Device Evaluations - Interventional Cardiology (2011) Volume 3, Issue 2
Endovascular aortic aneurysm repair: association between anatomical fixation and outcomes using the Powerlink device
- Corresponding Author:
- Dieter Raithel
Department of Vascular and Endovascular Surgery
Nuremberg, Southern Hospital, Nuremberg 90471, Germany
Tel: +49 913 154 700
Fax: +49 913 154 715
E-mail: dieter.raithel@rzmail.uni-erlangen.de
Abstract
Keywords
abdominal aortic aneurysm, endovascular repair, juxtarenal, percutaneous
Since the first endovascular repair of abdominal aortic aneurysm (endovascular aneurysm repair [EVAR]) was reported in 1991 [1], this less invasive approach has steadily increased in prevalence among vascular specialists who treat these patients. Randomized trials have demonstrated significant perioperative benefits versus open surgical repair, including increased survival, reduced morbidity, significantly better clinical utility outcomes and faster time to recovery [2–4]. Among recent reports involving meta-analysis or population-based observational analysis, these findings are replicated; interestingly, although long-term all-cause mortality is not different among treatment groups, endovascular repair was found to yield reduced longterm aneurysm-related mortality [5] or reduced laparotomy-related interventions compared with open surgery [6]. Yet it is estimated that approximately 25–40% of patients with abdominal aortic aneurysm are not suitable candidates for EVAR owing to anatomical challenges such as limited access vessel availability, a narrow distal aorta, or complex proximal neck characteristics such as severe angulation, short length, irregular shape, significant thrombus, or competing angles in the suprarenal and infrarenal segments [7].
As experience with EVAR has progressed, so have the device technologies and techniques. Initially, bifurcated stent grafts were designed to parallel surgical grafts, having short bodies and long limbs. This design requires that the devices are hung in the proximal infrarenal aorta to achieve both fixation and seal using a combination of radial force and either infrarenal or suprarenal placement, with or without penetrating hooks or barbs. The main device is modular in structure, meaning the repair requires cannulation of the contralateral gate and implant/attachment of the stent graft limb on that side. Other components are then added to complete the repair. Although devices have improved over time, an increasing incidence of postoperative complications can occur in challenging anatomy, such as thrombosis, modular component separation, stent fracture or dislodgement, distal migration and endoleaks with the potential for aneurysm enlargement. The most prevalent and concerning are migration, defined by the Society for Vascular Surgery [8] as device movement of greater than 10 mm, or lesser device movement necessitating secondary intervention, and type I endoleak, owing to the increased risk of aneurysm rupture. Migration has a reported incidence of up to 45% [9–11], and has been shown in biomechanical analyses to be the result of both persistent downward flow upon the stent graft bifurcation (affecting neck fixation), and transverse forces, resulting in device lateral movement (affecting neck fixation, component stability and iliac fixation) [12]. These forces are substantially magnified with increasing proximal neck diameter. This is particularly illustrated in the 17.5% migration rate within 2 years for a commercially available proximal fixation device intended to treat proximal necks up to 32 mm in diameter [10]. Newer proximal fixation device designs are being introduced to attempt to further mitigate this risk [13]. Type I endoleak is closely associated with migration, and is certainly the most significant predictor of aneurysm increase, risk of rupture, and need for secondary intervention or conversion to open repair. In their recent analysis of patients with type I endoleak following endografting that could not be satisfactorily repaired endovascularly, researchers at the Cleveland Clinic report a 19% mortality rate subsequent to open repair [14]. Clearly is it essential to implement devices, methods and techniques that increase resistance to migration and prevent endoleak in patients undergoing endovascular repair.
In analyses of EUROSTAR Registry outcomes, the most prevalent predictors of stent graft migration and type I endoleak are angulated and short aortic necks, larger neck diameter, neck thrombus and complicated iliac artery anatomy [15,16]. The presence of a calcified proximal neck or severe thrombus in the implantation site, specifically at the intended seal zone, compromise the area and reduce the effectiveness of stent graft proximal fixation anchoring and apposition, thereby increasing the potential for migration and sequelae. In the late postoperative period, predominantly reported causes of stent graft migration include aortic neck dilation, aneurysm remodeling leading to longitudinal changes and attendant stent graft lateral movement, and stent compression secondary to initial presentation (short, angulated neck) with blood flow between the stent and the aortic wall, causing stent graft instability and migration [17,18].
As the EVAR approach has expanded, physicians have sought to achieve ever better outcomes in the increasingly difficult and complex patient population. This led to an evolution in thinking beginning in 2000 that challenged the conventional wisdom at the time of modeling endografts after surgical grafts. It was then that a Powerlink® (Endologix, Inc., CA, USA) unibody bifurcated stent graft device was made available in Europe and began widespread clinical study in the USA. This device design made contralateral gate cannulation unnecessary and eliminated the distal component separation failure mode. Capitalizing on the columnar strength afforded by the long main body of this device with integrated limbs, physicians implanted the device at the aortoiliac bifurcation to naturally prevent distal migration and the associated sequelae, and coined this technique ‘anatomical fixation’. Completion of the repair with proximal sealing below the renal arteries involved placement of an aortic extension as needed to accommodate patient anatomy. Prospective clinical trial results have served to validate the concept and to objectively characterize the outcomes in both standard and challenging anatomy patient groups. Owing to the integrated 19 French (Fr) introducer sheath and contralateral 9Fr percutaneous access, bilateral percutaneous EVAR with the use of suture mediated closure devices is readily facilitated by this device and technique. The first prospective, randomized, multicenter trial of this approach is underway. In addition, further expansion of the anatomical fixation technique to more complex aneurysms is made possible by the use of the bifurcated device as the foundational platform. The addition of an off-the-shelf fenestrated proximal extension stent graft and compatible covered renal stents permits the timely endovascular repair of more complex juxtarenal and pararenal aneurysms. Clinical evaluations of this integrated system are beginning in 2011.
