Research Article - Imaging in Medicine (2018) Volume 10, Issue 5
Effects of NF-kB and the role of inflammatory response factors on the hepatocytic injury after TAE- An experimental study
Bimbadhar Valluru†, Wang Bei Ran†, Du Wei*, Yu Yi Jun, Wu Chun Hua & Kalyan SharmaDepartment of Radiology, The First Affiliated Hospital of Dali University, SAR China
†These authors contributed equally to this work.
- Corresponding Author:
- Du Wei
Department of Radiology
The First Affiliated Hospital of Dali University, SAR China
E-mail: duweifsk@qq.com
Abstract
Objective: To investigate the effects of NF-κB and the role of inflammatory response factors on the normal liver tissue around the tumors in rabbit VX2 hepatocellular carcinoma models after TAE.
Methods: Seventy VX2 hepatoma rabbit models were prepared and success rate was 85.7%. Computed tomography (CT) and Magnetic resonance imaging (MRI) was performed after 21 days of modelling. Sixty rabbits were randomly chosen and divided into three groups, 20 in each group: TAE group, Contrast/Angiography group, and Control group respectively. TAE group was treated with Transarterial embolization (TAE) and the Contrast group was subjected to hepatic arteriography and DSA, while the Control group served as a control group. The immune-histochemistry analysis was used to detect the expression of NF-κB in normal liver tissue around the tumors of each group. The levels of inflammatory factors like TNF-α, IL-10 of each group were detected by Enzyme-linked immunosorbent assay (ELISA) in the normal liver tissue around the tumor. Using SPSS 22.0 statistical analysis software, measurement data was analyzed using t-test, count data were compared using the χ2-test; P <0.05 was considered as statistically significant.
Results: The positive expression rates of NF-κB in the TAE group, contrast group, and control group were 75% (15/20), 35% (7/20) and 20% (4/20) respectively. The concentrations of TNF-α were 11.72 ± 0.65 (ng/L), 9.60 ± 0.31 (ng/L) and 8.82 ± 0.81 (ng/L); the concentrations of IL-10 were 2.18±0.13 (ng/L), 1.67 ± 0.12 (ng/L), 1.66 ± 0.10 (ng/L) respectively. Compared with the control group and contrast groups, TAE group showed a significant increase in the expression of NF-κB, and the levels of TNF-α and IL-10 were also increased.
Conclusion: The hepatocytic injury, degrading function after TAE could be due to the interaction of NF-κB and inflammatory factors- TNF-α and IL-10.
Keywords
transarterial emobolization ▪ hepatocellular carcinoma ▪ NF-κB, TNF-α ▪ IL-10 ▪ IR ▪ TACE.
Abbreviations
TAE: Trans Arterial Embolization; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; DSA: Digital Substraction Angiography; ELISA: Enzyme Linked Immunsorbent Assay; NF- κB: Nuclear Factor kappa B; TNF-α: Tumor Necrosis Factor alpha; IL-10: Interleukin-10.
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related deaths in the world. While metastases are more common in Western countries, primary liver cancers are frequently diagnosed in Asia and Africa. The incidence and mortality rates of liver cancer patients in China account for 50% of the world [1]. Radical segmental resection or hepatectomy is associated with a better prognosis for patients with primary liver cancer. It is the primary choice of management, but only 15-20% of the patients (considerably lower resection rates) are suitable for a surgical resection because of insidious onset, rapid growing tumors and early metastasis [1]; at the time of diagnostic assessment, most patients lose the opportunity because of the extent of tumor is beyond 40% of residual liver tissue remaining and poor hepatic function is the main factor responsible for higher morbidity and mortality [2]. But local ablative treatments such as MRguided or laser interstitial thermotherapy (LITT), microwave coagulation (MWC) or radiofrequency ablation (RFA) are also frequently used techniques. However, only a few patients are candidates for these selective interventions [3-5].
Transcatheter arterial embolization (TAE) or Transarterial chemoembolization (TACE) is a minimally invasive, loco-regional therapeutic interventional procedure, which is also referred to as liver-directed therapy [6] because it intervenes to focally target the large tumors within the liver. About 99% of the liver tumors attain their blood supply from the feeding arteries arising from the hepatic artery and embolization of these branches provides a theoretical support in the treatment of hepatocellular carcinoma (HCC) [7]. It is based on the synergistic effect of arterial occlusion and local chemotherapy enhancing the effectiveness of chemotherapeutics [8,9]. Though commonly used in clinical practice, it is found that patients often have varying degrees of hepatic dysfunction after undergoing TAE even when done by super selective arterial interventions because it leads to slow deposition of embolic material in the tiny arterioles and capillaries in the adjacent vascular distributive regions. Embolization can also cause low perfusion injury and further trigger acute ischemic hypoxia within the paracancerous normal liver tissue (normal liver tissue adjacent to tumor). Recent studies have also shown that some serious complications like an acute liver failure, liver infarction or hypoxic injury, postembolization syndrome etc., are possible in patients undergoing TAE [10] and preventing such complications is the main challenge for interventional surgeons. Understanding these complex physiological mechanisms of such complications associated with TAE can help the interventional surgeons in finding out new therapeutic interventional techniques in the effective management of HCC.
When the body is under physiologic stress, especially during acute inflammation due to a stimulus (pathology) from various etiologies, early manifestations include rubor (redness), calor (heat), tumor (swelling), dalor (pain) and functio laesa (loss of function). This inflammatory response (IR) is one of the first and most common pathophysiological reactions that can occur in any organ systems or tissues, which can be presented as pyrexia (fastigium), leukocytosis and generalized symptoms.
Tumor Necrosis Factor alpha (TNF-α) is a cell signaling cytokine protein that is involved in systemic inflammation and one of the cytokines that make up the acute phase reaction. It is produced chiefly by activated mononuclear macrophages [11]. On the other hand, Interleukin 10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF) is primarily produced by monocytes (triggering PD-1) and type 2 T helper cells which have multiple, pleiotropic effects in immunoregulation and inflammation. As a result of inflammation, the content of the inflammatory factors like TNF-α will increase which ultimately causes cell degeneration and further destroys the target local tissue cells.
