistry in amphetamine-induced model of schizophrenia
- Corresponding Author:
- Enass youssef Osman
Tanta University Faculty of Pharmacy
Tanta, EGYPT
E-mail: enassyoussefosman@yahoo.com
Abstract
Abbreviations
MDA (malondialdeyde), TNF- α (tumor necrosis factor alpha), DA (dopamine), 5HT (serotonin), ROS (reactive oxygen species), RNS (reactive nitrogen species), NO (nitric oxide), CSF (cerebrospinal fluids), GSH (glutathione), IL-6 (interleukin-6), IFN-γ (interferongamma), PUFA (polyunsaturated fatty acids), COX-2 (cyclooxygenase-2),PGs (prostaglandins), FA(fatty acids).
Keywords
oschizophrenia, celecoxib, omega-3, oxidative stress, neuro-inflammation, TNF-α, MDA
Introduction
Schizophrenia is a debilitating psychiatric disease affecting approximately 1% of the world population [1]. The symptoms of schizophrenia are classified into positive symptoms, which represent distortion of normal functioning and include delusion and hallucinations, paranoia, agitation; negative symptoms, which represent a deficit in functioning and include social withdrawal, affective flattening, and lack of motivation and cognitive symptoms which comprise deficits in learning, memory, attention, and executive functions [2-3].
Despite the advance in the understanding of the pathophysiology of the disease, schizophrenic patients still suffer from poor prognosis. The exact mechanism by which schizophrenia develops remains unknown. Genetic factors, neurotransmitter disturbances especially dopamine (DA), serotonin (5-HT) and glutamate in addition to environmental factors have been implicated in the etiology of schizophrenia [4].
The commonly used pharmacological treatments for schizophrenia were based on modulation of neurotransmitters, DA for the “typical”, 5-HT, norepinephrine, acetylcholine, and histamine for the “atypicals”. The typical antipsychotics are generally less effective against negative than positive symptoms of schizophrenia. They also produce small and inconsistent effects on cognitive functioning. They can produce extrapyramidal side effects (EPS) at therapeutic doses, including acute (parkinsonism, dystonia, akathisia) and later-onset tardive dyskinesia (TD). In addition to EPS, typical antipsychotics also cause increase serum prolactin concentration, dysphoria/anhedonia and depressed mood. These side effects can be unpleasant for the patient and frequently an important reason for noncompliance with medication and can lead to subsequent relapse [5]. Atypicals comprise a class of antipsychotics with a higher ratio of affinity for serotonin 5-HT2A receptor relative to dopamine D2. This explains the enhanced efficacy and reduced EPS of this class of drugs. Clozapine, risperidone, olanzapine and ziprasidone are examples of atypical antipsychotics [6].
Although atypical antipsychotics have been shown to be more effective than typicals in treating negative symptoms, studies revealed rather moderate advantage, for atypical versus typical drugs in the treatment of negative symptoms [7]. Atypicals have unique adverse effects that are of potential concern such as weight gain, diabetes mellitus, prolonged QTc interval and possible secondary cardiovascular complications. These side effects are associated with potential long-term health risks of patients as well as decreased adherence to treatment regimens, and eventually may lead to relapse [8]. Therefore there is a need for the use of novel drugs which act by mechanisms other than the commonly used antipsychotics may help to treat negative and neurocognitive symptoms. This may be also associated with less adverse effects and increases the patients adherence to the medication and decreases relapse.
Oxidative stress is the loss of balance between cellular antioxidant defense mechanisms and the production of endogenous reactive oxygen species (ROS) and reactive nitrogen species (RNS). Brain is more vulnerable to the toxic effects of ROS due to its high rate of oxidative metabolic activity, low levels of protective antioxidant enzymes and high ratio of membrane polyunsaturated fatty acids [9]. Several authors have reported elevated levels of malondialdehyde (MDA) and nitric oxide (NO), along with lower levels of the antioxidant molecule glutathione (GSH) in the plasma, cerebrospinal fluid (CSF) and peripheral tissues of schizophrenia patients [10]. However, the relation between oxidative stress and behavioral impairment in schizophrenia has not been well clarified.
The role of neuro-inflammation in schizophrenia has also been studied. Microglia hypothesis of schizophrenia stated that microglia which are derived from peripheral macrophages respond to minor pathological changes in the brain by releasing pro inflammatory cytokines such as IL-6, TNF-α and IFN-γ. Prolonged microglial hyperactivity may lead to neuronal degeneration neuronal apoptosis and brain damage [11]. One of the most important cytokines in the pathophysiology of schizophrenia is TNF-α. It plays a key role in orchestrating the complex events involved in inflammation and immunity [11-12]. Although the majority of the studies have reported elevated levels of TNF-α in schizophrenic patients [13], some studies have observed either decreases [11] or no change in TNF-α concentration.
Omega-3 polyunsaturated fatty acids (PUFA) play a vital role in brain function as well as normal growth and development [14]. Being located at the core of the walls of brain cells, they provide the flexibility necessary to receive the signals from other cells [15]. Reduced cell membrane PUFA levels has been found in schizophrenia [16]. The therapeutic effects of omega-3 PUFAs in schizophrenia may result from altered membrane fluidity and receptor responses following their incorporation into cell membranes [17]. They also interact with the dopaminergic and serotonergic systems through modulation of receptor-coupled arachidonic acid release [18].
Celecoxib is a non-steroidal anti-inflammatory drug acting by cyclooxygenase-2 (COX-2) inhibition. COX-2 is the inducible form of cyclooxygenase enzymes. COX-2 catalyses the first step in the synthesis of prostanoids which include prostaglandins (PGs), prostacyclin, and thromboxanes. In addition to their involvement in inflammatory cascade, PGs participate to synaptic plasticity through several mechanisms, including modulation of adrenergic, noradrenergic, and glutamatergic neurotransmission, remodeling of actin in the cytoskeleton thus influencing the shape of spines and dendrites [19]. Studies have shown that COX-2 inhibition can be added to the a beneficial add-on therapy to the commonly used antipsychotics in schizophrenia used antipsychotics [20]. Although their wellestablished therapeutics benefits, celecoxib and omega-3 have not been extensively studied in treatment of schizophrenia.
Considering the role played by the on inflammation and oxidative stress in the pathophysiology of schizophrenia, it seems meaningful to study the effects of COX-2 inhibition and antioxidants using an add-on design together with a well-proven neuroleptic medication in schizophrenic model. Celecoxib is a selective cyclooxygenase- 2 inhibitor, which accesses the CNS easily and has few adverse side effects. Omega-3 fatty acids are broad spectrum antioxidants with proven beneficial effects in neuropsychiatric disorders with little side effects. Risperidone was selected because it is an atypical neuroleptic with high efficacy in the therapy for both positive and negative symptoms of schizophrenia. In addition a wealth of experience with risperidone treatment has been collected to date. The effects of the combination of celecoxib or omega-3 FAs and risperidone on inflammatory, oxidative markers as well as the behavior of animals were studied in the current study.