Endologix Powerlink system
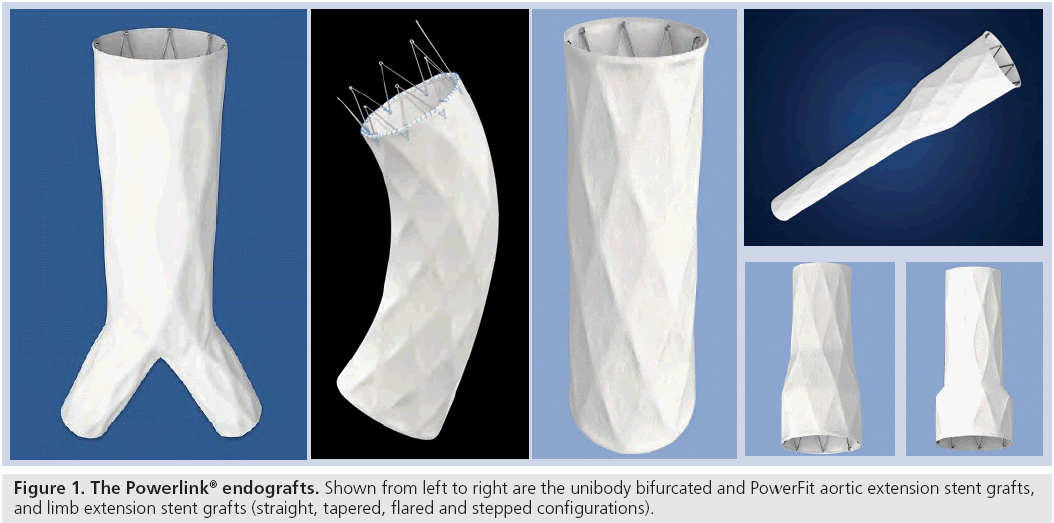
The Powerlink main body device is a unibody bifurcated endovascular graft consisting of a fully supported, high density, low porosity expanded polytetrafluoroethylene graft with an endoskeleton constructed as a single-wire, cobalt–chromium alloy body with limbs. The graft is attached to the stent only at the proximal and distal ends using surgical suture, a feature that results in excellent resistance to stent fracture or fatigue. Accessory proximal extensions are constructed in a similar manner, with both infrarenal configuration and suprarenal PowerFit™ configurations available (Figure 1). Accessory limb extensions in straight, stepped, flared and tapered configurations are available to permit customization to patient anatomy as needed. Device availability permits the treatment of patients with proximal aortic neck diameters of 18–32 mm and distal iliac sealzone diameters of 10–23 mm. The low profile 19Fr delivery system introducer requires only one surgically exposed femoral artery for deployment. Unique to this device, contralateral access is obtained percutaneously (9Fr) through a precannulated contralateral limb. These features enable use of the device in patients with one small or severely diseased iliac access vessel, where with available proximal fixation devices, this is not possible. The Powerlink design and access flexibility thus increases the number of patients that can be electively treated endovascularly. Moreover, the use of the integrated introducer sheath for accessory delivery and deployment and ancillary device introduction reduces the need for exchanges, thereby minimizing the potential for vessel intimal injury.
Endovascular aneurysm repair with the Powerlink system
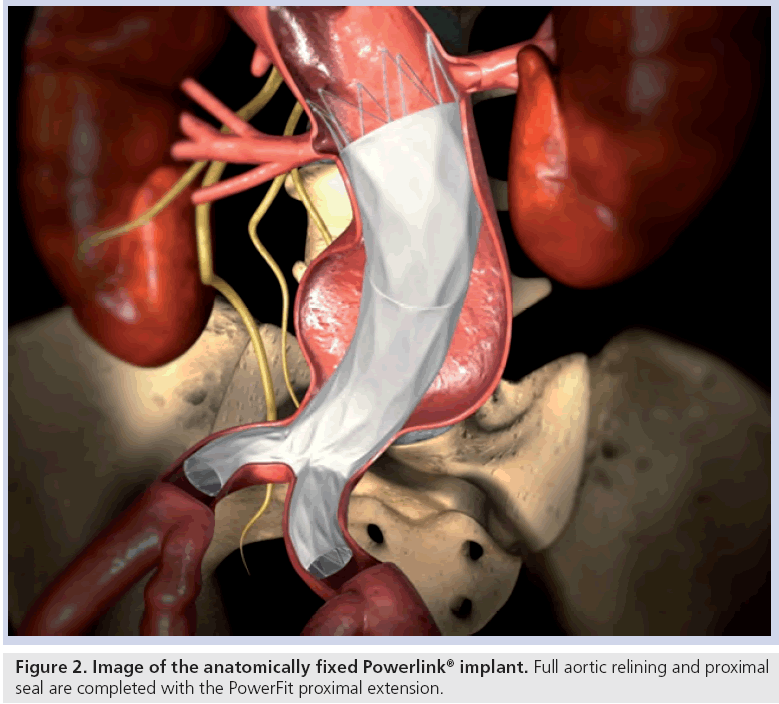
Stent graft selection is based on preoperative and intraoperative measurements of aortic nonaneurysmal neck diameter, aortic length from the most caudal renal artery to the aortic bifurcation and length from the aortic bifurcation to the hypogastric arteries. Procedures are performed in suitably equipped operating rooms or endovascular suites with operating room availability. Proper fluoroscopic imaging equipment and tools include a mobile C arm, automated contrast injector, intraoperative angiography and, if preferred, intravascular ultrasound. Bifurcated and, as needed, proximal extension stent graft models are chosen per established sizing algorithms to preserve at least one hypogastric artery, while achieving proximal seal and fully lining the infrarenal aorta. After angiography and aortic length verification with a marker catheter, the bifurcated stent graft delivery system contralateral guidewire is placed and the integrated 19Fr introducer sheath is advanced over the stiff guidewire into the aorta. Under fluoroscopic visualization, the introducer sheath is retracted to expose the constrained bifurcated stent graft. The constrained device is then placed upon the aortoiliac bifurcation, after which the main body and each limb of the device are deployed using a simple yet metered control cord mechanism. This results in implantation of the device at the aortoiliac bifurcation (i.e., anatomical fixation). Based on the patient anatomy and intraoperative angiography, an accessory proximal extension is placed as needed to achieve adequate overlap with the bifurcated device and seal in the proximal neck (Figure 2). Limb extensions can similarly be placed as needed to accommodate patient anatomical needs. Balloon dilatation may be performed if desired.
