Mini Review - Interventional Cardiology (2022) Volume 14, Issue 1
Endocardial radiofrequency ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy: A review of literature and comment on risks
- Corresponding Author:
- Thorsten Lawrenz
Department of Cardiology and Intensive Care Medicine,
Klinikum Bielefeld gemGmbH,
University hospital OWL,
Teutoburger Straße 50, 33604 Bielefeld,
Germany,
E-mail: thorsten.lawrenz@klinikumbielefeld.de
Received date: 30-Nov-2021, Manuscript No. FMIC-21-48753; Editor assigned: 02-Dec-2021, PreQC No. FMIC-21-48753 (PQ); Reviewed date: 16-Dec-2021, QC No. FMIC-21-48753; Revised date: 23-Dec-2021, Manuscript No. FMIC-21-48753 (R); Published date: 28-Dec-2021, DOI: 10.37532/1755-5310.2022.14(1).444
Abstract
Endocardial Radiofrequency Ablation of Septal Hypertrophy (ERASH) has been developed for patients suffering from Hypertrophic Obstructive Cardiomyopathy (HOCM) who are not eligible for Septal Myectomy (SM) or Alcohol Septal Ablation (ASA).
The existing data regarding clinical outcomes of ERASH is scarce. Therefore, we reviewed the literature on acute and long-term outcomes of ERASH with focus on potential procedure-related risks and complications.
In the published studies ERASH effectively reduced the LVOTG and improved diseaserelated symptoms in acute and chronic follow-up. 17.1% of the overall 99 reported patients had a procedure-related high-degree AV block. A paradoxical increase in obstruction, a life-threatening complication, occurred in 7.1% of the patients treated with ERASH. It was associated with progressive obstruction of the left ventricular outflow tract and mitral regurgitation due to pronounced systolic anterior movement of the anterior mitral valve leaflet. PIO led to death in 2 patients.
In conclusion, ERASH is feasible and effective for the treatment of patients with HOCM irrespective of the underlying coronary anatomy. However, the incidence of complications in the published studies was higher compared to ASA and SM and, thus, ERASH should only be applied to those patients who are not suitable for ASA and SM.
Keywords
Endocardial radiofrequency ablation • Septal hypertrophy • Hypertrophic Obstructive cardiomyopathy • Paradoxical increase in obstruction
Abbreviations
ASA: Alcohol Septal Ablation; ERASH: Endocardial Radiofrequency Ablation of Septal Hypertrophy; FU: Follow-Up; HOCM: Hypertrophic Obstructive Cardiomyopathy; LVOTG: Left Ventricular Outflow Tract Gradient; PIO: Paradoxical Increase in Obstruction; RF: Radiofrequency; RV: Right Ventricular; SAM: Systolic Anterior Movement; SM: Septal Myectomy
Introduction
Hypertrophic cardiomyopathy is a common myocardial disease with an estimated prevalence of 0.2% in the younger population [1]. The presence of Left Ventricular Outflow Tract (LVOT) obstruction is associated with more pronounced symptoms and a higher risk for heart failure and death [2]. Septal Myectomy (SM) and catheterbased Alcohol Septal Ablation (ASA) are recommended to reduce the LVOT gradient (LVOTG) in drug-refractory Hypertrophic Obstructive Cardiomyopathy (HOCM) [3]. However, ASA is not feasible in some patients due to unfavorable coronary anatomy of the obstructing septal bulge [4,5]. Thus, Endocardial Radiofrequency Ablation of Septal Hypertrophy (ERASH) has been developed for patients who are not eligible for ASA and reject SM [6]. The existing data regarding outcomes of ERASH is scarce and based on only few studies including small numbers of patients [7-14]. The aim of this article is to review the literature on acute and long-term outcomes after ERASH with focus on potential procedure-related risks and complications.
Literature Review
Overview of literature
ERASH was first described in 2004 in a 45-year-old male patient with severe HOCM who was treated at our institution [6]. Later, we reported the long-term outcomes of 19 patients in 2011 [14]. Our review of literature identified 7 more study cohorts with overall 99 patients in whom ERASH was performed (Table 1) [7-14]. The largest analysis refers to 41 patients published in 2021 [10]. The studies included adults and children (age between 2 and 81 years) who had persistent symptoms despite previous therapeutic attempts or in whom ASA or SM were not feasible (Table 1) [7- 14]. All patients had severe HOCM with interventricular septal diameters >18 mm and LVOTGs >50 mmHg at baseline [7-14].