Materials and Methods
Animals
All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of Ethics
Committee of faculty of Pharmacy, Tanta University. Adult white male albino rats obtained from the animal house of the National Institute of Ophthalmology, Cairo, Egypt, and weighing 150-180 gm were used in the present study The animals were kept in climate-controlled (22 °C) with light–dark cycle of 12 h; water and food were provided ad libitum throughout the treatment.
Drugs and reagents
Amphetamine sulfate (d-isomer) was purchased as a white powder from Sigma Aldrich Co (U.S.A). 2,5- dimethyl–celecoxib was purchased as a white powder from Sigma Aldrich Co (U.S.A.). Omega-3 fatty acid was purchased as soft gelatin capsules (180 mg eicosapentanenoic acid (EPA) + 120 mg docosahexaenoic acid (DHA)/ capsule) from Sedico Co. (Egypt). Risperidal was purchased as 1 mg/ml solution from Janseen Cilag (France). Rat Tumor necrosis factor alpha (TNF-α) ELISA kit was purchase from Bioscience Co (U.S.A).All other chemicals were of analytical grade.
Drug Administration
The study used a total of 98 animals, which were divided randomly into seven groups of 14 rats.
Group I: Rats were injected with control vehicle (saline and DMSO) s.c or i.p, respectively at a dose volume of 0.5 ml/100g body weight each for five consecutive days. The group was co-currently carried out with Amphetamine- treated groups to serve as a control group. Group II: Rats are injected with amphetamine (2.5 mg/kg, s.c) every other day for a total of five doses [21]. Group III: Rats are injected with risperidone (0.1mg/kg, i.p) [21] 20 minutes before amphetamine every other day for a total of five doses. Group IV: Rats are injected with celecoxib (5 mg/kg ,i.p.) 20 minutes before amphetamine every other day for a total of five doses [20]. Group V: Rats received omega-3 FA (0.1 mg, p.o.) 20 minutes before amphetamine every other day for a total of five doses [22]. Group VI: Rats are injected with a combination of risperidone and celecoxib 20 minutes before amphetamine every other day for a total of five doses. Group VII: Rats are injected with a combination of risperidone and omega -3 FA 20 minutes before amphetamine every other day for a total of five doses. Randomly chosen rats were divided in two main categories (7 rats each). The first category was used for evaluation of the behavioral parameters 24hr after the last dose treatment and the second category was used for estimation of biological parameters.
Behavioral testing
Swimming test
A rectangular glass tank (140cm×70 cm diameter × 60cm high) was filled to 30cm deep with water that was made opaque by addition of milk powder at a temperature (26°C). A stainless steel ramp was placed within the pool, submerged approximately 2cm below the surface of the water. The platform was placed in the center of the west quadrant for each trial. Before the start of training, animals were habituated to the pool without a platform 1 min per day for 3 days. The experimenters and extramaze cues remained constant throughout testing [23]. To test for spatial memory as an indicator for the cognitive performance of rats, a simple water maze apparatus and procedure was designed as follows: The rats were trained to locate the hidden platform in the water pool. During training, animals were required to locate the submerged platform by using extramaze cues. The rat was placed into the pool, facing the wall of the tank and allowed 120 seconds to locate and climb onto the submerged platform on which it was allowed to stay for 30 sec., before the next training. trial. If it failed to find the platform within 120 sec., it was guided gently onto platform. The behavior of the animal in the swimming pool was observed in order to evaluate the latency time which is the time to reach the hidden platform was recorded on each day of testing. The mean latency for each group of rats was estimated (12 trials per day for 3 days namely session1,2 and 3 with a 10-min interval between trials for rest). Swimming speed is also estimated as distance travelled by rats per sec.
Y-maze task
This task assesses recent memory related to the optimal foraging strategies in the wild and depends on the integrity of prefrontal and hippocampal systems [24]. Each arm of the Y maze was 22 x 7 cm. The mouse was placed in one arm and allowed to move freely through the maze for a 5 min test session. The sequence of arm entries was recorded. An alternation was defined as the number of triads containing entries into all three arms divided by the maximum possible alternations [25]. For this and all subsequent tasks, the maze was cleaned with a diluted alcohol solution and dried with a paper towel.
Social interaction test
It was carried out in a neutral arena consisting of a clean standard rat cage [26]. The test rat use was placed in the novel cage 2 min before stimulus rat to avoid an interaction of the orientation response to the cage and to the stimulus rat. Stimulus animals were 23–28 day-old rat of the same sex as the test rat. Stimulus rats were derived from the same strain background. The test rat was exposed to the same juvenile rat for 2 min over two trials with an inter-trial interval of 20 min. For the third dishabituation trial, the subject was exposed to a novel juvenile mouse for 2 min. The time spent in social investigation for each trial was recorded. Social investigation was defined as direct, active, olfactory exploration of the stimulus rat, specifically nosing and sniffing of the head and anogenital regions, close following, and pursuit. Each stimulus rat was used only once per day for 3 sessions.
Biochemical analysis
Preparation of samples
Rats were killed by cervical dislocation then, brains were carefully removed. Brain samples from some rats were kept frozen at -20ºC until used. Before the procedures rat brain tissues were homogenized in phosphate buffer solution, PBS (Intertrade, USA) with protease inhibitor cocktail (Sigma-Aldrich, USA) and centrifuged at 5000 rpm for 5 min. The supernatant was separated for the assays.
MDA measurement
The method of Yoshioka et al., [27] was adopted. The lipid peroxide products were estimated in the brain homogenate by determination of the levels of thiobarabituric acid reactive substances (TBARS) that were measured as malodialdehyde (MDA). The latter is a decomposition product of the process of lipid peroxidation and is used as a measure of this process. It depends on colorimetric determination of the pink color resulting from the reaction of TBARS with thiobarbituric acid in acidic medium, at high temperature (100°C). To increase the specificity and sensitivity of the assay, the resultant color product was extracted in n-butanol and measured at 535 nm to exclude the interfering substances. Two hundred fifty milligrams (0.25 gm) of the brain was washed with sodium chloride (0.9 %) and homogenized in 10 volumes of ice cold potassium chloride solution (1.15%) using polytron homogenizer (PT 3100). To 0.5 ml of the homogenate, 3 ml of TCA (0.5%) and 1 ml TBA (0.6%) were added, mixed and then the mixture was heated for 45 minutes in a boiling water bath. After cooling, 4 ml of n-butanol was added and mixed vigorously. The n-butanol layer was separated by centrifugation at 3000 rpm for 15 minutes. The absorbance of the pink colored product was measured at 535 nm against blank containing water instead of the sample, using double-beam spectrophotometer (Shimadzu UV-PC 1601, Japan). The concentration of TBARS in the brain samples was expressed as nmol/gm tissue using a standard curve
TNF-α measurement
Brain TNF- α contents were measured by a commercial TNF- α sandwich-ELISA kit, according to the manufacturer’s instructions.
Statistical analysis
Data management and analysis were performed using Minitab computer software (version 13).
