Device Evaluations - Interventional Cardiology (2012) Volume 4, Issue 2
Endovascular aspiration thrombectomy in acute ischemic stroke therapy: the Penumbra system
- Corresponding Author:
- Mayank Goyal
Department of Clinical Neurosciences
University of Calgary
Calgary, Alberta, Canada
E-mail: mgoyal@ucalgary.ca
Abstract
Keywords
aspiration thrombectomy, endovascular thrombolysis, stroke intervention
Stroke is a global health problem and a leading cause of disability [1]. On average, every 40 s someone in the USA has a stroke, with a total of approximately 800,000 annually [2]. About 80% of strokes result from an acute occlusion of a cerebral blood vessel, triggering a cascade of cellular reactions that culminate in ischemic infarction and loss of function of the affected brain area. Restoration of the flow to the affected brain can limit brain injury and reverse symptoms, but only if done in a timely manner. Current therapies that are approved by the US FDA include only one intravenous (iv.) thrombolytic therapy, tissue plas minogen activator (tPA). While only eight patients need to be treated to prevent one major disability or death, more than half of those treated in the NINDS trial were dependent or dead, implying a substantially unmet need [3].
In a recently updated pooled analysis of eight randomized trials of iv. tPA (3670 patients), bene-fits derived from the treatment was noticeably time dependent [4]. Patients treated with iv. tPA derived the highest odds of favorable 3 month outcome (defined as modified Rankin score 0–1) when the treatment was initiated within 90 min of symptom onset (odds ratio: 2.55; 95% CI: 1.44–4.52). This benefit diminished steadily as the treatment was delayed, until treatment beyond 4.5 h from onset was associated with increased mortality (adjusted odds of mortality: 1.49; 95% CI: 1.00–2.21).
Why iv. tPA is not enough
Nearly half (48%) of patients with severe stroke (NIH Stroke Scale [NIHSS] >20) died in 3 months of their stoke despite iv. tPA treatment within 3 h from symptom onset in the NINDS tPA stroke trial [3]. This is likely related to the low rates of recanalization with iv. tPA alone in proximal occlusions or larger clots as only a third to a half of patients treated with iv. tPA achieve recanalization in middle cerebral artery (MCA) occlusions [5,6]. This rate is even lower with more proximal occlusions or larger clot burden [7–10]. In addition, 12–34% of those who achieve some degree of recanalization with iv. tPA suffer early reocclusion [11–13]. Reocclusion was more common among patients with partial recanalization, which was encountered in about half of those receiving iv. tPA [11].
Status of endovascular stroke therapy preceding Penumbra system
This low rate of recanalization even with timely-administration of iv. tPA created an unmet need for achieving better recanalization rates. The delivery of thrombolytic agents, through a microcatheter, directly to the site of occlusion was promising to offer a relatively higher recanalization rate, without increasing the rate of symptomatic intracranial hemorrhage (ICH). The early trials of endovascular stroke throm-bolysis used this approach [14]. This was tested in one of the earliest clinical trials of intra-arterial (IA) thrombolytic therapy: the PROACT I and II trials [15,16]. Acute stroke patients with angio-graphically proven MCA occlusion were randomized within 6 h of onset to receive IA recombinant pro-urokinase and low-dose iv. heparin versus iv. heparin alone. For the primary out-come, 40% of those who received pro-urokinase achieved a favorable neurological outcome (modified Rankin’s scale [mRS] ≤2) compared with 25% in the control group (p = 0.04). Recanalization was achieved in 66% of the treatment group versus 18% of the control group (p < 0.001) [16]. Higher rates of ICH were seen in the pro-urokinase patients (10%) as opposed to 2% of the controls (p = 0.06). With a mean time to start treatment of 5.3 h, PROACT II, combined a relatively higher recanalization with favorable outcome.
Similar results to PROACT II were observed in the Phase I, EMS bridging trial, which randomized 35 patients to receive either iv./IA tPA (17 patients) or placebo/IA tPA (18 patients) within 3 h of onset. A higher rate of full recanalization was achieved in the iv./IA group (54%) compared with only 10% in the placebo/IA group [17]. This did not reflect on the outcome, as both groups had similar 7, 10 and 90 day outcomes, although there were more deaths in the iv./IA group. However, this pilot study was not powered to examine differences in efficacy between the two treatment groups.
Other studies have demonstrated the safety of endovascular acute stroke therapy. The IMSI Pilot Study was an open-label, single-arm feasibility and safety study of a combined iv./IA approach [5]. It enrolled 80 patients (median baseline NIHSS score of 18) within 3 h of symptom onset. All patients received iv. tPA (0.6 mg/kg, 60 mg maximum) at a median time of 140 min. Subsequently, 77 patients underwent cerebral angiography and 62 of them received IA tPA. Patients who did not receive IA thrombolysis had either no visible thrombus or distal thrombus not amenable to an IA approach. For the 62 patients treated with IA tPA, the rate of partial or complete recanalization (thrombolysis in myocardial infarction [TIMI] 2 or 3) was 56%. Five patients (6.3%) developed symptomatic ICH less than 36 h from onset of treatment. Among patients who achieved TIMI 2 or 3 flow, 34% had a favorable outcome (mRS 0–1 at 3 months) as compared with 12% of those who achieved only TIMI 0 or 1 flow (p = 0.013).