The factors of inflammation are mediated by TNF-α and the anti-inflammatory response is marked by IL-10 which could be mentioned as disproportionate resistance that occurs during an inflammatory process [12,13]. Under normal physiological states, Nuclear Factor- kappa B (NF-кB) is a dimer, mainly distributed in the cytoplasm that is composed of P105, P100, P50, p52, RELA/p65, c-REL, and RELB7 proteins, and functions as a transcriptional activator protein factor [14]. This plays a critical role in the regulation of gene expression of inflammation related to malignant tumors; inhibitors of this pathway are good for cancer treatment. It is activated by several stimuli as inflammatory cytokines, bacterial endotoxins, hyperglycemia, and hypoxia, thereby activating the survival pathway [15]. Previous studies have shown that NF-κB and inflammatory reaction (IR) plays an important role in the process of cell necrosis [16]. Other inflammatory factors like TNF-α and IL-10 are also regulated by NF- κB [17].
The VX2 liver cancer rabbit models are commonly used to evaluate the efficacy of locoregional anti-cancer therapy experimentally [18]. VX2 tumors can be induced by the Shope cottontail rabbit papillomavirus and is an anaplastic squamous cell carcinoma [19]. These tumors grow rapidly and produce glycolytic large lesions that are appreciable on radiological imaging. So interventional radiologists have found this tumor to be a very useful surrogate in the study of loco-regional liver-directed therapies, mainly for Hepatocellular carcinoma, as it shares physiological similarities with advanced human cancers [20]. So, in this study, we set up rabbit VX2 hepatoma models in parallel to TAE intervention module and observed the expression of NF-κB as well as the changes in the content of TNF-α and IL-10 in the paracancerous tissue. The main aim of this study is to explore this possible role of NF-κB and inflammatory factors like TNF-α and IL-10 in stimulating hepatocytic injury after TAE.
Materials and Methods
■ Materials
Experimental animals: Two pre-modeled rabbits with subcutaneous VX2 tumors in the hind limbs were donated by Affiliated Hospital of Southeast University. Seventy (70) healthy New Zealand white rabbits, both male and female (45:15), with an average weight of 2.8 ± 0.28 kgs (median-2.91 kgs) were provided by the Experimental Animal Center of Dali University; also were fed regularly ad libitum by the designated personnel and were taken good human care by providing clean housing, caging with contiunous supply of water, adequate lighting during whole experimentation. All the rabbits are evaluated prior to experimentation/ modeling as per guidelines set by the Biomedical Ethical committee of Affiliated Hospital of Dali University.
Essential equipment, experimentation instruments, and drug: The whole experiment utilized the following materials that were preequipped such as 16 row Spiral Computed Tomography Unit (Phillips), 3.0 Tesla Magnetic Resonance Imaging System (Toshiba Taitan), Digital subtraction Angiography (DSA) Unit, Interventional Surgery Unit (GE INNOVA- 3100IQ), all the units maintained at The First Affiliated Hospital of Dali University; predesigned experimental rabbit operation table and surgical drapes suitable for rabbits; consumables like multiple gauge syringes, ophthalmic scissors & forceps, kidney trays, plastic containers, Sterile kiosk (solitary workstation for tumor thawing and isolation), arterial clamps, 18G puncture needle, 4F cobra C3 hyrophilic angiographic catheter, 3F, 2.4F sp microcatheter, 45- degree angled hydrophilic micro-guide seldinger wire; 3% phenobarbital sodium for anaesthesia, Gentamycin Sulfate Injection, 0.9% NaCl (Normal Saline) as required, iohexol for sterilization. NF-κB anti-mouse/rabbit polyclonal antibodies (purchased from MlBo Company, Wuhan), two anti-mouse/rabbit reagents, and DAB color reagent (purchased from Dako), IL- 10, TNF-α anti-rabbit double sandwich test kit (purchased from Ancient Biological and Science Technological Laboratories Co. Ltd, Shanghai) were also pre-arranged.
■ Methods
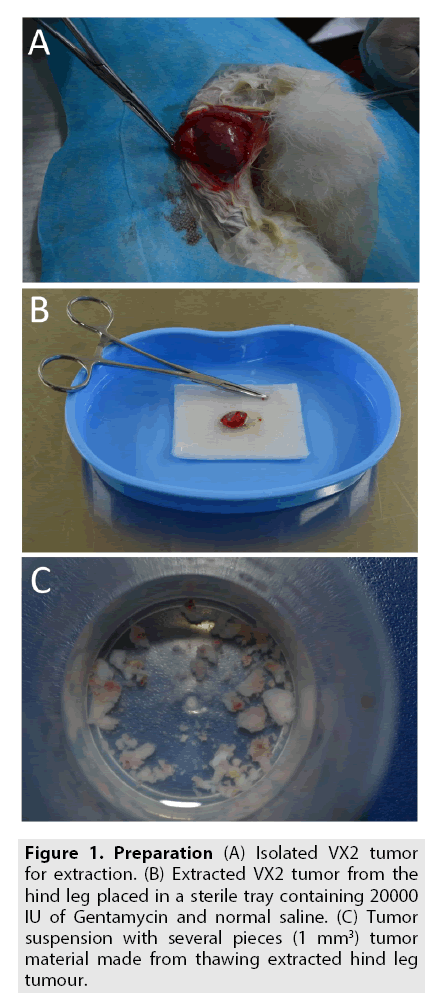
Preparation of the VX2 tumor models in rabbits: One rabbit bearing a VX2 tumor on the hind legs was randomly selected for tumor extraction. 3% phenobarbital sodium (30 mg/ kg) was injected slowly (5 ml/min) through the dorso lateral vein that runs along the pinna until sufficient anesthetic response was achieved. The rabbit was fixed in supine position on the operation table (predesigned); the surgical site was sterilized and draped, exposing the tumor on one of the hind limbs. A vertical incision was made to reach the epidermis and the tumor was carefully extracted isolating it from all the fascial attachments (FIGURE 1A). Surrounding necrotic tissue that spreads till subcutaneous layers were debrided surgically and the incision was closed by subcutaneous interrupted suture technique. The extracted VX2 tumor was placed into a kidney tray containing a mixture of 50 ml normal saline with 20000 IU of Gentamycin (FIGURE 1B). The tumor was then thawed for 15 min using a thawing glass apparatus in the sterile kiosk at 36o to 37°C; then transferred to a sterile plastic container with normal saline. The resultant material was further cut into 1-mm3 pieces using ophthalmic scissors to prepare the tumor tissue suspension (FIGURE 1C). A consistent concentration of the mixture (15-20 tumor particles/ml) from the tumor suspension was aspirated into a 1 ml syringe with an 18G puncture needle head. Six healthy rabbits were randomly selected (out of groups) and the tumor particles were injected subcutaneously (at 45° angle insertion) into the vastus medialis muscle of both the hind legs (0.5 ml for each side) for further preservation and inoculation.