Comparisons among groups were performed using Kruskal–Wallis test for behavioral tasks. Data from the biochemical analyses are reported as ANOVA followed by Tukey post hoc test and expressed as mean±S.D. In all comparisons, p<0.05 indicated statistical significance.
Results
Behavioral tests
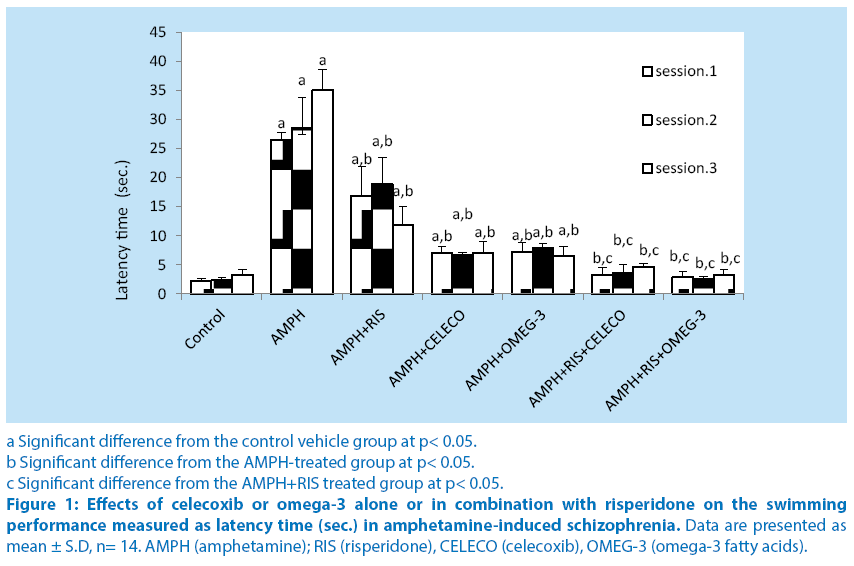
When performance in the water maze task was analyzed, it was found that treatment of rats with amphetamine showed a significant increase in latency time at sessions 1,2 and 3 (1120.3%, 1123.2% and 951.1%) compared with their respective control vehicle group. Pretreatment of rats with risperidone before amphetamine caused significant decrease in latency time at sessions 1, 2 and 3 (37.4%, 33.9% and 66.2%, respectively) compared with their respective amphetamine–treated group. Pretreatment of rats with celecoxib (before amphetamine caused significant decrease in latency time at sessions 1, 2 and 3 (73.6%, 76.6% and 80%, respectively) compared with their respective amphetamine– treated group. Pretreatment of rats with omega-3 FA before amphetamine caused significant decrease in latency time at sessions 1, 2 and 3 (73%, 72.5% and 81.4%, respectively) compared with their respective amphetamine– treated group. Schizophrenic rats treated with a combination of risperidone and celecoxib caused significant decrease in latency time at sessions 1, 2 and 3 (80%, 80.5% and 60.5%, respectively) compared with the respective schizophrenic group treated with RIS alone.Schizophrenic rats treated with a combination of risperidone and omega-3 FA caused significant decrease in latency time at sessions 1, 2 and 3 (83%, 76.7% and 71.8%, respectively) compared with the respective schizophrenic group treated with RIS alone.Intra-comparing the two treatment regimens of amphetamine-treated rats, injection of celecoxib or omega-3 fatty acids revealed significant alterations in latency time from that of risperidone treatment. (Figure 1).
a Significant difference from the control vehicle group at p< 0.05.
b Significant difference from the AMPH-treated group at p< 0.05.
c Significant difference from the AMPH+RIS treated group at p< 0.05.
Figure 1: Effects of celecoxib or omega-3 alone or in combination with risperidone on the swimming performance measured as latency time (sec.) in amphetamine-induced schizophrenia. Data are presented as mean ± S.D, n= 14. AMPH (amphetamine); RIS (risperidone), CELECO (celecoxib), OMEG-3 (omega-3 fatty acids).
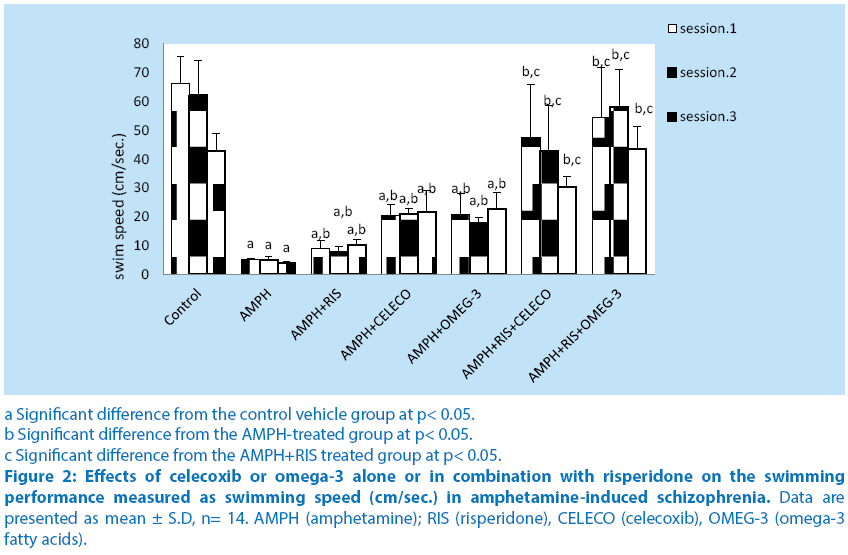
Treatment of rats with amphetamine showed a significant decrease in swimming speed at sessions 1,2 and 3 (92%, 91.8% and 91%) compared with their respective control vehicle group.
Pretreatment of rats with risperidone before amphetamine caused significant increase in swimming speed at sessions 1, 2 and 3 (68.5%, 54.4% and 154.3%, respectively) compared with their respective amphetamine–treated group. Pretreatment of rats with celecoxib before amphetamine caused significant increase in swimming speed at sessions 1, 2 and 3 (284.6%, 316.4% and 437.2%, respectively) compared with their respective amphetamine–treated group. Pretreatment of rats with omega-3 FA before amphetamine caused significant increase in swimming speed at sessions 1, 2 and 3 (292.7%, 255.2% and 463.1%, respectively) compared with their respective amphetamine– treated group. Schizophrenic rats treated with a combination of risperidone and celecoxib before amphetamine caused significant increase in swimming speed at sessions 1, 2 and 3 (47.3%, 446.6% and 195.3%, respectively) compared with the respective schizophrenic group treated with RIS alone. Schizophrenic rats treated with a combination of risperidone and omega-3 FA caused significant increase in swimming speed at sessions 1, 2 and 3 (506%, 644.6% and 324.3%, respectively) compared with the respective schizophrenic group treated with RIS alone. Intra-comparing the two treatment regimens of amphetamine-treated rats, injection of celecoxib or omega-3 fatty acids revealed significant alterations in swimming speed from that of risperidone treatment ( Figure 2).
a Significant difference from the control vehicle group at p< 0.05.
b Significant difference from the AMPH-treated group at p< 0.05.
c Significant difference from the AMPH+RIS treated group at p< 0.05.