Procedural aspects
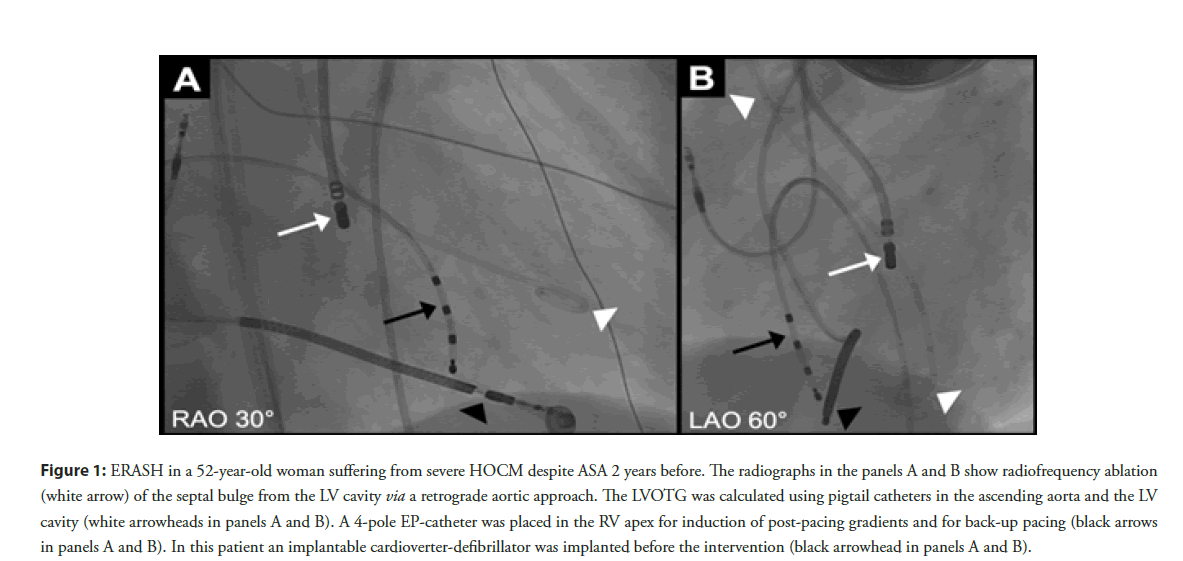
During the procedure patients were either under conscious sedation [11,14] or general anesthesia [7-9,12]. ERASH was most frequently performed from the LV cavity via an either retrograde aortic or more rarely transseptal approach (Table 1 and Figure 1) [7-14]. However, our working group also performed ERASH from the Right Ventricular (RV) cavity in 37% of the cases due to safety considerations in patients with very severe LVOT obstruction (Table 1) [10,14]. A three-dimensional mapping system was often used to identify the septal bulge and the bundle of his [7-11,13,14]. Moreover, some authors performed transesophageal [9,12] or intracardiac echocardiography [7,10,11,13,14] to visualize the appropriate position of the catheter tip in the target region.
Endocardial ablation was performed using Radiofrequency (RF) energy to induce a localized myocardial damage of the obstructing septal bulge (Figure 1) [6]. Most authors used 4-mm irrigatedtip catheters for ablation [7-14]. The delivered RF energy differed between 30 and 70 W (mean 50 W) in the published literature (Table 1) [7,8,10-13].
For continuous monitoring of the LVOTG during the procedure 2 catheters were placed in the ascending aorta and the LV cavity (Figure 1) [10,14]. An electrophysiology catheter placed in the RV apex enabled the provocation of gradients by premature paced beats and served for back-up pacing during the intervention and in the post-procedural monitoring phase [10,14].
Figure 1: ERASH in a 52-year-old woman suffering from severe HOCM despite ASA 2 years before. The radiographs in the panels A and B show radiofrequency ablation (white arrow) of the septal bulge from the LV cavity via a retrograde aortic approach. The LVOTG was calculated using pigtail catheters in the ascending aorta and the LV cavity (white arrowheads in panels A and B). A 4-pole EP-catheter was placed in the RV apex for induction of post-pacing gradients and for back-up pacing (black arrows in panels A and B). In this patient an implantable cardioverter-defibrillator was implanted before the intervention (black arrowhead in panels A and B).