Anatomical fixation trial results
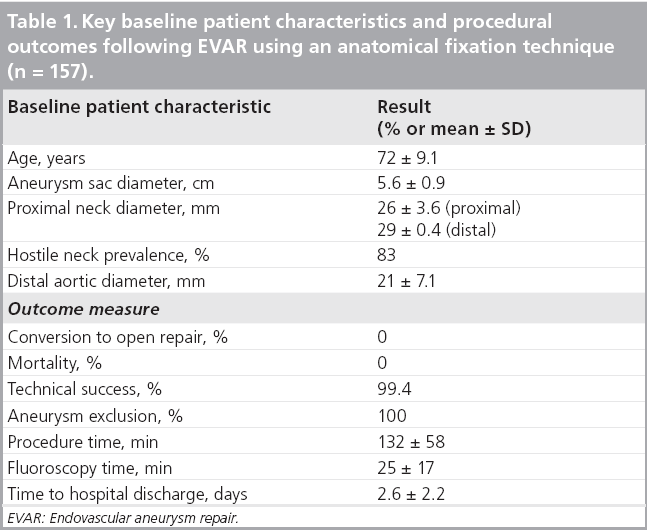
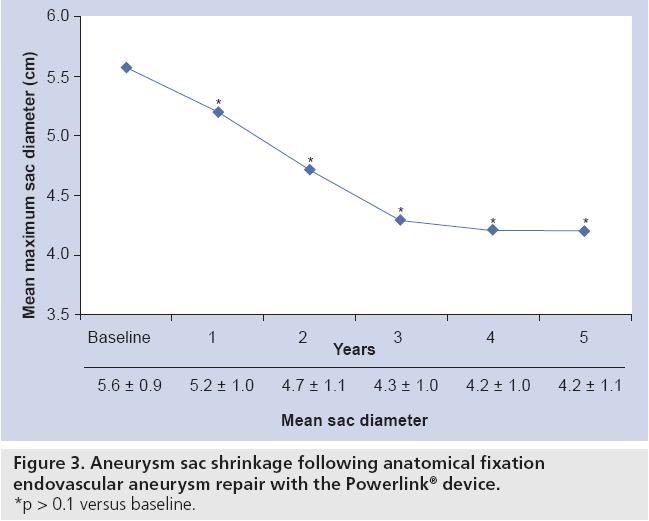
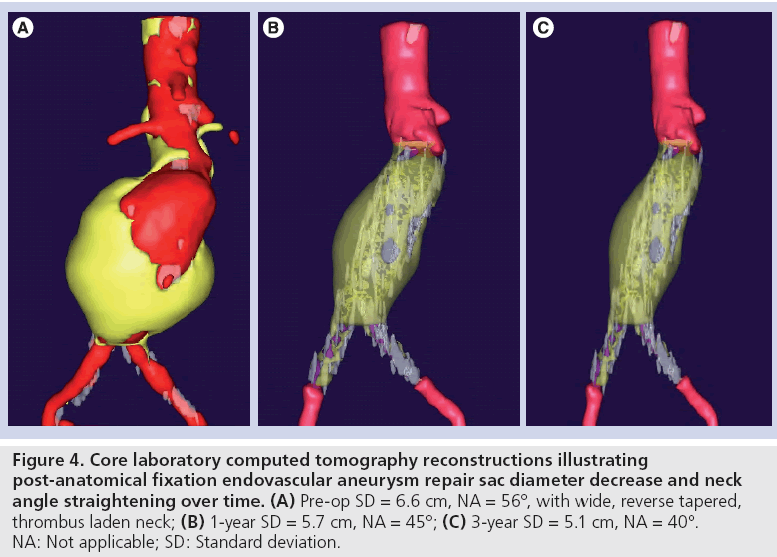
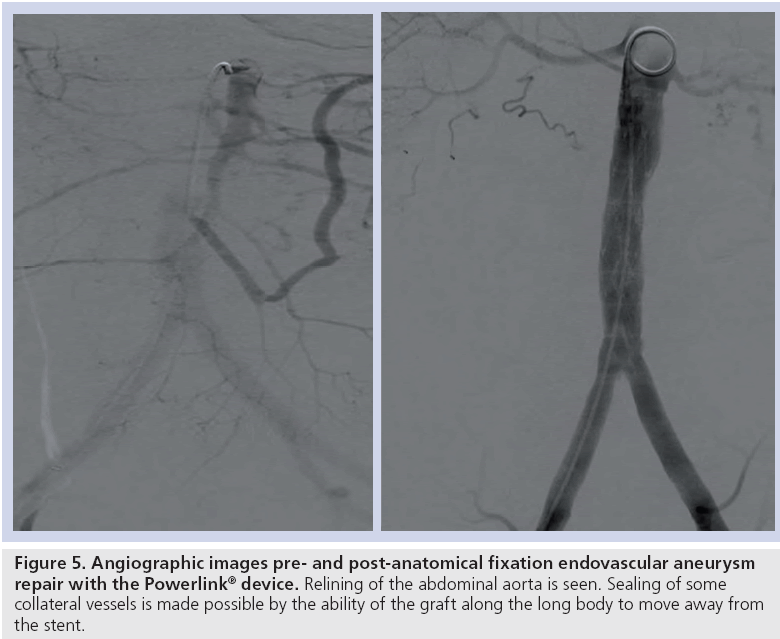
The results of the multiple prospective, multicenter US trials of the Powerlink stent graft system in 315 patients demonstrate safety and durability for aneurysm exclusion [19–23]. In 192 eligible and consenting patients in whom the device was implanted with a proximal fixation technique (82%) or an anatomical fixation technique (18%) [19,20], results through 5 years demonstrated 97.9% freedom from aneurysm-related mortality, stable or significantly reduced sac diameter (≥5 mm reduction) in 93% of patients and a migration rate of 4.2%. Subgroup analysis found that all migrations and aneurysm-related deaths were in the proximal fixation group. This led to the design and completion of additional trials to specifically test the anatomical fixation technique with this unibody bifurcated device with concomitant placement of a large diameter aortic extension [21] or a suprarenal extension [22]. Independently, each trial demonstrated 100% freedom from aneurysm-related mortality and migration beginning at the primary end point follow-up (1-year) and continuing to 5 years. The combined experience with anatomical fixation and this device system in 157 patients was consolidated into an analysis published recently [23]. Pooling of these data were justified by the authors owing to the use of a common protocol among study sites; implantation of the same Powerlink bifurcated device in all patients, and rigorous monitoring of the study. The results provide not only proof of concept in terms of procedural outcomes (Table 1), but also safety and durability for long-term aneurysm exclusion, even in the presence of hostile neck anatomy (Box 1). Patient selection by trial investigators resulted in a substantial number of patients reported by the core laboratory with challenging proximal neck characteristics. A short sealzone, angulated neck, severe thrombus or a reverse taper was found in 83% of patients. This is in contrast to the lower rate of such challenging anatomy (59%) in the Powerlink proximal fixation group, substantiating that the anatomical fixation cohort was at higher risk for endovascular repair. In terms of challenging distal anatomy, a narrow distal aorta was predominant in the cohort, presumably because this is not a procedural challenge for a unibody main device that is anatomical fixed. Conversely, this is a known significant challenge for proximal fixation devices which require that both limbs fit into the bifurcation without kinking or obstruction. All patients received the Powerlink bifurcated device via anatomical fixation at the aortoiliac bifurcation. Of the cohort, 50% had a wide aortic neck (up to 32 mm in diameter) and received a Powerlink XL proximal extension to complete the repair. Approximately one third of the patients received a proximal extension with a suprarenal configuration, with