Figure 2: Effects of celecoxib or omega-3 alone or in combination with risperidone on the swimming performance measured as swimming speed (cm/sec.) in amphetamine-induced schizophrenia. Data are presented as mean ± S.D, n= 14. AMPH (amphetamine); RIS (risperidone), CELECO (celecoxib), OMEG-3 (omega-3 fatty acids).
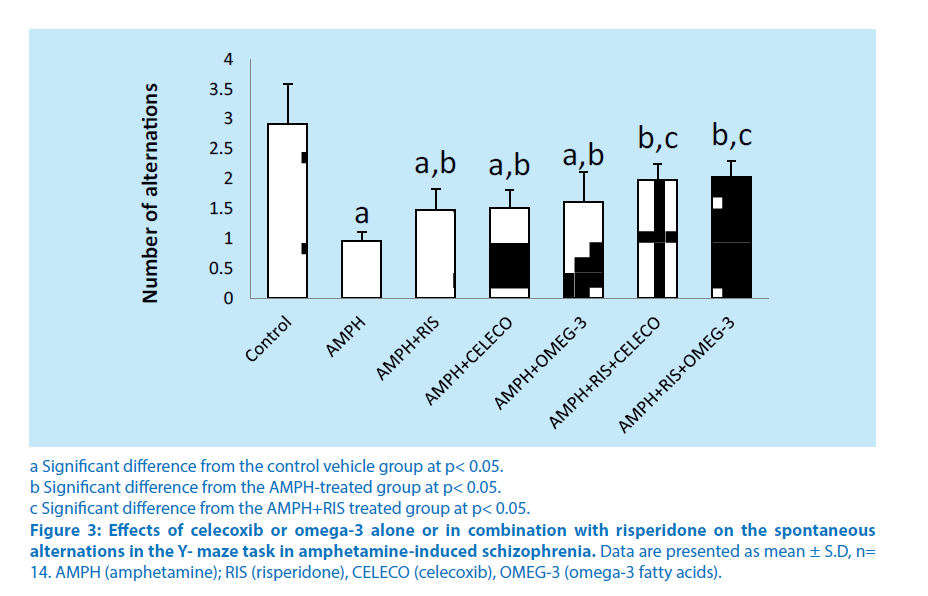
Regarding the Y-maze task, treatment of rats with amphetamine showed a significant decrease in the number of alternations (66.8%) compared with control vehicle group. Pretreatment of rats with risperidone before amphetamine caused significant increase in the number of alternations (53.4%) compared with amphetamine–treated group. Pretreatment of rats with celecoxib before amphetamine caused significant increase in the number of alternations (57%) compared with amphetamine–treated group. Pretreatment of rats with omega-3 FA (before amphetamine caused significant increase in the number of alternations (67.3%) compared with amphetamine–treated group. Schizophrenic rats treated with a combination of risperidone and celecoxib before amphetamine caused significant increase in the number of alternations (33.7%) compared with the schizophrenic group treated with RIS alone. Schizophrenic rats treated with a combination of risperidone and omega-3 FA caused significant increase in the number of alternations (37.1%) compared with the schizophrenic group treated with RIS alone. Intra-comparing the two treatment regimens of amphetamine-treated rats, injection of celecoxib or omega-3 fatty acids revealed non-significant change in the number of alternations from that of risperidone treatment (Figure 3).
a Significant difference from the control vehicle group at p< 0.05.
b Significant difference from the AMPH-treated group at p< 0.05.
c Significant difference from the AMPH+RIS treated group at p< 0.05.
Figure 3: Effects of celecoxib or omega-3 alone or in combination with risperidone on the spontaneous alternations in the Y- maze task in amphetamine-induced schizophrenia. Data are presented as mean ± S.D, n= 14. AMPH (amphetamine); RIS (risperidone), CELECO (celecoxib), OMEG-3 (omega-3 fatty acids).
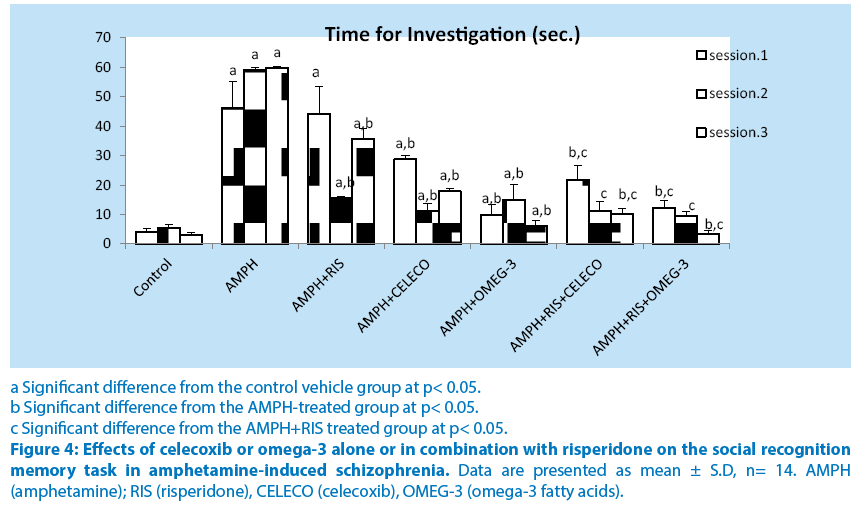
Treatment of rats with amphetamine showed a significant increase in the time required to investigate a foreign rat at sessions 1,2 and 3 (1058.3%, 972.8% and 1889%) compared with their respective control vehicle group. Pretreatment of rats with risperidone before amphetamine caused non-significant decrease in the time required to investigate a foreign rat at sessions 1 (4.7%) but caused significant decrease in time required to investigate a foreign rat at sessions 2 and 3 (73.7% and40.2%, respectively) compared with their respective amphetamine– treated group. Pretreatment of rats with celecoxib before amphetamine caused significant decrease in the time required to investigate a foreign rat at sessions 1, 2 and 3 (37.8%, 81.1% and 70.2%, respectively) compared with their respective amphetamine–treated group. Pretreatment of rats with omega-3 before amphetamine caused significant decrease in the time required to investigate a foreign rat at sessions 1, 2 and 3 (78.4%, 74.6% and 90%, respectively) compared with their respective amphetamine– treated group. Schizophrenic rats treated with a combination of risperidone and celecoxib before amphetamine caused significant decrease in the time required to investigate a foreign rat at sessions 1, 2 and 3 (51%, 27% and 71.5%, respectively) compared with the respective schizophrenic group treated with RIS alone. Schizophrenic rats treated with a combination of risperidone and omega-3 FA 1, 2 and 3 (72.1%, 38.7% and 90.2%, respectively) compared with the respective schizophrenic group treated with RIS alone. Intra-comparing the two treatment regimens of amphetamine-treated rats, injection of celecoxib or omega-3 fatty acids revealed significant alterations in the time required to investigate a foreign rat from that of resperidone treatment (Figure 4).
a Significant difference from the control vehicle group at p< 0.05.
b Significant difference from the AMPH-treated group at p< 0.05.
c Significant difference from the AMPH+RIS treated group at p< 0.05.