Among all published studies ERASH was feasible and not limited by technical restrictions even when performed in children [7-14].
Efficacy and clinical outcome
ERASH effectively reduced the LVOTG and improved diseaserelated symptoms in acute and long-term follow-up in all studies (FU; Table 1) [7-14]. Some analysis also revealed a reduction of the interventricular septal diameter (Table 1) [7,13]. The longest post-procedural FU was 12 months [8,11].
With our recently published analysis of a 6-months-FU in a cohort of 41 patients we were able to confirm the efficacy of the procedure [10]. The LVOTG was significantly reduced immediately after ERASH and during FU resulting in improved exercise capacity measured as the 6-minutes-walk-distance [10]. However, 3 patients of our study cohort needed further septal reduction therapies due to insufficient LVOTG reduction and persisting symptoms [10].
Complications
Although the above mentioned results indicate overall good efficacy of ERASH regarding symptom improvement and reduction of the LVOTG, harms and risks of the procedure may be underestimated due to only small numbers of patients included in most of the trials [7-14]. In Table 2 we summarize the major procedure-related complications which were observed in the reviewed studies. 17 of the reported 99 patients (17.2%) needed a pacemaker due to a procedure-related high-degree AV block [7-14]. Thus, the rate of pacemaker dependency was higher compared to ASA (10%) and SM (5%) [15]. In 3 patients (3.0%) a pericardial effusion due to catheter perforation occurred [8,10]. In 1 of these patients pericardiocentesis was necessary, while 1 patient required surgical repair and 1 was treated conservatively [8,10]. Ventricular fibrillation during the procedure was observed in 3 patients (3.0%) [9,12].
| Authors | Year | Pts. | Age in years mean (min-max) | Baseline IVSD in mm mean | FU IVSD in mm mean | Baseline LVOTG at rest in mmHg mean | FU LVOTG at rest in mmHg mean | Mean RF power in Watt min-max | LV-ablation site in % |
|---|---|---|---|---|---|---|---|---|---|
| Cooper, et al. [7] | 2016 | 5 | 59 (44-79) | 18.3 | 16.8 | 64.2 | 12.6 | 50-60 | 100 |
| Crossen, et al. [8] | 2016 | 11 | 62 (50-81) | 21.0 | 20.0 | 66.7 | 10.0 | 50 | 100 |
| Emmel, et al. [9] | 2005 | 2 | 8 (5-11) | N | N | 55.0 | 25.0 | N | 100 |
| Lawrenz, et al. [6,10,14] | 2004, 2011, 2021 |
41 | 59 | 21.6 | N | 65.1 | 29.5 | 40-75 | 63 |
| Riedlbauchová, et al. [13] | 2013 | 1 | 63 | 22.0 | 14.0 | 99.0 | 15.0 | 35-40 | 100 |
| Shelke, et al. [11] | 2016 | 7 | 43 (21-62) | N | N | 81.0 | 42.9 | 30-40 | 100 |
| Sreeram, et al. [12] | 2011 | 32 | 11 (2-17) | N | N | 96.9 | 32.7 | 60 | 100 |
Abbreviations: FU: Follow-Up; IVSD: Interventricular Septal Diameter; LVOTG: Left Ventricular Outflow Tract Gradient, N: Not documented; Pts.: Patients; RF: Radiofrequency
Table 1: Baseline characteristics and procedural aspects in the reviewed studies.
| Authors | Year | Pts. | AV-block | VF | Pericardial effusion | PIO | Death | Reason for death | Therapy of PIO |
|---|---|---|---|---|---|---|---|---|---|
| Cooper, et al. [7] | 2016 | 5 | 1 | 0 | 0 | 1 | 1 | retroperitoneal haemorrhage | dexamethasone, RV pacing |
| Crossen, et al. [8] | 2016 | 11 | 2 | 0 | 1 | 1 | 0 | - | diuretics, betablockers |
| Emmel et al. [9] | 2005 | 2 | 0 | 1 | 0 | 0 | 0 | - | - |
| Lawrenz et al. [6,10,14] | 2004, 2011, 2021 |
41 | 12 | 0 | 2 | 4 | 1 | PIO | Impella®pump, SM, mitral valve replacement, prednisolone |
| Riedlbauchová, et al. [13] | 2013 | 1 | 0 | 0 | 0 | 0 | 0 | - | - |
| Shelke, et al. [11] | 2016 | 7 | 0 | 0 | 0 | 0 | 0 | - | - |
| Sreeram, et al. [12] | 2011 | 32 | 2 | 2 | 0 | 1 | 1 | PIO | extracorporal membrane oxygenation |
Abbreviation: FU: Follow-Up; PIO: Paradoxical Increase in Obstruction; Pts.: Patients; SM: Septal Myectomy; VF: Ventricular Fibrillation
Table 2: Procedure-related complications in the reviewed studies.