Figure 1: (A) Isolated VX2 tumor for extraction. (B) Extracted VX2 tumor from the hind leg placed in a sterile tray containing 20000 IU of Gentamycin and normal saline. (C) Tumor suspension with several pieces (1 mm3) tumor material made from thawing extracted hind leg tumour.
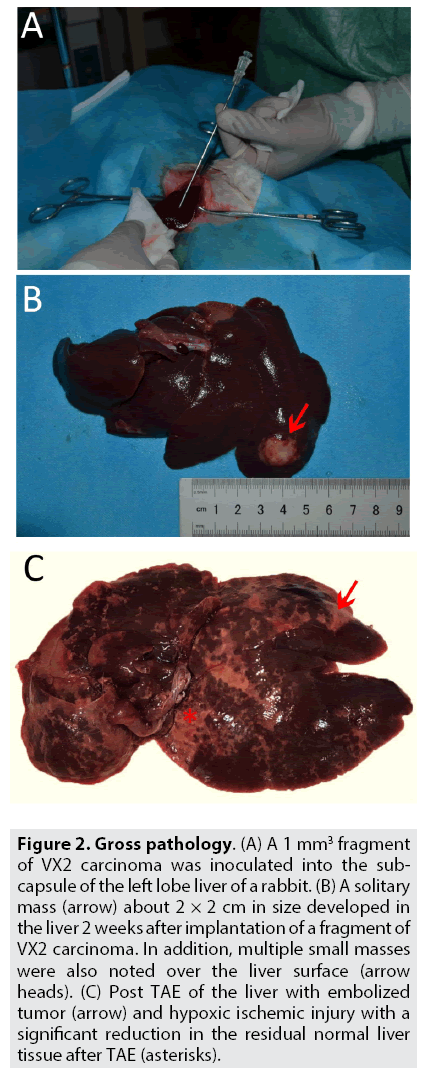
Experimental animals - Preparation of the VX2 liver cancer models: Healthy rabbits were randomly selected and anesthetized using the same method cited supra. The rabbits were sterilized and adequately draped exposing the abdomen. To implant the VX2 carcinoma fragment into the liver, a standard sub-xiphoid laparotomy with an incision 3-5 cm in length was performed. The left lobe of the liver was pulled out gently by holding it with a sterile cotton gauge/swab; a 1 mm3 fragment of VX2 carcinoma was inoculated into the sub capsule of the lateral part of the left lobe of the liver because it has a larger surface area (FIGURE 2A). The puncture site of the liver was compressed with a sterile cotton gauze for 3-5 mins to prevent any unanticipated hemorrhage. Before the wound was closed in layers, 0.5 ml of Gentamycin (40000 IU) was dripped into the peritoneal cavity to prevent infection. All the laparotomized VX2 liver cancer model rabbits underwent the same procedure and were injected with i.m gentamycin injection (40000 IU) [21] for consecutive 3 days, to prevent any post-surgical systemic infection.
Figure 2: Gross pathology. (A) A 1 mm3 fragment of VX2 carcinoma was inoculated into the subcapsule of the left lobe liver of a rabbit. (B) A solitary mass (arrow) about 2 × 2 cm in size developed in the liver 2 weeks after implantation of a fragment of VX2 carcinoma. In addition, multiple small masses were also noted over the liver surface (arrow heads). (C) Post TAE of the liver with embolized tumor (arrow) and hypoxic ischemic injury with a significant reduction in the residual normal liver tissue after TAE (asterisks).
All the modelling was performed at the Experimental Animal Laboratory of Dali University.