the remaining patients either receiving an infrarenal extension or requiring only the bifurcated device for the repair. Procedural technical success was 99.4%, with one patient requiring femoral-tofemoral bypass due to inadvertent limb damage intraprocedurally. All patients were discharged with complete aneurysm exclusion at a mean of 2.6 days. Within 30 days, no mortality occurred and 3.2% of patients were identified with a serious adverse event requiring medical treatment. After 30 days, patients continued in the trials with follow-up at 6 months, 1 year and annually for 5 years. The all-cause mortality rate observed within 1 year was 7.0%, with a similar annual rate subsequently. Independent adjudication of causation determined none were aneurysm-related but were related to comorbidities (i.e., primarily cardiac and cancer related). No conversion to open surgery, aneurysm rupture, migration, or type III (junctional) endoleak has been observed. Core laboratory evaluations of contrast-enhanced computed tomography angiography (CTA) scans at 30 days and at each subsequent follow-up have been performed and have verified significant aneurysm sac shrinkage through 5 years, with any individual sac increase attributable to refractory type II endoleak (Figure 3). In addition, the majority of patients are observed with neck straightening relative to the aneurysm sac. This appears to be a finding unique to the Powerlink device that is consistently observed over multiple trials and may potentially be attributed to the device’s columnar strength with full relining of the abdominal aorta (Figures 4 and 5). Further follow-up and analysis is warranted to investigate this relationship and to establish the absence of aortic neck dilatation as a contributing factor. These findings may be important to endovascular durability and the avoidance of late complications. As noted above, others who have studied proximal fixation devices describe neck shortening and attendant endograft kinking, buckling or lateral movement as a risk factor for graft occlusion, endoleak and separation of endograft components [17]. It seems intuitive that an endograft configuration having a balance between longitudinal column strength and flexibility is more likely to maintain its properties over time and resist migration under aortic flow conditions. Type I proximal endoleak was identified and repaired endovascularly in five patients, all of whom had at least two proximal neck hostile characteristics that are known risk factors for endoleak. In addition, two patients (0.6% of limbs) were identified with a limb occlusion and underwent successful repair. This low rate of secondary intervention in light of the challenging anatomy treated compares very favorably to rates for proximal fixation devices with similar patient characteristics. In a recent analysis of Powerlink patient serial CTA scans over 5 years, it was found that the low rate of secondary interventions was strongly correlated to the first (1 month) CTA scan results [24].