Of the 99 patients reported there were 3 procedure-related deaths [7,10,12,14]. One patient died due to retroperitoneal haemorrhage despite urgent surgical repair [7]. The other two patients died from a Paradoxical Increase in Obstruction (PIO) of the LVOT [10,12]. This complication occurred in 7 of the 99 patients (7.1%) who underwent ERASH in published studies [7-14].
Discussion
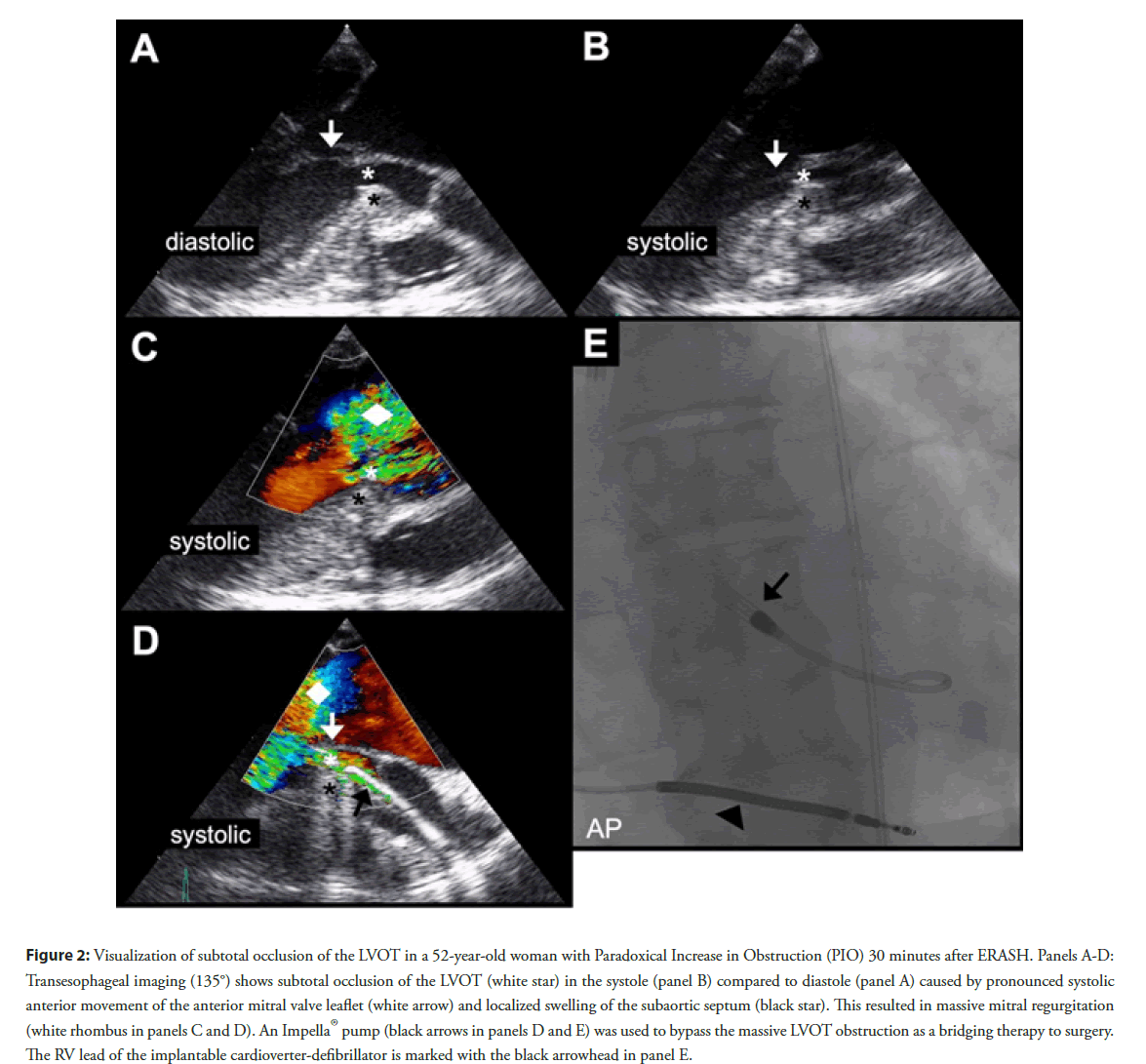
With this article we aim to attract attention to PIO, an ERASHspecific complication, which has not been noted after ASA or SM [15]. In our recently published patient cohort the affected patients experienced chest pain and dyspnea and 2 of the 4 patients developed rapid cardiogenic shock within 30 minutes after the procedure [10]. Echocardiography revealed a progressive obstruction of the LVOT and high-degree mitral regurgitation due to pronounced Systolic Anterior Movement (SAM) of the anterior mitral valve leaflet (Figure 2). We suspect ablation induced edema of the septal bulge as the underlying cause. In the 2 cases of severe PIO an Impella® pump was used as bridge to surgery (Figure 2) but, unfortunately, one of these patients died [10]. The other patient, a 52-year-old woman (Figure 2), was treated with highdose corticosteroids in addition to the Impella®pump and was referred for urgent SM one day after ERASH. Figure 2 illustrates the transesophageal echocardiography images indicating severe mitral regurgitation due to pronounced SAM of the anterior mitral valve leaflet. The subtotally narrowed LVOT was bypassed using the Impella®pump (Figure 2). The other two patients with mild PIO in our study survived without necessity of mechanical circulatory support [10]. Cooper, et al. also reported 1 patient with PIO who was treated with high-dose corticosteroids and RV pacing and survived without sequelae [7]. In another cohort of 11 patients undergoing ERASH Crossen, et al. described a patient with marked dynamic LVOTG and pulmonary congestion which was successfully treated with betablockers and diuretics [8]. In a group of children, in whom ERASH was performed, PIO occurred in a 4-year-old-girl who developed acute cardiac failure and died despite immediate implantation of an extracorporal membrane oxygenator [12].