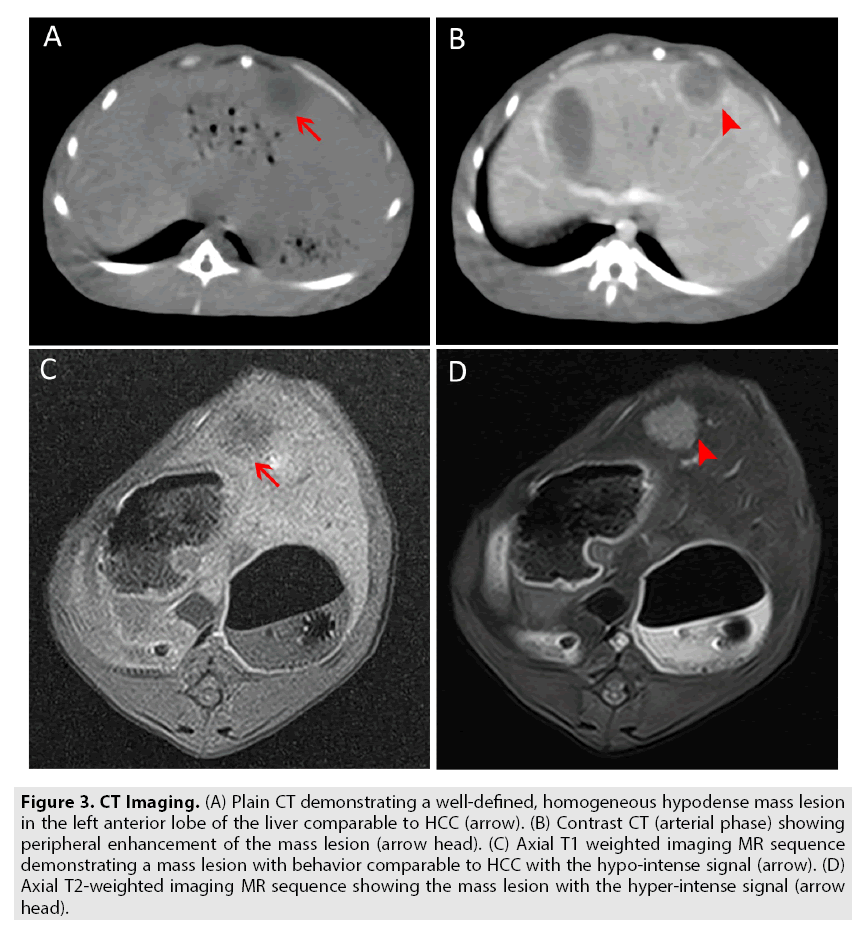
Grouping and procession of experimental models: Each rabbit, modeled with VX2 liver cancer was scanned with CT & MRI after 7, 14, 21, 28 days of inoculation to track the tumor growth respectively. The overall success rate was 85.7% (60/70). Total sixty rabbits were randomly selected. The size, location, and radiographic charecteristics were recorded; the size of the lesion 1.5-2.0 cm on the 21st day, number of lesions, location and imaging characteristics consistent with primary liver cancer was considered as a standard for grouping to replicate different stages of HCC (FIGURE 3A-3D).
Figure 3: CT Imaging. (A) Plain CT demonstrating a well-defined, homogeneous hypodense mass lesion in the left anterior lobe of the liver comparable to HCC (arrow). (B) Contrast CT (arterial phase) showing peripheral enhancement of the mass lesion (arrow head). (C) Axial T1 weighted imaging MR sequence demonstrating a mass lesion with behavior comparable to HCC with the hypo-intense signal (arrow). (D) Axial T2-weighted imaging MR sequence showing the mass lesion with the hyper-intense signal (arrow head).
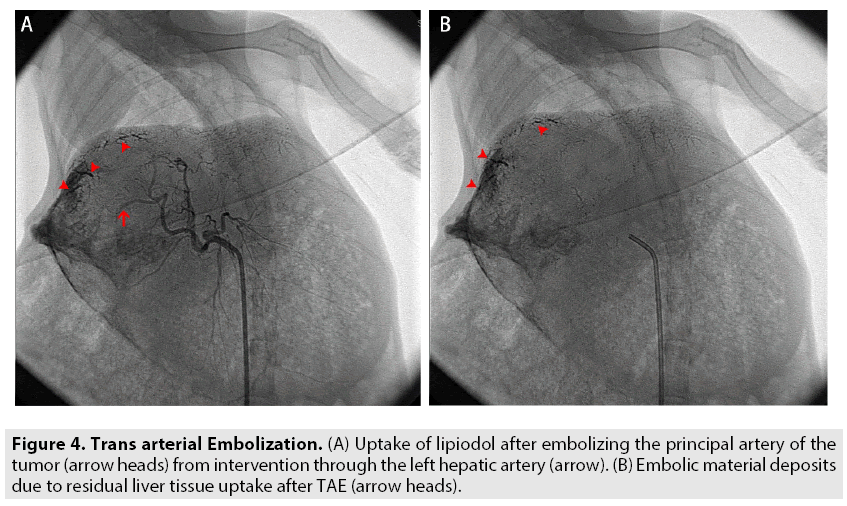
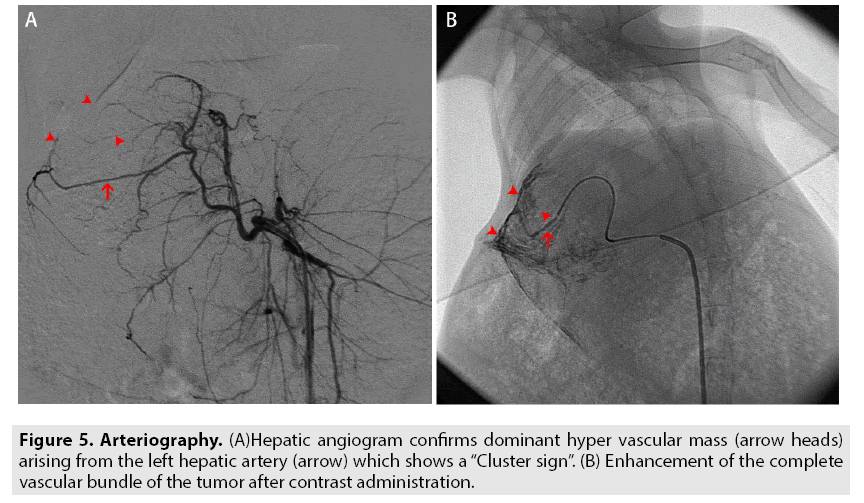
All the sixty rabbits were divided into 3 groups namely: TAE group, Angiography- contrast group, and Control group, accumulating 20 rabbits in each. For TAE group, a transarterial embolization (TAE) was performed by injecting 0.2 ml poppy seed iodized oil (Lipiodol) into the principal hepatic artery of the tumor using "seldinger technique" at the interventional surgery unit (FIGURE 4A and 4B). For the angiography-contrast group, angiography was performed through DSA by injecting non-ionic iodine contrast agent into the hepatic artery (FIGURE 5A and 5B). The control group did not receive any interventional procedures, but the progression of the tumor was monitored on CT/MRI for the consecutive 28 days.