Figure 4: Core laboratory computed tomography reconstructions illustrating post-anatomical fixation endovascular aneurysm repair sac diameter decrease and neck angle straightening over time. (A) Pre-op SD = 6.6 cm, NA = 56°, with wide, reverse tapered, thrombus laden neck; (B) 1-year SD = 5.7 cm, NA = 45°; (C) 3-year SD = 5.1 cm, NA = 40°. NA: Not applicable; SD: Standard deviation.
This means that if the first Powerlink postoperative computed tomography has no abnormal findings (endoleaks or limb ischemia), future long-term surveillance could be performed using duplex ultrasound. This specific finding has not been replicated with other devices. Others evaluating proximal fixation device outcomes have found that absence of endoleak at the 1 month and 1 year time points are highly predictive of long-term freedom from aneurysm related mortality [25].
International single-center results in relatively unselected patients provide further confirmation of these controlled trial results. Following this implantation algorithm in 79 patients who were evaluated for an average of 3 years, researchers in Italy observed no migration, aneurysm rupture or late conversions [26]. It should be noted that these authors also reported on 126 patients who received a bifurcated device alone without proximal sealing with a proximal extension (i.e., contrary to the anatomical fixation algorithm). Results were not as favorable using this suboptimal technique, with a 6.3% migration rate at a mean of 45 months. Using the proper anatomical fixation technique, our 7‑year experience with this device found no migration in consecutive patients receiving the Powerlink System [27]. These findings are favorable compared with migration rates between 3.0 and 17% reported within 4 years for proximal fixation endografts relying on combinations of radial force or penetrating hooks or barbs applied at the infrarenal or suprarenal segment [9–12]. Interestingly, in more recent reports, the important role of distal fixation in realizing long-term stability and freedom from migration has been emphasized [28,29]. By anatomically fixing the unibody stent graft at the aortoiliac bifurcation with placement of an aortic extension as needed to achieve proximal seal, the issues related to achieving proximal and distal fixation are removed, leaving for consideration the proper device sizing to achieve proximal and distal seal.
Further anatomical considerations
Considering these exceptional results following anatomical fixation of the unibody stent graft in the context of challenging aortic neck anatomy, it is becoming increasingly clear that anatomies that may otherwise pose challenges for proximal fixation treatment approaches may benefit substantially from this approach. For example, proximal fixation devices artificially elevate the bifurcation into the aorta, requiring contralateral limb cannulation some distance from the patient’s aortoiliac bifurcation. In the presence of a narrow distal aorta or bilobe or saccular aneurysms, such maneuvers can be challenging or unachievable. An example of this is highlighted in a recent report regarding a newer device, where the distal aortic diameter averaged 33 mm in enrolled patients, more than 50% increased over that of patients enrolled in the Powerlink anatomical fixation trials. Even with this large distal diameter, intraoperative limb stenosis requiring stenting occurred in 11% of patients, with intervention within 30 days for limb occlusion or thrombosis performed in 2.2% of patients [13], leading the authors to suggest prophylactic intraoperative limb stenting. It is not known if the long-term rate of limb kinking/ occlusion will be similar to the 4.3% rate reported for other currently available devices [30]. Interestingly, endografts having fully supported limbs (e.g., Excluder and Powerlink) appear to have greater resistance to intraoperative stenosis and kinking and the lowest reported rates of limb kinking or occlusion through long-term follow-up (1.7 [31] and 1.2% [23]). Because the Powerlink device includes a precannulated contralateral limb requiring only 9Fr percutaneous access, anatomical fixation implantation eliminates the cannulation concern. This is further relevant to patients with limited vascular access, prevalent in this population. In a multinational registry analysis of patients presenting with coronary artery disease, cerebrovascular disease or peripheral arterial disease, patients with abdominal aneurysms were found to have a threefold increased incidence of peripheral arterial disease compared with those without abdominal aneurysms [32]. One cannot deny that preserving the aortoiliac bifurcation after EVAR to enable future peripheral interventions is an important consideration. The anatomically fixed Powerlink device relining the abdominal aorta offers the opportunity for such subsequent peripheral interventions post-EVAR (Figure 6).
Total percutaneous aneurysm repair
Several single-center published sources describe the development, feasibility and initial safety of a bilateral percutaneous approach to EVAR. Two comprehensive reviews of the literature available are published [33,34]; however no multicenter randomized trials, or for that matter nonrandomized trials, have yet been completed. The primary impetus for the development of a percutaneous endovascular aneurysm repair (PEVAR) approach is to further reduce surgical wound and lymphatic complications and the associated morbidity and attendant patient discomfort in standard EVAR procedures. Among the potential benefits of a percutaneous approach are improved clinical utility measures (i.e., shorter procedure time, time to ambulation and time to hospital discharge), reduced wound complications and increased patient satisfaction. Conversely, the success of percutaneous EVAR is highly dependent upon appropriate patient selection and meticulous physician technique following an extensive learning period in the application of both EVAR and percutaneous methods. Although the closure devices add to the procedural cost, it may be justified if total costs could be reduced owing to shorter procedure time, hospital stays and reduced wound complications. In addition, PEVAR facilitates the usage of local anesthesia, which is of particular importance to the treatment of EVAR patients with prevalent comorbidities that are recognized risk factors for general anesthesia. In the largest comparison of EVAR outcomes in patients treated with local or general anesthesia within the EUROSTAR Registry, Ruppert and associates found the local anesthesia group to have significantly reduced procedure time (116 vs 133 min, p < 0.0001), hospital stay (3.7 vs 6.2 days, p < 0.0001) and systemic complications (6.6 vs 13%, p = 0.0015) compared with the general anesthesia group [35]. Accurate vessel targeting, proper closure device placement, minimization of sheath size and avoidance of sheath exchanges are important for avoiding potentially serious complications. The availability of the unibody bifurcated device delivered with an integrated 19Fr introducer sheath has led physicians to initiate a formal validation of the bilateral percutaneous technique in EVAR. In a single-center experience in 114 access sites using this endovascular system, a technical success rate of 98% was achieved with a low incidence of postoperative complications [36]. The authors attributed this to the lowprofile delivery system and to the avoidance of exchanges afforded by the integrated sheath. The first prospective, multicenter, randomized controlled trial of percutaneous EVAR (the PEVAR Trial) is underway using the Powerlink device and is expected to complete within the next year.