Figure 2: Visualization of subtotal occlusion of the LVOT in a 52-year-old woman with Paradoxical Increase in Obstruction (PIO) 30 minutes after ERASH. Panels A-D: Transesophageal imaging (135°) shows subtotal occlusion of the LVOT (white star) in the systole (panel B) compared to diastole (panel A) caused by pronounced systolic anterior movement of the anterior mitral valve leaflet (white arrow) and localized swelling of the subaortic septum (black star). This resulted in massive mitral regurgitation (white rhombus in panels C and D). An Impella®pump (black arrows in panels D and E) was used to bypass the massive LVOT obstruction as a bridging therapy to surgery. The RV lead of the implantable cardioverter-defibrillator is marked with the black arrowhead in panel E.
The reported cases of PIO following ERASH emphasize that PIO constitutes a serious and life-threating complication. The occurrence of PIO requires urgent treatment to prevent cardiogenic shock and death. Therefore, the institutions performing ERASH should provide the ability of mechanical circulatory support to manage PIO and establish the infrastructure for an urgent referral to SM.
Moreover, we need to identify predictors of PIO to reduce this procedural risk. In our recently published analysis the patients with PIO did not have differences regarding the interventricular septal diameter compared to the patients without PIO [10]. There was a trend for higher baseline LVTOGs in patients with PIO compared to those without but this difference was not statistically significant [10]. Interestingly, all PIOs occurred only in patients in whom ERASH was performed from the LV cavity [7,8,10,12]. The 4 patients with PIO at our institution were treated using a high-power approach with a mean RF power of 69.2 W whereas PIO was not observed in the low-power series [10]. The 2 patients with PIO reported by Cooper, et al. and Sreeram, et al. were treated with a RF power of 60 W [7,12]. However, Crossen, et al. describe a case of PIO following ERASH using a maximum output of 50 W [8]. Despite of the still limited experience with PIO and the lack of precise instructions for the prevention and treatment of this severe complication due to the small number of studies we recommend to perform ERASH using only low RF energy <60 W, based on our experience.