The completion of the IMSI study 1.5 years earlier than anticipated encouraged investigating the emerging endovascular technology in the IMSII trial [18] while plans were ongoing for a large randomized Phase III (IMSIII) trial [19]. The IMSII trial enrolled 81 subjects with a median baseline NIHSS score of 19 and a median onset to iv. tPA initiation of 142 min.
In 33 patients, an investigational, ultrasound-enhanced thrombolysis using the EKOS ultrasound system was used. It includes a 0.014 inch microcatheter with an end hole infusion and an ultrasound element at the distal end. The ultrasound energy source is a reusable control unit. This approach aims to accelerate chemical thrombolysis by sending low-energy ultra-sound waves to alter the structure of the thrombus and allows for better penetration by tPA. Results showed that 69% of those who received IA tPA plus ultrasound treatment had TIMI 2 or 3 recanalization, which compared favorably with the 56% recanalization rate from the IMSI trial. Mortality rates (16%) were similar in both trials, although the rate of symptomatic ICH was higher in the IMSII (11%) compared with IMSI (6%).
A number of concerns over IA tPA use and efficacy started to surface and prompted the search for alternative means for IA therapies. The rate of recanalization with IA tPA were still suboptimal [20]. The anatomical characteristic of thrombi in the vascular bed leaves only a small surface area for IA tPA to act on. In addition, thrombi containing calcium or surrounded by endothelium are resistant to the action of tPA which does not penetrate these components of thrombi [21]. In addition, tPA is usually slowly infused at the thrombus interface, which delays recanalization time and may allow ongoing coagulation cascade activation leading to enhanced aggregation and potentially reocclusion [22].
The first mechanical thrombectomy device approved by the FDA was the Merci Retrieval System® (Concentric Medical, Inc, CA, USA). The device is a tapered nitinol wire that at its distal end takes the form of five helical loops. This corkscrew configuration aims to allow for better engagement and extraction of thrombi under a negative pressure created by manual suction at the guidecatheter, and a transient flow arrest that is applied when a balloon guide catheter placed proximal to the thrombus is inflated [23]. Following the publication of the first report describing the safety of the Merci device in 30 patients [23], its safety, as well as efficacy, was replicated in a number of prospective studies [24–27]. This included an international, multicenter, single-arm trial (Multi MERCI) that enrolled 164 patients (mean age of 68 years and median NIHSS of 19) within 8 h of stroke symptom onset. Successful recanalization (TIMI 2 or 3, interpreted by the site investigator) was achieved in 57.3% of those treated with the Merci system alone and in 69.5% if IA tPA or other mechanical adjunctive therapies were used. Symptomatic (increase in NIHSS of ≥4 points) ICH occurred in 9.8% while significant procedural complications (procedure complication with an increase in NIHSS of ≥4 points or death, groin complication requiring surgery or blood transfusion) occurred in 5.5%.
The Penumbra system
▪ The need for another mechanical device
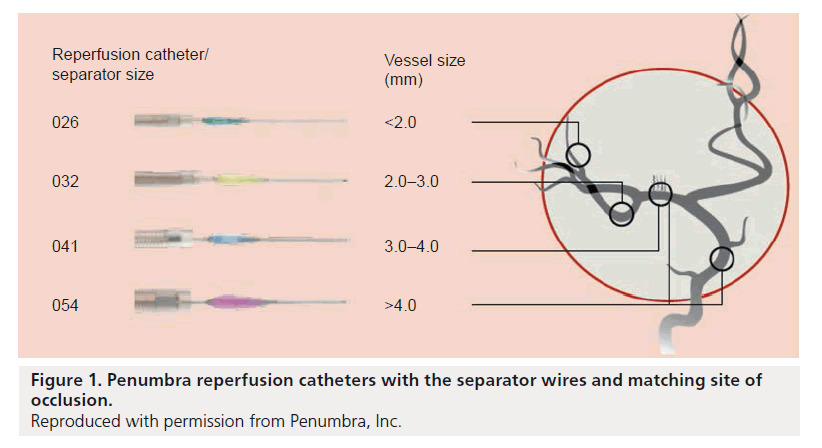
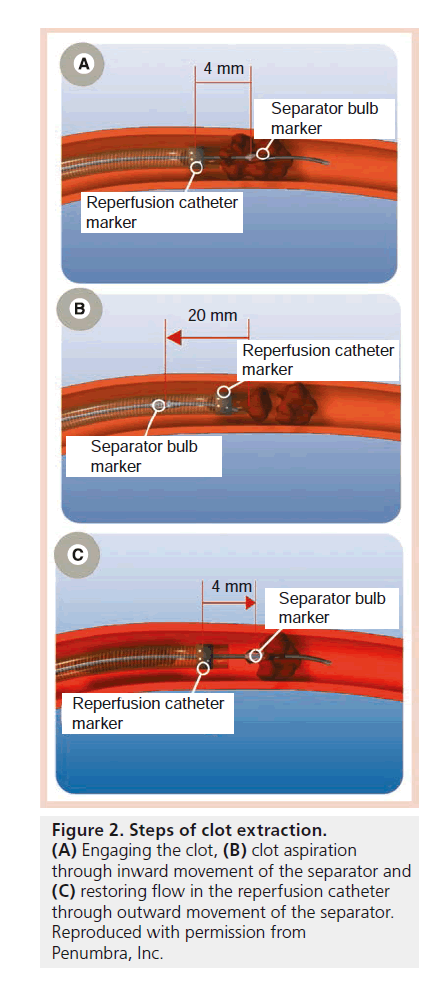