Technological advancement in complex aneurysm repair
The complexity of abdominal aortic aneurysm is commonly characterized based on location and involvement of visceral vessels. Infrarenal aneurysms generally involve the infrarenal aorta and may involve the aortoiliac vasculature. A subset of infrarenal aneurysms extend up to the level of, but do not involve, the renal arteries (juxtarenal aneurysms) or extend further to involve one of both of the renal arteries (pararenal aneurysms). As noted previously, a substantial proportion of infrarenal aneurysms are not suitable for endovascular repair owing to unfavorable proximal neck anatomy (e.g., highly angulated, dilated, short or encroaching on or involving the renal arteries). In regulatory studies of endovascular infrarenal aneurysm repair, patients were carefully selected to ensure neck length and angulation requirements were met in order to optimize outcomes. Shorter lengths or greater angulation have been reported since the original trials to increase the risk of migration and type Ia endoleak and associated need for intervention [37,38].
Owing to the increased risk of renal complications, mesenteric ischemia and other complications following open repair of juxtarenal or pararenal aneurysms compared with infrarenal aneurysms [39], researchers have sought to extend a totally endovascular technique to repair of these aneurysms. In this treatment, it is essential to maintain the patency of the renal arteries and other visceral vessels. Up to now, only homemade or customized fenestrated stent grafts with the use of commercially available uncovered or covered vascular stents have been used for the repair of juxtarenal aneurysms. The key limitation to this approach is the need to customize the design and manufacture of each stent graft to a particular patient anatomy. This requires a lengthy period of time for planning, manufacture and delivery of the device, and is very costly. As a result, physicians have begun to seek other options, such as hybrid debranching techniques or chimney techniques. However, these options remain suboptimal. The concept of an off-the-shelf alternative to customization has been postulated; however, this has yet to be realized [40]. More recently, an off-the-shelf fenestrated stent graft system has been developed based on the Powerlink stent graft. Integral to this system are the bifurcated stent graft, anatomically fixed at the aortoiliac bifurcation, a fenestrated proximal extension with proprietary design and compatible covered renal stents. All devices are constructed from cobalt–chromium alloy for exceptional durability and have a high density polytetrafluoroethylene covering. Initial estimates suggest this system will be applicable to 80% or more of patients with juxtarenal or pararenal aneurysm, without need for customization. Initial clinical experience will be available by the end of 2010. Multicenter clinical trials are expected to commence in 2011 and if successful, would significantly increase the numbers of patients who could be treated endovascularly, significantly reduce the time from diagnosis to treatment and would represent significant improvement in cost–effectiveness over the currently available options.
Conclusion
Endovascular abdominal aortic aneurysm repair methods have developed steadily over the last decade. The technique of anatomical fixation using a unibody device has emerged to address key failure modes associated with modular proximal fixation devices, namely distal migration and associated sequelae. Clinical trials using this device and technique have demonstrated its performance and utility in challenging anatomies involving access vessels, aneurysm morphology and proximal neck characteristics. Randomized trial validation of percutaneous approaches to endovascular aneurysm repair is on the near horizon. An off-the-shelf endovascular option to permit endovascular repair of juxtarenal and pararenal aneurysms using an anatomical fixation technique is expected to overcome current challenges with customized stent grafts and is in early stages of clinical investigation.
Future perspective
The field of EVAR has matured in recent years and is progressing into ever more challenging anatomy than treated in early trials. The problems of distal migration or endoleak remain, particularly in the more challenging anatomies. Clearly, utilization of the aortoiliac bifurcation as a strong foundation on which to place the Powerlink bifurcated stent graft followed by achievement of proximal seal with an aortic extension if needed is a simple yet elegant way of mitigating the risk of migration and endograft destabilization. The anatomically fixed bifurcated stent graft combined with a well-designed off-the-shelf proximal extension and visceral branch grafts, starting clinical evaluation at this time, has strong potential to address a significant unmet clinical need and to address global demands for a cost-effective endovascular treatment option. Furthermore, the prospective clinical validation of a total percutaneous approach to EVAR will serve to advance this technique and its use in properly selected patients. Other proximal fixation devices are in development or available in some regions that feature lower-profile delivery systems and enhanced proximal fixation attachment mechanisms. A novel sac anchoring endovascular prosthesis (the Nellix device) is in final stages of regulatory review in Europe. This technology obliterates the aneurysm sac with a polymer-filled endobag and paving endoframe lumens. Although it has the potential to significantly reduce or eliminate the incidence of any endoleak, and thus reduce the surveillance necessary with other endovascular prostheses, broader, longer term study will be necessary to validate this fully [41]. The next horizon yet to be addressed is the thoracic aorta, particularly the ascending aorta, where no adequate endovascular options yet exist. Although in final proprietary development at this time, a novel endografting approach utilizing a thoracic anatomical fixation technique with in situ fenestration and branch preservation is on the horizon.
Financial and competing interests disclosure
D Raithel serves as a consultant of Endologix, Gore, BSc LeMaitre and a shareholder of Endologix. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Modular stent graft devices mimic surgical grafts that have historically been used for open repair.
▪ Proximal modular device fixation and anatomical unibody device fixation are the two current endovascular approaches to abdominal aortic aneurysm repair.