Figure 4: Effects of celecoxib or omega-3 alone or in combination with risperidone on the social recognition memory task in amphetamine-induced schizophrenia. Data are presented as mean ± S.D, n= 14. AMPH (amphetamine); RIS (risperidone), CELECO (celecoxib), OMEG-3 (omega-3 fatty acids).
Biochemical measurements
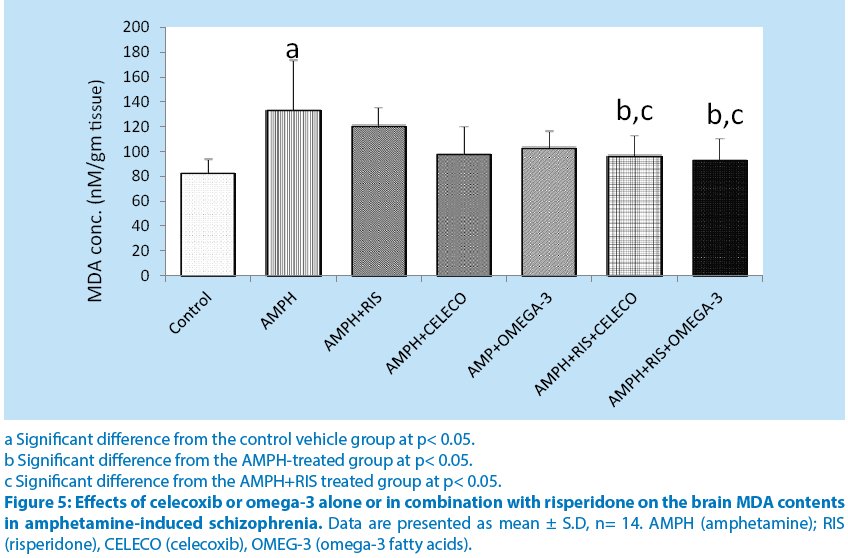
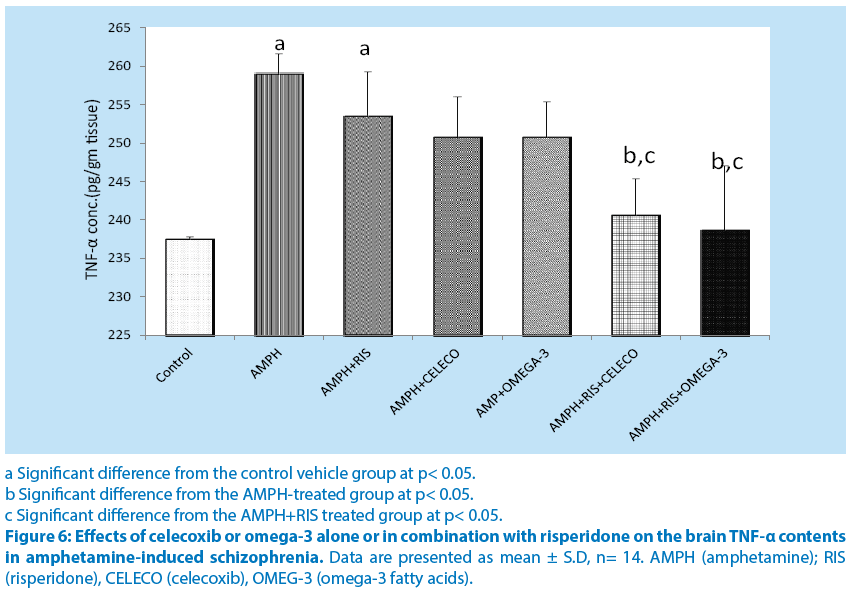
Brain MDA and TNF-α contents were significantly higher n amphetamine group compared to control group. Risperidone, celecoxib and omega-3 PUFAs alone caused non-significant decrease in MDA and TNF-α contents compared to amphetamine group. Combinations of riisperidone with either celecoxib or omega-3 PUFAs caused significant decreases in MDA and TNF-α contents.(Figure 5 and 6).
a Significant difference from the control vehicle group at p< 0.05.
b Significant difference from the AMPH-treated group at p< 0.05.
c Significant difference from the AMPH+RIS treated group at p< 0.05.
Figure 5: Effects of celecoxib or omega-3 alone or in combination with risperidone on the brain MDA contents in amphetamine-induced schizophrenia. Data are presented as mean ± S.D, n= 14. AMPH (amphetamine); RIS (risperidone), CELECO (celecoxib), OMEG-3 (omega-3 fatty acids).
a Significant difference from the control vehicle group at p< 0.05.
b Significant difference from the AMPH-treated group at p< 0.05.
c Significant difference from the AMPH+RIS treated group at p< 0.05.
Figure 6: Effects of celecoxib or omega-3 alone or in combination with risperidone on the brain TNF-α contents in amphetamine-induced schizophrenia. Data are presented as mean ± S.D, n= 14. AMPH (amphetamine); RIS (risperidone), CELECO (celecoxib), OMEG-3 (omega-3 fatty acids).
Discussion
To our knowledge, this is the first study to show the use of celecoxib or omega-3 for positive, negative and cognitive symptom prevention in amphetamine- induced animal model of schizophrenia.
Swimming test is used to test spatial orientation and recognition which is a part of declarative memory. The results of the present study shows that treatment of rats with amphetamine showed a significant increase in latency time and significant decrease in swimming speeds at all test sessions. It is well known that amphetamine damages brain areas which includes the hippocampus which is critical to declarative memory [28]. These results are in agreement with previous studies [29-31] whose major findings were that latencies and swimming speeds in were sharply affected due to treatment with methylamphetamine. The speed of swimming seems to have the positive correlation to the motivation of the animal to find the platform in the swimming test [32-33].
Groups received RIS alone showed significant reduction in latency time and significant increase in swimming speeds at all sessions compared to AMHP-treated group. Similar to the these results were the results of previous studies [34-36] which demonstrated that that RIS has a positive effect on cognition in the swimming test. Risperidone acted via blockade of 5-HT2A. Serotonergic system play a key role in behaviors that involve a high cognitive demand. Groups treated with celecoxib showed significant decrease in the latency time and increase in the swimming speed compared to AMPH –treated group. The effects of combination were also significant to group treated with RIS alone. These results are in agreement with previous findings [37-39] which found that celecoxib reduced immobility in forced swim test.
In the present study, AMPH-treated group showed significant decrease in the number of alternations in the arms of Y-maze compared with control vehicle group. These data are similar to previous results [40-41]. Groups treated with celecoxib or omega-3 FAs showed significant increase in the number of alternations in the Y-maze. The effects of combination were also significant to group treated with RIS alone and this effect was in agreement with previous studies [42-43] which found that celecoxib and omega-3 FAs, respectively, improve the behavior in Y-maze.