Conclusion
In the published studies ERASH was feasible and effective for the treatment of LVOT obstruction in patients with HOCM irrespective of the underlying coronary anatomy. However, the incidence of complications in the published studies was higher compared to ASA and SM. PIO, a life-threatening complication, occurred in 7.1% of the patients treated with ERASH. Thus, ERASH should only be applied to those patients who are not suitable for ASA and SM.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
This research was not supported by any funding.
References
- Maron BJ, Gardin JM, Flack JM. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study: Coronary artery risk development in (Young) Adults. Circulation. 92(4): 785-789 (1995).
[CrossRef] [Google scholar] [Pubmed]
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 348(4): 295-303 (2003).
[CrossRef] [Google scholar] (All versions) [Pubmed]
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 142(25): e533-e557 (2020).
[CrossRef] [Google scholar] [Pubmed]
- Faber L, Seggewiss H, Welge D, et al. Echo-guided percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: 7 years of experience. Eur J Echocardiogr. 5: 347-355 (2004).
[CrossRef] [Google scholar] [Pubmed]
- Chan W, Williams L, Kotowycz MA, et al. Angiographic and echocardiographic correlates of suitable septal perforators for alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Can J Cardiol 30: 912-919 (2014).
[CrossRef] [Google scholar] [Pubmed]
- Lawrenz T, Kuhn H. Endocardial radiofrequency ablation of septal hypertrophy. A new catheter-based modality of gradient reduction in hypertrophic obstructive cardiomyopathy. Z Kardiol. 93(6): 493-499 (2004).
[CrossRef] [Google scholar] [Pubmed]
- Cooper RM, Shahzad A, Hasleton J, et al. Radiofrequency ablation of the interventricular septum to treat outflow tract gradients in hypertrophic obstructive cardiomyopathy: A novel use of CARTOSound® technology to guide ablation. Europace. 18: 113-120 (2016).
[CrossRef] [Google scholar] [Pubmed]
- Crossen K, Jones M, Erikson C. Radiofrequency septal reduction in symptomatic hypertrophic obstructive cardiomyopathy. Heart Rhythm. 13: 1885-1890 (2016).
[CrossRef] [Google scholar] (All versions) [Pubmed]
- Emmel M, Sreeram N, deGiovanni JV, et al. Radiofrequency catheter septal ablation for hypertrophic obstructive cardiomyopathy in childhood. Z Kardiol. 94(10): 699-703 (2005).
[CrossRef] [Google scholar] (All versions) [Pubmed]
- Lawrenz T, Lawin D, Radke K, et al. Acute and chronic effects of endocardial radiofrequency ablation of septal hypertrophy in HOCM. J Cardiovasc Electrophysiol. 32(10): 2617-2624 (2021).
[CrossRef] [Google scholar] [Pubmed]
- Shelke AB, Menon R, Kapadiya A, et al. A novel approach in the use of radiofrequency catheter ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy. Indian Heart J. 68(5): 618-623 (2016).
[CrossRef] [Google scholar] [Pubmed]
- Sreeram N, Emmel M, Giovanni JV de. Percutaneous radiofrequency septal reduction for hypertrophic obstructive cardiomyopathy in children. J Am Coll Cardiol. 58(24): 2501-2510 (2011).
[CrossRef] [Google scholar] [Pubmed]
- Riedlbauchová L, Janoušek J, Veselka J. Ablation of hypertrophic septum using radiofrequency energy: An alternative for gradient reduction in patient with hypertrophic obstructive cardiomyopathy? J Invasive Cardiol. 25(6): E128-132 (2013).
- Lawrenz T, Borchert B, Leuner C, et al. Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: acute results and 6 months’ follow-up in 19 patients. J Am Coll Cardiol. 57(5): 572-576 (2011).
[CrossRef] [Google scholar] [Pubmed]
- Osman M, Kheiri B, Osman K, et al. Alcohol septal ablation vs. myectomy for symptomatic hypertrophic obstructive cardiomyopathy: Systematic review and meta-analysis. Clin Cardiol. 42(1): 190-197 (2019).
[CrossRef] [Google scholar] [Pubmed]