▪ Anatomical challenges posed by limited access, narrow distal aortas, and difficult aneurismal and proximal necks have been successfully addressed with the anatomical fixation technique using a unibody device.
Endologix Powerlink® system
▪ The bifurcated stent graft is delivered via an integrated, hydrophilically coated, low-profile introducer sheath ipsilateral and a 9Fr percutaneous sheath contralaterally. Completion of the repair is achieved through the introducer sheath, without the need for exchanges.
▪ Trial experience to date demonstrates safety and effectiveness for abdominal aortic aneurysm repair in both standard and challenging patient anatomies.
▪ Clinical proof-of-concept data for endovascular repair of juxtarenal and pararenal aneurysm using an off-the-shelf anatomical fixation system will be available over the next several months.
▪ This is the first endovascular stent graft technology and technique to undergo a prospective, randomized trial for percutaneous aneurysm repair.
Comparisons with modular devices
▪ Anatomical fixation with the unibody device permits treatment of patients with narrow distal aortas, bilobe or saccular/angulated aneurysms and hostile neck characteristics that are otherwise challenging or unaddressable by modular devices.
▪ Limb occlusion and thrombosis are far less frequently observed with an anatomically fixed, fully supported unibody device than with modular devices.
Future perspective
▪ Percutaneous endovascular aneurysm repair with concomitant local anesthesia, particularly in patients at high risk for surgery, is on the horizon as a clinically validated approach.
▪ An off-the-shelf totally endovascular approach to juxtarenal and pararenal aneurysm repair based on anatomical fixation is under clinical investigation and is on the horizon.
▪ Future comparisons focusing on cost–effectiveness and long-term performance in reducing the need for secondary procedures will likely be made among endovascular devices, rather than in comparison to open repair.
▪ A thoracic anatomical fixation endografting approach with branch vessel preservation is on the horizon.
References
Papers of special note have been highlighted as:
▪ of interest
- Parodi JC, Palmaz JC, Barone HD: Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 5(6), 491–499 (1991).
- Greenhalgh R, Allison D, Bell P et al.: Endovascular versus open repair of abdominal aortic aneurysm. N. Engl. J. Med. 362(20), 1863–1871 (2010).
- Prinssen M, Verhoeven E, Buth J et al.: A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysm. N. Engl. J. Med. 351(16), 1607–1618 (2004).
- Lederle F, Freischlag J, Kyriakides T et al.: Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA 302(14), 1535–1542 (2009).
- Lovegrove R, Javid M, Magee T, Galland R: A meta analysis of 21 178 patients undergoing open or endovascular repair of abdominal aortic aneurysm. Br. J. Surg. 95, 677–684 (2008).
- Schermerhorn M, O’Malley J, Jhaveri A, Cotterill P, Pomposelli F, Landon B: Endovascular vs. open repair of abdominal aortic aneurysm in the Medicare population. N. Engl. J. Med. 358, 464–474 (2008).
- Carpenter JP, Baum RA, Barker CF et al.: Impact of exclusion criteria on patient selection for endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 34, 1050–1054 (2001).
- Chaikof EL, Brewster DC, Dalman RL et al.: The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guideline. J. Vasc. Surg. 50, 2S–49S (2009).
- Zarins CK, Bloch DA, Crabtree T, Matsumoto A, White R, Fogarty T: Stent graft migration after endovascular aneurysm repair: importance of proximal fixation. J. Vasc. Surg. 38, 1264–1272 (2003).
- Lee JT, Lee J, Aziz I et al.: Stent graft migration following endovascular repair of aneurysms with large proximal necks: anatomical risk factors and long-term sequelae. J. Endovasc. Ther. 9, 652–664 (2002).
- Lee ES, Zarins CK: Endograft migration: incidence, causes, and clinical significance. Perspect. Vasc. Endovasc. Surg. 16(3), 1–5 (2004).
- Li Z, Kleinstreuer C: Analysis of biomechanical factors affecting stent-graft migration in an abdominal aortic aneurysm model. J. Biomechanics 39, 2264–2273 (2006).
- Torsello G, Troisi N, Tessarek J et al.: Endovascular aortic aneurysm repair with the endurant stent graft: early and 1‑year results from a European multicenter trial. J. Vasc. Interv. Radiol. 21, 73–80 (2010).
- Kelso RL, Lyden SP, Butler B, Greenberg RK, Eagleton J, Clair DG: Late conversion of aortic stent grafts. J. Vasc. Surg. 49, 589–595 (2009).
- Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RL: Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J. Vasc. Surg. 32(4), 739–749 (2000).
- Hobo R, Buth J: Secondary interventions following endovascular aortic abdominal aneurysm repair using current endografts: a EUROSTAR report. J. Vasc. Surg. 43, 896–902 (2006).
- Wolf YG, Hill BB, Lee WA, Corcoran CM, Fogarty TJ, Zarins CK: Eccentric stent graft compression: an indicator of insecure proximal fixation of aortic stent graft. J. Vasc. Surg. 33, 481–487 (2001).
- Rafii BY, Abilez OJ, Benharash P, Zarins CK: Lateral movement of endografts within the aneurysm sac is an indicator of stent graft instability. J. Endovasc. Ther. 15, 335–343 (2008).
- Carpenter JP: Midterm results of the multicenter trial of the Powerlink bifurcated system for endovascular aortic aneurysm repair. J. Vasc. Surg. 40, 849–859 (2004).
- Wang G, Carpenter JP: The Powerlink bifurcated system for endovascular aortic aneurysm repair: six-year results. J. Vasc. Surg. 48, 535–545 (2008).