Figure 4: Trans arterial Embolization. (A) Uptake of lipiodol after embolizing the principal artery of the tumor (arrow heads) from intervention through the left hepatic artery (arrow). (B) Embolic material deposits due to residual liver tissue uptake after TAE (arrow heads).
While working on conventional imaging (CT/MRI), DSA and interventional procedures, radiological protocols, and standard sterilization techniques were followed.
Post procedural handling of experimental samples and randomization: Modeled rabbits from each group were euthanized after 28- 35 days for further collection of samples. An incision was made laterally following along the left hypochondrium to mid-epigastrium and extending further along the midline till lower abdomen adequately to gain access to the site of the liver. The liver was completely isolated. The tumor tissue along with surrounding residual normal liver tissue (paracancerous specimens) was separated and cut into pieces >2 cm. Such para-cancerous tissues samples were collected from each group and marked accordingly. Gross pathological specimens for observation were collected (only 2 out of groups) which shows the successful implantation of the VX2 tumor and aprreciable hypoxic ischemic injury after TAE (FIGURE 2B-2C). Each sample was placed in a 5 ml plastic test tube and transferred to a liquid nitrogen cooling tank for the next 15 min; then refrigerated at -800 C for the next 12 h. Randomization was performed after specimen collections, and stored in a sealed, sequentially numbered containers and transferred for further analysis.
HE staining: Thinly sliced sections from each sample were embedded with liquid paraffin and fixated with HE stain. Pathologic analysis of the liver provided a direct reference standard for comparing conventional imaging, angiography and TAE results among the groups to evaluate positivity rate, expression of NF-κB and inflammatory factors.
The determination of expression of NF- кB: All the samples were double stained and analyzed using Envision TM immunohistochemical analysis technique to determine the expression of NF-кB. In accordance to the positive expression of cells and positive color intensity at the detected protein expression sites as observed under 400× (10 × 10 grid) of magnification per FOV (field of view), scores were given as follows:
a) Cell positive expression scoring: if the positive expression of cells was less than 5% -0 points; if the cell expression of cells was less than 25% -1 point; 25-50% -2 points; and >50% -3 points were given respectively.
b) Postive color intensity at the detected protein expression sites scoring: Points were given according to the color of the stain as intensity represented the amount of expressed protein. If the cells did not stain-o points; if cells stained with a light yellow-1 point; yellowishbrown- 2 points; and dark brown-3 points were given respectively.
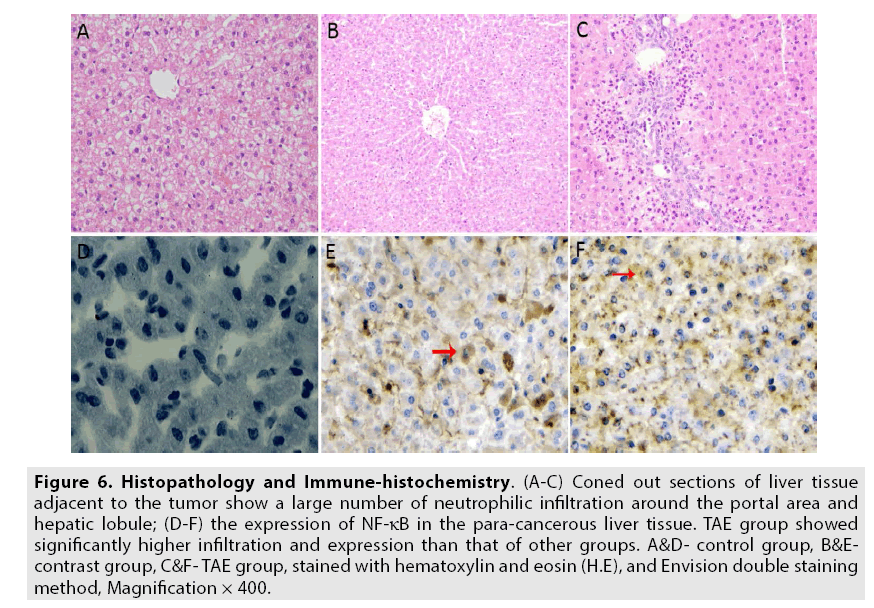
Finally, the average of the sum of both scorings was taken as the basic criterion for determining the results. Overall average <2 points is considered as negative expression (-); 2~3 points- a weak positive expression (+); 4~5 points- a moderately positive expression(++); and 6~7- a strongly positive expression (+++) & (++++) accordingly. The corresponding results are displayed in FIGURE 6A-6F and TABLE 1.
Figure 6: Histopathology and Immune-histochemistry. (A-C) Coned out sections of liver tissue adjacent to the tumor show a large number of neutrophilic infiltration around the portal area and hepatic lobule; (D-F) the expression of NF-κB in the para-cancerous liver tissue. TAE group showed significantly higher infiltration and expression than that of other groups. A&D- control group, B&Econtrast group, C&F- TAE group, stained with hematoxylin and eosin (H.E), and Envision double staining method, Magnification × 400.
| Group | Number of cases | NF-кB expression | Positivity Rate | |||
| - | + | ++ | +++ | |||
| Control Group | 20 | 16 | 1 | 2 | 1 | 20% |
| Angiography Group | 20 | 13 | 2 | 3 | 2 | 35% |
| TAE Group | 20 | 5 | 2 | 6 | 7 | 75% |
Table 1: The expression of NF-кB in the paracancerous tissue specimens.
The detection of the protein content of TNF-α and IL-10: Enzyme-linked immunosorbent assay (ELISA) was utilized to detect the level of TNF-α and IL-10 protein contents in all the specimens according to the instruction manual in the kit.