In the present study, AMPH-treated group was shown to significantly reduce the social interaction compared to the control-vehicle group. These findings are in consistent with previous findings [44-45]. Celecoxib and omega- 3FAs showed significant decrease in the time required for the investigation of a novel animal. The effects of combination were also significant to group treated with RIS alone. These results are in accordance with previous studies [46-47] which revealed that celecoxib or omega3 FAs, respectively have positive effects on the social interaction.
The effect of celecoxib is probably due to its inhibition of the cyclooxygenase-2 (COX-2) enzyme which is responsible for production of the prostaglandins (PGs). COX-2 and PGs are involved in physiological mechanism of memory. Also, celecoxib causes reduction of the brain levels of inflammatory cytokines (TNF–α and IL-1β) and induction of inducible nitric oxide synthase in the brain. This suggests that celecoxib, attenuates neurodegeneration and improves cognitive impairment. This provides evidence on the relevance of persistent inflammation as a triggering factor for schizophrenia.
In the present study, treatment of schizophrenic rats with omega-3 fatty acids resulted in significant reduction in latency time and increase in swimming speed in Morris water maze at all test sessions. The effects of combination were also significant to group treated with RIS alone. Studies [48-49] revealed that administration of omega-3 Fas reduced immobility and increased swimming behavior in forced swim test. Omega-3 fatty acids are essential components of CNS membrane phospholipid-acyl [50]. An optimal fluidity is required for neurotransmitter binding and signaling within the cell [51]. Also, omega-3 fatty acids can increase adenyl cyclase activity. This pathway is used by 5-HT1 receptors, alpha-2 adrenergic and betaadrenergic receptors, and both D1 and D2 (dopamine) receptors. Omega-3 fatty acids are well-documented inhibitors of proinflammatory cytokines, particularly TNF-α and IL-1 which are well known players in mood and cognition respectively [52-53]. Also, omega-3FAs increased the hippocampal calmodulin genes expression. The increased level of calmodulin enhances the signal transduction and communication between neurons during memory formation [54]. In addition, Omega-3 fatty acids induced suppression of PGE2, thromboxane A2 and histamine [55] which are involved in antiinflammatory effects Chronic administration of omega-3 fatty acids can cause an increase in brain-derived neurotrophic factor (BDNF), which plays a role in the plasticity and survival of nervous system [56].
Schizophrenia is associated with mitochondrial dysfunction and high levels of genes responsible for oxidative stress [57-58]. Lipid peroxidation measured as malondialdehyde is a good measure of oxidative stress. Many previous studies found that schizophrenia is associated with increased lipid peroxidation [59-60].
The present study provides a strong evidence for involvement of oxidative stress in the pathophysiology of schizophrenia since treatment with AMPH resulted in a significant increase in brain MDA levels. Excessive dopamine production due to AMPH use which is observed in the current study represents the main source of oxidative stress in the brain, due to redox potential of dopamine. Dopamine may be metabolized via monoamine oxidase with production of H2O2 and dihydroxphenylacetic acid [61-62], or can go through nonenzymatic hydroxylation in the presence of Fe2+ and H2O2 leading to the formation of 6-hydroxydopamine (6-OHDA) [63].
Inflammation represents another source of oxidative stress in the present study. Increased levels of TNF-α was observed in the AMPPHtreated group. TNF- α activates NF-kB which in turn activates the production of cytokines, such as IL-6 and IL-8, and T-cell derived cytokines, such as interferon gamma [64]. The release of monoamine neurotransmitters provides tonic sympathetic control on cytokine production and hence on the balance of pro-inflammatory/ anti-inflammatory cytokines. The extent of lipid peroxidation is positively correlated with the severity of symptoms [65]. Combination of celecoxib or omega-3 FAs with risperidone produced significant decreases in brain MDA and TNF-α levels compared to RIS alone.
Several mechanisms may explain the antioxidant properties of COX-2 inhibitors. One depends on the enhanced release of cytokines that promote the formation and release of reactive oxygen species (ROS) and nitric oxide from microglial cells [66]. In this context, proinflammatory cytokines influence brain activity by inducing the expression of COX-II in brain vascular cells, which transduces inflammatory signals into a prostaglandin signaling cascade [67]. Another mechanism is based on the release of excitatory amino acids, aspartate and glutamate, that induce free radical formation during their physiological action [68]. A third mechanism is related to mobilization of mitochondrial calcium, which in turn, activates the arachidonic acid cascade that produces ROS. COX isoenzyme leads to an increase in the peroxynitrite levels in rat brain. COX inhibitors could also scavenge NO and hydrogen peroxide and prevent excitotoxicity and subsequent oxidative stress [69-70].
The therapeutic effects of omega-3 FAs may result from altered membrane fluidity and receptor responses following their incorporation into cell membranes [71]. They also interact with the dopaminergic and serotonergic systems, which both have been associated with the pathophysiology of schizophrenia [72]. They inhibit IL-6, IL-1b and TNF-α expression in human endothelial cells and macrophages when stimulated with bacterial endotoxin lipopolysaccharide (LPS) [73-75]. The increase in brain cytokine production is linked with schizophrenia symptoms [76]. Furthermore, they increase glutathione in the temporal lobes of first-episode psychosis patients [77-79]. The evidence that PUFAs can reduce symptoms in schizophrenia, may have neuroprotective properties, and do not have clinically relevant adverse effects make them an ideal candidate for indicated prevention of schizophrenia. To summarize, the present study shows that administration of celecoxib or omega-3 alone or in combination with risperidone prevent positive, negative and cognitive symptoms in amphetamine animal model of schizophrenia. Their effects on behavior were attributable to their antioxidants and anti-inflammatory effects since they were able to prevent elevation of MDA and TNF-α.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgment
The authors would like to thank the staff members of the neuropsychiatry department, Faculty of Medicine, Tanta university. Egypt, for their assistance in the methodology.
References
- Howes OD, Kambeitz J, Kim, E. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69 (8): 777- 786 (2012).
- Strauss G, Waltz J, Gold J. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull. 40 (2): S107–S116 (2014).
- Tandon R, Gaebel W, Barch D. Definition and description of schizophrenia in the DSM-5. Schizophrenia Research 150: 3-10 (2013).
- Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatrica Scandinavica 130(4): (2014).
- Harrow, M and Jobe, T. Does long-term treatment of schizophrenia with antipsychotic medications facilitate recovery?. Schizophr. Bull. 39(5): 962-965 (2013).
- Harrow M, Jobe T, Faull R. Do all schizophrenia patients need antipsychotic treatment continuously throughout their lifetime? A 20-year longitudinal study. Psychol Med. 42(10): 2145-2155 (2012).
- Gareri P, Segura-Garcia C, Manfredi V. Use of atypical antipsychotics in the elderly: a clinical review. Clin Interv Aging. (9): 1363-1373 (2014).