- Jordan WD Jr, Moore WM Jr, Melton JG, Brown OW, Carpenter JP: Secure fixation following EVAR with the Powerlink XL system in wide aortic necks: results of a prospective, multicenter trial. J. Vasc. Surg. 50, 979–986 (2009).
- Harlin SA, Beasley RE, Feldman RL, Thompson CS, Williams JB: Endovascular AAA repair using an anatomical fixation technique and concomitant suprarenal orientation: results of a prospective, multicenter trial. Ann. Vasc. Surg. 24, 921–929 (2010).
- Carpenter JP, Garcia MJ, Harlin SA et al.: Contemporary results of endovascular repair of abdominal aortic aneurysms: effect of anatomical fixation on outcomes. J. Endovasc. Ther. 17, 153–162 (2010).
- Patel M, Carpenter JP: The value of the initial post-EVAR computed tomography angiography scan in predicting future secondary procedures with the Powerlink stent graft. J. Vasc. Surg. 52(5), 1135–1139 (2010).
- Sternbergh WC, Greenbergh RK, Chuter TA, Tonnesson BH: Redefining postoperative surveillance after endovascular aneurysm repair. J. Vasc. Surg. 48, 278–285 (2008).
- Coppi GC, Silingardi R, Tasselli S, Gennai S, Saitta G, Veraldi GF: Endovascular treatment of abdominal aortic aneurysms with the Powerlink Endograft System: influence of placement on the bifurcation and use of a proximal extension on early and late outcomes. J. Vasc. Surg. 48, 795–801 (2008).
- Qu L, Hetzel G, Raithel D: Seven years’ single center experience of the Powerlink bifurcated endograft for endovascular aortic aneurysm repair. J. Cardiovasc. Surg. 48, 13–19 (2007).
- Heikkinen M, Alsac M, Arko F, Metsanoja R, Zvaigzne A, Zarins CK: The importance of iliac fixation in the prevention of stent graft migration. J. Vasc. Surg. 43, 1130–1137 (2006).
- Benharash P, Lee JT, Abilez OJ, Crabtree T, Bloch D, Zarins CK: Iliac fixation inhibits migration of both suprarenal and infrarenal aortic endografts. J. Vasc. Surg. 45(2), 250–257 (2007).
- Cochennec F, Becquemin JP, Desgranges P, Allaire E, Kobeiter H, Roudot-Thoraval F: Limb graft occlusion following EVAR: clinical pattern, outcomes and predictive factors of occurrence. Eur. J. Vasc. Endovasc. Surg. 34(1), 59–65 (2007).
- Alterman DM, Stevens SL: The excluder aortic endograft. Persp. Vasc. Surg. Endovasc. Ther. 20(2), 136–148 (2008).
- Baumgartner I, Hirsch AT, Abola MT et al.: Cardiovascular risk profile and outcome of patients with abdominal aortic aneurysm in out-patients with atherothrombosis: data from the reduction of atherothrombosis for continued health (REACH) registry. J. Vasc. Surg. 48, 808–814 (2008).
- Starnes BW, Andersen CA, Ronsivalle JA, Stockmaster N, Mullinex P, Statler JD: Totally percutaneous aortic aneurysm repair: experience and prudence. J. Vasc. Surg. 43(2), 270–276 (2006).
- Hogg ME, Kibbe MR: Percutaneous thoracic and abdominal aortic aneurysm repair: techniques and outcomes. Vascular 14, 270–281 (2006).
- Ruppert V, Leurs LJ, Steckmeier B, Buth J, Umscheid T: Influence of anesthesia type on outcome after endovascular aortic aneurysm repair: an analysis based on EUROSTAR data. J. Vasc. Surg. 44, 16–21 (2006).
- Krajcer Z, Gregoric I: Totally percutaneous aortic aneurysm repair: methods and outcomes using the fully Integrated IntuiTrak endovascular system. J. Cardiovasc. Surg. 51(4), 493–501 (2010).
- Leurs LJ, Kievit J, Dagnelie PC, Nelemans PJ, Buth J: Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J. Endovasc. Ther. 13, 640–648 (2006).
- AbuRahma A, Campbell J, Stone PA et al.: The correlation of aortic neck length to early and late outcomes in endovascular repair patients. J. Vasc. Surg. 50, 738–748 (2009).
- Fulton JJ, Farber MA, Marston WA, Mendes R, Mauro M, Keagy BA: Endovascular stent-graft repair of pararenal and type IV thoracoabdominal aortic aneurysms with adjunctive visceral reconstruction. J. Vasc. Surg. 41, 191–198 (2005).
- Nordon IM, Hinchcliffe RJ, Manning B et al.: Toward an “off-the-shelf ” fenestrated endograft for management of short-necked abdominal aortic aneurysms: an analysis of current graft morphological diversity. J. Endovasc. Ther. 17, 78–85 (2010).
- Donayre CD, Zarins CK, Krievins DK et al.: Initial clinical experience with a sacanchoring endoprosthesis for aortic aneurysm repair. J. Vasc. Surg. 53(3) 574–582 (2011).
▪ Demonstrates the long-term safety and durability of the Powerlink® stent graft system for aortic aneurysm repair.
▪ Details the exceptional results of the only prospective, multicenter trial of anatomical fixation in the endovascular repair of wide aortic necks.
▪ Details the consolidated results of anatomical fixation with the Powerlink® device over three trial cohorts using the same patient selection criteria and protocol methods.
▪ Provides scientific justification for implementation of a reduced surveillance protocol (e.g., duplex ultrasound) following implantation of the Powerlink stent graft.
▪ Provides clinical validation of a totally percutaneous endovascular technique for implant of the Powerlink stent graft using the IntuiTrak delivery system.