■ Statistical analysis
Measurement data from all groups were analyzed using a t-test, count data were analyzed using χ2- test and compared by ANOVA as moduled into SPSS Statistics (Ver.22.0; Chicago IL) software package for Windows (Microsoft). If P<0.05, the differences were statistically significant.
Results
■ Analysis of NF-кB expression in VX2 liver cancer model groups
The immune-histochemical analysis results showed that there was a strongly positive expression of NF-кB in 15 cases of the specimens from the paracancerous tissue of the TAE group, the positive rate was 75%; 7 cases had varied positive expression in the angiography group (35%) and the 4 cases were positive in the control group (20%).
The Chi-square test (χ2 - test) results as compared to each group showed that: 1) Angiography group as compared to the control group, (P>0.05) there are no significant statistical differences; 2) TAE group as compared to the angiography group, (P<0.001) there were significant statistical differences and 3) TAE group as compared to the control the group, (P=0.011), there were significant statistical differences as shown in TABLE 1.
■ Analysis of the detection of the protein content of TNF-α and IL-10
The protein content of TNF-α and IL-10 were dominantly high in the TAE group than the angiography group, while the control group has the lowest expression. The results of ANOVA are as follows: 1) Angiography group as compared to the control group, (P>0.05) there were no significant statistical differences; 2) TAE group as compared to the control group, (P<0.05), there were significant statistical differences; and 3) TAE group as compared to the angiography group, (P<0.05) there were significant statistical differences as shown in TABLE 2.
| Group | TNF-α | IL-10 |
|---|---|---|
| Control Group | 8.82+0.81 | 1.66+0.10 |
| Angiography Group | 9.60+0.31* | 1.67+0.12* |
| TAE Group | 11.72+0.65**# | 2.18+0.13**# |
Table 2: The content of TNF-α and IL-10 in the cells adjacent to the cancerous tissue *comparison between the contrast and the control group (P>0.05), ** comparison of TAE group with the control group (P<0.05), # comparison of TAE group with the contrast group (P<0.05).
When compared to the TAE group, many models among the other two groups had significant differences in the positivity rate and had negative expressions of NF-κB, while comparable disproportionate content levels of TNF-α and IL-10 are also evident.
Discussion
The hepatic artery proper that arises from the common hepatic artery, runs alongside the portal vein and the common bile duct to form the portal triad which provides the main vascular supply to the liver. While normal liver tissue receives 75% of its blood supply from the portal vein and 25% from the hepatic artery, the liver tumors are almost exclusively supplied by the feeding arteries arising from the hepatic artery and the liver parenchyma obtains the majority of its blood supply from the portal vein [22]. Therefore, theoretically, embolization (vascular compromise) of the hepatic artery can lead to selective necrosis of the liver tumor, leaving normal liver parenchyma virtually unaffected [23]. It also improves the chances of survival by prolonging the lifetime through assisted compensatory physiologic blood flow from the portal vein. So it has been widely used in the treatment of intermediate and advanced HCC patients, which are most likely inoperable at that stage. However, due to abundant vascular supply from the hepatic portal systems: hepatic sinusoid and the medial branch of the liver, and tumoral branches respectively (FIGURE 5); when the hepatic arterial blood flow is restricted due to embolization, it leads to the inevitable entry of the lipiodol into the residual normal liver tissue adjacent to the cancerous lesion through the anastomosis and blocks surrounding arteries (FIGURE 4). So as a result, though cancer has been eliminated through selective TAE, such patients have a poor prognosis because this vascular disturbance stimulates the paracancerous normal liver tissue to produce a large number of reactive oxygen species (ROS) and activates inflammatory factors namely TNF-α and IL-10 triggering a series of complex endogenous reactions like apoptosis and necrosis that ultimately deteriorates the liver function [24]. Consequently, it has higher mortality rates owing to the eventual development of complete liver failure [25].
TNF being an endogenous pyrogen, it is able to induce fever, apoptotic cell death, cachexia, inflammation and to inhibit tumorigenesis. The tumor necrosis factor receptor superfamily 1A (TNFR1A) is a ubiquitous membrane receptor that binds TNF-α which triggers the destruction of lysosomes so that the endogenous cells are exposed to destructive enzymes [26]. This receptor can also activate the transcription of NF-кB that mediates apoptosis and function as a regulator of inflammation. By the entry of embolic material into the paracancerous normal liver tissue, TNF- α activates the tissue epithelial cells, increases the permeability of capillaries that lead to aggregation of edematous fluid which eventually hinders cell perfusion and gaseous exchange, as a result aggravating the tissue damage [27]. In our study, the strong toxic apoptotic effects of TNF-α are represented by a simultaneous rise in their levels. NF-кB was also elevated significantly after TAE, we can infer that NF-кB also triggered an inflammatory response following a different pathway. This suggests that both NF-кB and TNF-α may play an important role in inducing liver injury and consequently deteriorating the liver function after TAE.
Similarly, the potential of one’s body ability to ward off a disease (resistance) is mediated by anti-inflammatory factors like IL-10 which redirects to remove all the involved inflammatory factors, thereby reducing the influential damage in an anticipation to restore the cell physiology to normal [28]. So theoretically, if proinflammatory and anti-inflammatory factors are at equilibrium, the internal environment in the target cell will relatively be stable and such injury can be repaired eventually; on the contrary, if any one of them is overexpressed which are dependant on conditions like the tumor size, stage of cancer (early-mid-late), metastatic spread, peritonitis, ascites, blood flow rate/pressure-the equilibrium cannot be attained, finally aggravating the damage of target cells [29]. Like TNF-α, IL-10 can also produce reliable immunosuppressive effects on endothelial cells, inhibit tumor metastasis [30,31], and strong anti-inflammatory effects on macrophages that eventually reduce tissue damage [28].