- D McGorry P, Nelson B, Phillips L. Randomized Controlled Trial of Interventions for Young People at Ultra-High Risk of Psychosis: Twelve-Month Outcome. J Clin Psychiatry. 74(4): 349-335 (2013).
- Paulsen Bda S, de Moraes Maciel R, Galina A. Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant. 21(7):1547- 1559 (2012).
- Coughlin, J. M., Ishizuka, K., Kano, S. (2013) Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol. Psychiatry (18), 10-11.
- Busse S, Busse M, Schiltz K, et al., (2013). Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: Further evidence for disease course-related immune alterations?. Brain, Behavior and Immunity. 26(8): 1273-1279.
- Naz M, Riaz M, Saleem M. Potential role of Neuregulin 1 and TNF-alpha (-308) polymorphism in schizophrenia patients visiting hospitals in Lahore, Pakistan. Mol Biol Rep. 38(7): 4709-4714 (2011).
- Stuarta M, Baunea B. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neuroscience and Biobehavioral Reviews. (42): 93-115 (2014).
- Borovcanin M, Jovanovic I, Radosavljevic G. Antipsychotics can modulate the cytokine profile in schizophrenia: Attenuation of the type-2 inflammatory response. Schizophrenia Research (147): 103-109 (2013).
- Francesconi L, Cereser K, Mascarenhas R. Increased annexin-V and decreased TNF-alpha serum levels in chronic-medicated patients with schizophrenia. Neurosci Lett (502): 143-146 (2011).
- Fusar-Poli P, Gregor B. Eicosapentaenoic Acid Interventions in Schizophrenia: Meta-Analysis of Randomized, Placebo-Controlled Studies. Journal of Clinical Psychopharmacology, 32 (2): 179-185 (2012).
- Calder AJ. Does facial identity and facial expression recognition involve separate visual routes?” in The Oxford Handbook of Face Perception, eds Calder A. J., Rhodes G., Johnson M. H., Haxby J. V., editors. (Oxford: Oxford University Press. Pp: 427-448 (2011).
- Jamilian H, Bagherzadeh K, Nazeri Z. Vitamin D, parathyroid hormone, serum calcium and phosphorus in patients with schizophrenia and major depression. Int J Psychiatry Clin Pract. 17(1): 30-34 (2013).
- Arroll M, Wilder L, Neil J. Nutritional interventions for the adjunctive treatment of schizophrenia: a brief review. Nutr J. 13(1): 91-100 (2014).
- Latour A, Grintal B, Champeil-Potokar G. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell (12): 76-84 (2013).
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 63(9): 901-910 (2004).
- Muller N, Krausea D, Dehning S. Celecoxib treatment in an early stage of schizophrenia: Results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophrenia Research. 121 (1-3): 118-124 (2010).
- Abekawa T, Koki I, Nakagawa S. Olanzapine and risperidone block a high dose of methamphetamineinduced schizophrenia like behavioral abnormalities and accompanied apoptosis in the medial prefrontal cortex. Schizophr Res. (101): 84-94 (2008).
- Abekawa T, Honda M, Ito K. Effects of NRA0045, a novel potent antagonist at dopamine D4, 5-HT2A, and α1 adrenaline receptors, and NRA0160, a selective D4 receptor antagonist, on phencyclidineinduced behavior and glutamate release in rats. Psychopharmacology. (169): 247-256 (2003) .
- Amminger G, Schafer M, Papageorgiou K. Long-Chain ω-3 Fatty Acids for Indicated Prevention of Psychotic Disorders, A Randomized, Placebo-Controlled Trial. Gen Psychiatry. 67(2): 146-154 (2010).
- Alaei H, Moloudi R, Sarkaki A. Effects of treadmill running on mid-term memory and swim speed in the rat with Morris water maze test. Journal of bodywork and movement therapies. 12(1): 72-75 (2008).
- Divac I, Wikmark R, Gade A. Spontaneous alternation in rats with lesions in the frontal lobes: An extension of the frontal lobe syndrome. Physiol Psychol. (3): 39-42 (1975).
- Stone W, Rudd R, Gold P. Glucose attenuation of scopolamine- and age-induced deficits in spontaneous alternation behavior and regional brain [3H]2- deoxyglucose uptake in mice. Psychobiology. 20(4): 270-279 (1992).
- Savonenko AV, Melnikova T, Laird FM. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. PNAS. 105(14): 5585-5590 (2008).
- Yoshioka T, Kawada K, Shimada T. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. American Journal of Obstetrics and Gynecology 135(3): 372-376 (1979).
- Berman S, O’NeillJ , Fears S. Abuse of Amphetamines and Structural Abnormalities in Brain. Ann N Y Acad Sci. (1141), 195-220 (2008).
- Galizio M, Byrd B, Robinson A. Repeated Acquisition in the Morris Swim Task: Effects of MDMA, Methamphetamine and Methylphenidate. Psychol Rec. 64(2): 143-150 (2014).
- Macuchova A, Nohejlova-deykun K, Slamberova R. Effect of Methamphetamine on Cognitive Functions of Adult Female Rats Prenatally Exposed to the Same Drug. Physiol Res 62: S89-S9 (2013).
- Williams M, Brown R, and Vorhees C. Neonatal methamphetamine administration induces regionspecific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 19(12): 3165-3170 (2004).
- Lubbers M, van den Bos V, Spruijt B. Mu opioid receptor knockout mice in the Morris Water Maze: A learning or motivation deficit?. Behavioural Brain Research (180): 107-111 (2007).
- Salamone J, Correa M, Nunes E. The Behavioral Pharmacology of Effort-related Choice Behavior: Dopamine, Adenosine and Beyond. J Exp Anal Behav. 97(1): 125-146 (2012).
- Rogoz Z. Effects of co-treatment with mirtazapine and low doses of risperidone on immobility time in the forced swimming test in mice. pharmacological reports (62): 1191-1196 (2010) .
- Ipek K, Celikyurt F, Yildiz A. Effects of Risperidone on Learning and Memory in Naive and MK-801-Treated Rats. Pharmacology (87): 187-194 (2011).
- Rogoz Z, Kabzinski M, Sadaj W. Effect of co-treatment with fluoxetine or mirtazapine and risperidone on the active behaviors and plasma corticosterone concentration in rats subjected to the forced swim test. pharmacological reports (64): 1391-1399 (2012).
- Costa-Nunes J, Cline B, Araujo-Correia M. Animal Models of Depression and Drug Delivery with Food as an Effective Dosing Method: Evidences from Studies with Celecoxib and Dicholine Succinate. BioMed Research International. 1-11 (2014).
- Maciel S, Silva R, Morrone F. Synergistic Effects of Celecoxib and Bupropion in a Model of Chronic Inflammation-Related Depression in Mice. PLOS One. 8(9): 1-15 (2013).
- Khanam R, Ahmed S, Akhtar M. Possible Modulating Effects of Celecoxib (COX II Inhibitor) on Antidepressant Action of Duloxetine (SNRI) in Stressed Mice. The Open Conference Proceedings Journal (3): 35-41 (2012).