In our study, correlated results among the three groups show that TAE group has a large number of neutrophils infiltrated among tumor cells and in the periphery hepatic lobules (FIGURE 6A-6C), while, the TNF-α and IL-10 contents are also elevated respectively. This suggests that evident serious injury in the paracancerous tissue has taken place. So, it is presumed that peri-inflammatory factors response may be the principal cause of ischemic hypoxia that occurs in the adjacent tissues was triggered only after TAE intervention. Therefore, it can be concluded that IR has taken place. Such a complicated signaling pathway ensures that, whenever TNF-α is released, various cells with vastly diverse physiological functions can all respond to inflammation appropriately.
When the cells are triggered by inflammatory factors such as TNF-α and reactive stimulus such as ischemia, reperfusion injury and, hypoxia etc.- it combines with the inhibitory protein IκBα that normally binds to NF-κB and inhibits its translocation [32]. Further, the oxidative phosphorylation of IκBα by IκB kinase (IKK) and is subsequently degraded, NF-κB is unbound from the receptor protein (sequestering), enters the nucleus- negatively regulating the transcriptional activity i.e inhibiting its translocation [32]. Angiotensin-II type 1 receptor (AT1) downstream signaling is recognized to lead to the activation of NF-κB [33], thereby regulating the reaction of inflammatory factors and finally producing a protective effect on the body [34]. Other studies have found that severe ischemia and anoxia can lead to the excessive activation of NF-κB and enhance the expression of the downstream inflammatory factor TNF-α, which further stimulates the synthesis of IL-10, antagonizing inflammation and elevating anti-inflammatory factors during IR process. This causes cell necrosis, eventually leading to tissue damage [35]. The results of our study show that as compared with the control group and the contrast groups; the positive expression rates of NF-κB was significantly increased in the adjacent tissues.
In various scenarios conducted in our experiment, as a result of various stimuli such as TAE intervention, contrast material (angiography), tumor implantation and progression etc.; the TAE group showed highest positivity rate of NF-κB along with a proportionate rise of TAE dependent TNF-α and IL-10 content levels owing to deposition of embolic material in the paracancerous normal liver tissue; but in the other two groups, low rates of NF-κB positive expression along with disproportionate, moderate, independent rise in the inflammatory cytokines (TNF-α, IL-10) content levels indicate the disproportionate resistance (IR and anti-inflammation reactions). These variations are due to the progression of the tumor during the determined timeline framed in the experimentation and various other associated pathophysiological changes depending on applied stimulus. This also suggests that NF-κB did not get elevated until TAE was performed.
Particularly after undergoing TAE, following a series of complex reactions mentioned, this study reveals the role of NF-κB in triggering IR to cause hepatocytic injury and activating inflammatory cytokines who further deteriorated overall hepatic function. However, more research to gain control over the IR process so as to reduce the extent of the liver injury or even exploring newer techniques to prevent TAE induced hepatic dysfunction is necessary which can improve the survival rate and decrease mortality.
Conclusion
We can conclude that the injury to the paracancerous liver tissue after TAE may be caused by ischemic reperfusion injury i.e hypoxia/ anoxia triggered by intraoperative embolic agents entering the residual liver tissue. This has lead to excessive activation of NF-κB and regulation of the downstream synthesis of inflammatory factors like TNF-α and IL-10 which lead to the inequilibrium of pro-inflammatory and anti-inflammatory factory along the IR process. By inhibiting the overexpression of NF-κB, we can reduce the excessive synthesis of TNF-α and IL-10, thereby preventing the inequilibrium of pro-inflammatory and anti-inflammatory factors. Our future projects endeavor to target NF-κB and research interventional methods with various embolization materials to prevent this damage to residual normal liver tissue and preserve the hepatic function that is degrading which is induced after TAE.
Declarations
■ Ethics approval and consent to participate
This publication and study protocols were approved by the Biomedical Ethics committee of The First Affiliated Hospital of Dali University. Consent to participate is not applicable.
■ Consent to publish
Not applicable
■ Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
■ Competing interests
The authors declare that they have no competing interests to disclose.
■ Funding
This work was supported by the National Natural Science Foundation of China (Grant No: 81241131, 81660300); The Department of Medical Imaging and Interventional Surgery, the First Affiliated Hospital of Dali University, key subjects for medical imaging and study.
■ Author contributions
BV and W-BR contributed to the manuscript by writing the original draft, reviews, editing, conception and design, modelling and sampling, data collection. DW contributed to conceptualization, experimentation, interventional surgical procedures, data curation, reviews. YY-J and WC-H contributed with materials and methods, interventions, post-processing, analysis. KS contributed to literature reviews, editing, randomization and statistical data compalition. All authors read and approved the final manuscript.
■ Acknowledgements
The authors would like to thank Dr. Wang Guang Ming, Department of Pathology and Immunehistochemistry, The First Affiliated Hospital of Dali Uniersity for providing high quality images and expertise in the pathological analysis. We would also like to thank Dr.Zhou Zhou along with Dr. Duan Wen Shuai in conducting and supervising the interventional and DSA procedures. The authors appreciate the support and participation of all the personnel of the Department of Radiology throughout this experiment.
References
- Sun H, Han B, Zhai H et al. Significant association between MTHFR C677T polymorphism and hepatocellular carcinoma risk: A meta-analysis. J.Tumor. Biology. 35: 189-193, (2014).
- Ma KW, Cheung TT. Surgical resection of localized hepatocellular carcinoma: patient selection and special consideration. J. Hepatocell. Carcinoma. 4: 1-9, (2016).
- Vogl TJ, Straub R, Eichler K et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology. 225: 367- 377, (2002).
- Midorikawa T, Kumada K, Kikuchi H et al. Microwave coagulation therapy for hepatocellular carcinoma. J. Hepatobiliary. Pancreat. Surg. 7: 252-259, (2000).
- Allgaier HP, Deibert P, Zuber I et al. Percutaneous radiofrequency interstitial thermal ablation of small hepatocellular carcinoma. Lancet. 353: 1676-1677, (1999).
- Olumide BG, Michael SA, Eric Wb et al. Locoregional and systemic therapy for hepatocellular carcinoma. J. Gastrointest. Oncol. 8: 215-228, (2017).