- Featherby T, Van den Buuse M, Lubman D. Persistent downregulation of hippocampal CREB mRNA parallels a Y-maze deficit in adolescent rats following semi-chronic amphetamine administration. British Journal of Pharmacology (154): 417-428 (2008).
- Oades R, Taghzouti K, Simon H. Dopaminesensitive alternation and collateral behaviour in a Y-maze: Effects of d-amphetamine and haloperidol. Psychopharmacology. 85(1): 123-128 (1985).
- El Sayed NS, Kassem LA, Heikal OA. Promising therapy for Alzheimer’s disease targeting angiotensinconverting enzyme and the cyclooxygense-2 isoform. Drug Discov Ther. (3): 307-315 (2009).
- Sopian N, Ajat M , Shafie N. Does Short-Term Dietary Omega-3 Fatty Acid Supplementation Influence Brain Hippocampus Gene Expression of Zinc Transporter-3?. Int J Mol Sci (16): 15800-15810 (2015).
- Corbett R, Camacho F, Woods AT. Antipsychotic agents antagonize non-45 N-methyl-d-asparate antagonist-induced behaviors. Psychopharmacology. (120): 67-74 (1995).
- Dodd F. Effects of Continuous D-Amphetamine and Phencyclidine Administration on Social Behaviour, Stereotyped Behaviour, and Locomotor Activity in Rats. Neuropsychopharmacology. (19): 18-25 (1998).
- Asadabadi M, Mohammadi M, Ghanizadeh A. (2012) Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: a randomized, doubleblind, placebo-controlled trial Psychopharmacology, 225(1), 2796-2798.
- James S, Montgomery P and Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst Rev. (2011).
- Laino C, Garcia Podesta P. Fluoxetine Potentiation of Omega-3 Fatty Acid Antidepressant Effect: Evaluating Pharmacokinetic and Brain Fatty Acid-Related Aspects in Rodents. Pharmacokinetics, Pharmacodynamics and Drug Transport and Metabolism. Journal of Pharmaceutical Sciences 103(10): 3316-3325 (2014).
- Moranis A, Delpech J, De Smedt-Peyrusse V. Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain, Behavior, and Immunity (26): 721-73 (2012).
- Bourre J, Piciotti M, Dumont O. Dietary linoleic acid and polyunsaturated fatty acids in rat brain and other organs. Minimal requirements of linoleic acid. Lipids (25): 465-472 (1990).
- Heron DS, Shinitzky M, Hershkowitz M. Lipid fluidity markedly modulates the binding of serotonin to mouse brain membranes. Proc Natl Acad Sci. (77): 7463-7467 (1980).
- Murray C, Lynch M. Evidence That Increased Hippocampal Expression of the Cytokine Interleukin- 1β Is a Common Trigger for Age- and Stress-Induced Impairments in Long-Term Potentiation. The Journal of Neuroscience. 18(8): 22974-2981 (1998).
- Tancredi V, D Arcangelo G, Grassi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neuroscience Letters. 146(2): 176-178 (1992).
- Rellos P, Pike A, Niesen F. Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLOS Biol. 8(7): 1-28 (2010).
- Iso H, Kobayashi M, Ishihara J. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese. The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation (113): 195-202 (2006).
- Ikemoto A, Nitta A, Furukawa S. Dietary n-3 fatty acid deficiency decreases nerve growth factor content in rat hippocampus. Neurosci Lett. (285): 99-102 (2000).
- Gawryluk JW, Wang JF, Andreazza AC. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. (16): 1-8 (2010).
- Tosica M, Ott J, Barral S. Schizophrenia and Oxidative Stress: Glutamate Cysteine Ligase Modifier as a Susceptibility Gene. AJHG. 79(3): 586-592 (2006).
- Zortea K, Fernandes B, Guimaraes L. Reduced serum non-enzymatic antioxidant defense and increased lipid peroxidation in schizophrenic patients on a hypocaloric diet. Neuroscience Letters (512): 43-47 (2012).
- Dietrich-Muszalska A, Kontek B. Lipid peroxidation in patients with schizophrenia. Psychiatry Clin Neurosci. 64(5): 469-475 (2010).
- Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem. 73(3): 1127-1137 (2010).
- Maker H, Weiss C, Brannan T. Amine-mediated toxicity: The effects of dopamine, norepinephrine, 5-hydroxytryptamine, 6-hydroxydopamine, ascorbate, glutathione and peroxide on the in vitro activities of creatine and adenylate kinases in the brain of the rat. Neuropharmacology. 25(1): 25-32 (1986).
- Berk M, Kapczinskie F, Andreazza A. Pathways underlying neuroprogression in and Biobehavioral Reviews (35): 804-817 (2011).
- Gordon S, Martinez FO. (2010) Alternative activation of macrophages: mechanism and functions. Immunity. 32(5): 593-604.
- Wood S, Pantelis C, Yung A. Brain changes during the onset of schizophrenia: implications for neurodevelopmental theories. Med J Aust. 190(4): 1-10 (2009).
- Woodroofe M. Cytokine production in the central nervous system. Neurology. 45(6): S6-S10 (1995).
- Kronfol Z, Remick DG. (2000) Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 157(5): 683-694.
- Moghaddam B. Stress Preferentially Increases Extraneuronal Levels of Excitatory Amino Acids in the Prefrontal Cortex: Comparison to Hippocampus and Basal Ganglia. Journal of Neurochemistry. 60(5): 1650- 1657 (1993).
- Maes M, Kubera M, Obuchowiczwa E. Depression’s multiple comorbidities explained by (neuro) inflammatory and oxidative and nitrosative stress pathways. Neuroendocrinology Letters. (32): 7-24 (2011).
- Hoffmann C. COX-2 in brain and spinal cord implications for therapeutic use. Curr Med Chem. 7(11): 1113-1120 (2000).
- Fentona W, Hibbelnb J, Knable M. Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biological Psychiatry. 47(1): 8-21 (2000).
- Berger G, Wood S, Wellard R. Ethyl-Eicosapentaenoic Acid in First-Episode Psychosis. A 1H-MRS Study. Neuropsychopharmacology (33): 2467-2473 (2008).
- Calder P. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 83(6): S1505-1519S (2006).
- Khalfoun B, Thibault F,Watier H. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. (400), 589-597 (1997).
- Weldon S, Mullen A, Loscher C. Docosahexaenoic acid induces an anti-nflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. The Journal of Nutritional Biochemistry. 18(4): 250-258 (2007) .
- O’Connor M, Harris Ja, McIntosh A. Specific cognitive deficits in a group at genetic high risk of schizophrenia. Psychological Medicine. 39(10): 1649-1655 (2009).
- Gysin R, Kraftsik R, Sandell J. Impaired glutathione synthesis in schizophrenia: Convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 104(42): 16621-16626 (2007).
- Steullet P, Neijt HC, Cuenod M. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience. (137): 807-819 (2006).