- Liang YJ, Yu H, Feng G et al. High-Performance Poly(lactic-co-glycolic acid) - Magnetic Microspheres Prepared by Rotating Membrane Emulsification for Transcatheter Arterial Embolization and Magnetic Ablation in VX2 Liver Tumors. ACS. Appl. Mater. Interfaces. 9: 43478-43489, (2017).
- Lu D, Abulimiti A, Wu T et al. Pulmonary surfactant-associated proteins and inflammatory factors in obstructive sleep apnea. Sleep. Breath. 22: 99-107, (2018).
- Epanchintsev A, Shyamsunder P, Verma RS et al. IL-6, IL-8, MMP-2, MMP-9 are overexpressed in Fanconi anemia cells through an NF-kappaB/TNF-alpha dependent mechanism. J.Mol. Carcinog. 54: 1686-1699, (2014).
- Alda LT, Marites P, Joe Ensor et al. Rabbit hepatic artery anatomical variations: Implications on experimental design. Acta. Radiol. 55: 1226-1233, (2014).
- Shope RE, Hurst EW. Infectious papillomatosis of rabbits. J. Exp. Med. 58: 607-624, (1933).
- Wang D, Bangash AK, Rhee TK et al. Monitoring embolization in rabbits with VX2 tumors-transcatheter intraarterial first-pass perfusion MR imaging. Radiology. 245: 130-139, (2007).
- Chen JH, Lin YC, Huang YS et al. Induction of VX2 carcinoma in rabbit liver: comparison of two inoculation methods. Lab. Anim. 38, 79-84 (2004).
- Wang LQ, Persson BG, Bergqvist L et al. Influence of dearterialization on the distribution of absolute tumor blood flow between hepatic artery and portal vein. Cancer. 74: 2454-2459, (1994).
- Jaeger HJ, Mehring UM, Castaneda F et al. Sequential transarterial chemoembolization for unresectable advanced hepatocellular carcinoma. Cardiovasc. Intervent. Radiol. 19: 388-396, (1996).
- Kennedy KA, Rockwell S, Sartorelli AC. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer. Res. 40: 2356-2360, (1980).
- Pan SS, Andrews PA, Glover CJ et al. Reductive activation of mitomycin C and mitomycin C metabolites catalyzed by NADPH-cytochrome P-450 reductase and xanthine oxidase. J. Biol. Chem. 259: 959-966, (1984).
- Izumi Miki, Satoru Murata, Fumio Uchiyama et al. Evaluation of the relationship between hepatocellular carcinoma location and trans arterial chemoembolization efficacy. J. World. J. Gastroenterol. 23: 6437-6447, (2017).
- Guo HQ, Che Chidan, Yan peng et al. Transcatheter super-selective hepatic arterial embolization for the treatment of polycystic liver disease: a preliminary study. J. Intervent. Radiol. 24: 676-679, (2015).
- Sakamoto I, Aso N, Nagaoki K et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 18: 605-619, (1980).
- Chen L, Deng H, Cui H et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 9: 7204-7218, (2018).
- Seleme MC, Kosmac K, Jonjic S et al. Tumor necrosis factor alpha-induced recruitment of inflammatory mononuclear cells leads to inflammation and altered brain development in murine cytomegalovirus infected newborn mice. J. Virol. 91: 1-22, (2017).
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8: 958-969, (2008).
- Schall TJ, Lewis M, Koller KJ et al. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 61: 361-370, (1990).
- Khoshdel A, Kheiri S, Omidvari P et al. Association between Interleukin-10-1082 G/A and Tumor Necrosis Factor-α 308 G/A Gene Polymorphisms and Respiratory Distress Syndrome in Iranian Preterm Infants. Mediators. Inflamm. 1: 638-645, (2017).
- Zheng LM, Ojcius DM, Garaud F et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J. Exp. Med. 184: 579-584, (1996).
- Men T, Yu C, Dan W et al. The impact of interleukin-10 (IL-10) gene 4 polymorphisms on peripheral blood IL-10 variation and prostate cancer risk based on published studies. Oncotarget. 8: 45994-46005, (2017).
- Palacz WM, Borko WP, Paul SM et al. Effect of apigenin, kaempferol, and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 93: 1205-1212, (2017).
- Zhang Y, Hu F, Wen J et al. Effects of sevoflurane on NF-кB and TNF-α expression in renal ischemia–reperfusion diabetic rats. J.Inflamm. Res. 66: 901-910, (2017).
- Bakhtiari E, Hosseini A, Boroushaki M et al. Angiotensin II receptor antagonist olmesartan and NF-kappaB inhibitor as cytotoxic and apoptotic agents in the MCF-7 human cell line. J. Chemother. 28: 314-320, (2016).
- Kim HJ, Hawke N, Baldwin AS et al. NF-kappaB and IKK as therapeutic targets in cancer. J. Cell. Death. Differ. 13: 738-747, (2006).
- Jingjing Hou, Shihao Jiang, Jiabao Zhao et al. N-Myc-Interacting Protein Negatively Regulates TNF-alpha-Induced NF-kappaB Transcriptional Activity by Sequestering NF-kappaB/p65 in the Cytoplasm. J.Sci. Rep. 7: 1-14, (2017).
- Nagai N, Izumi NK, Oike Y et al. Suppression of diabetes- induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kB pathway. J.Invest. Ophthalmol. Vis. Sci. 48: 4342-4350, (2007).
- Li W, Wang X, Niu X et al. Protective Effects of Nobiletin Against Endotoxic Shock in Mice Through Inhibiting TNF-α, IL-6, and HMGB1 and Regulating NF-κB Pathway. J.Inflammation. 39: 786-797, (2016).
- Chandra K, Gogia A, Kakar A et al. To study co-relation of inflammatory marker TNF-α in diabetic mellitus patients and healthy nondiabetic adults. J.Curr. Med. Res. Pract. 6: 8-11, (2016).