Despite the encouraging results of the MERCI study [27], the unmet clinical need for improving outcomes in acute ischemic stroke patients persisted as only 28% of patients treated in the MERCI study achieved mRS ≤2 at 90 days. Also, recanalization could only be achieved in approximately two-thirds of the patients. Additionally in our experience, use of the MERCI device was quite painful. We found that it was sometimes difficult to use MERCI under local anesthesia and we were increasingly using general anesthesia (which has the potential to introduce further delays and increase the possibility of an adverse outcome) [28]. The pursuit continued for devices that can achieve timely and complete recanalization, and therefore less brain damage, less stroke-related deficits and better clinical outcomes. The Penumbra system (PS; Penumbra, CA, USA) emerged as a new device for mechanically removing thrombi in the setting of acute ischemic stroke. The device consists of a reperfusion catheter, available in four different sizes (Figure 1), that is navigated to the site of occlusion to aspirate and extract thrombi with the aid of a separator wire that is moved in and out of the reperfusion catheter to help break up the thrombus inside the catheter tip and facilitate its aspiration (Figure 2). The catheter is attached to suction tubing that is connected to an aspiration pump that delivers 25 mmHg of suction (Figure 3).
Figure 1. Penumbra reperfusion catheters with the separator wires and matching site of occlusion.
Reproduced with permission from Penumbra, Inc.
Figure 2. Steps of clot extraction.
(A) Engaging the clot, (B) clot aspiration through inward movement of the separator and (C) restoring flow in the reperfusion catheter through outward movement of the separator. Reproduced with permission from Penumbra, Inc.
The unique design of the PS has a number of advantages. Thrombi are removed using a process of continuous aspiration and extraction. Aspiration can begin at the thrombus interface eliminating the need to catheterize distal to the thrombus. The reperfusion catheter is available in different sizes to facilitate accessibility depending on the target vessel size: 026 (0.026 inch inner lumen, 3.9 F outer diameter tapered to 2.8 F distally), 032 (0.032 inch inner lumen, 4.1 F outer diameter tapered to 3.4 F distally), 041 (0.041 inch inner lumen, nontapered 4.1 F outer diameter) and 054 (0.054 inch inner lumen, 6.0 F outer diameter tapered to 5.0 F distally). The FDA has recently cleared two new models as well: 4MAX (0.041 inch inner lumen, 6.0 F outer diameter tapered to 4.4 F distally) and 3MAX (0.035 inch inner lumen, 4.8 F outer diameter tapered to 4.0 F distally).
▪ Evidence for the PS
The initial experience with the device was described in a prospective, single arm trial that enrolled 23 patients from six European centers within 8 h from stroke symptoms onset [29]. Only 20 patients were treated with the PS (mean age: 60 years, mean NIHSS score: 21) as three patients could not be treated with the device due to vascular tortuosity and the identification of 21 target vessel occlusions. The use of PS resulted in 100% (21 out of 21) recanalization (TIMI 2–3). There were two procedural complications (a groin hematoma and an intra-procedural subarachnoid hemorrhage) and two cases of symptomatic ICH (a 4 point drop on the NIHSS score). The 30 day all-cause mortality rate was 45% (nine out of 20).
The PS received FDA approval in December 2007 based on the result of the Penumbra Pivotal Stroke Trial [30]. This was a prospective, singlearm study conducted in 24 centers in Europe and the USA and enrolled 125 patients with a mean age of 63.5, mean NIHSS score of 17.6 and a mean time to presentation of 1.9 h. Successful recanalization (TIMI 2–3) was achieved in 81.6% of patients but only 25% achieved mRS ≤2 at 90 days. Nineteen procedural complications occurred in 16 (12.8%) patients. These included vasospasm (four patients), reocclusion of the target artery (three), dissection (three), perforation (three), ICH (two), subarachnoid hemorrhage (one), anemia (one), embolization of a previously uninvolved vessel (one) and stroke in a new territory (one). Symptomatic ICH occurred in 14 (11.2%) patients.
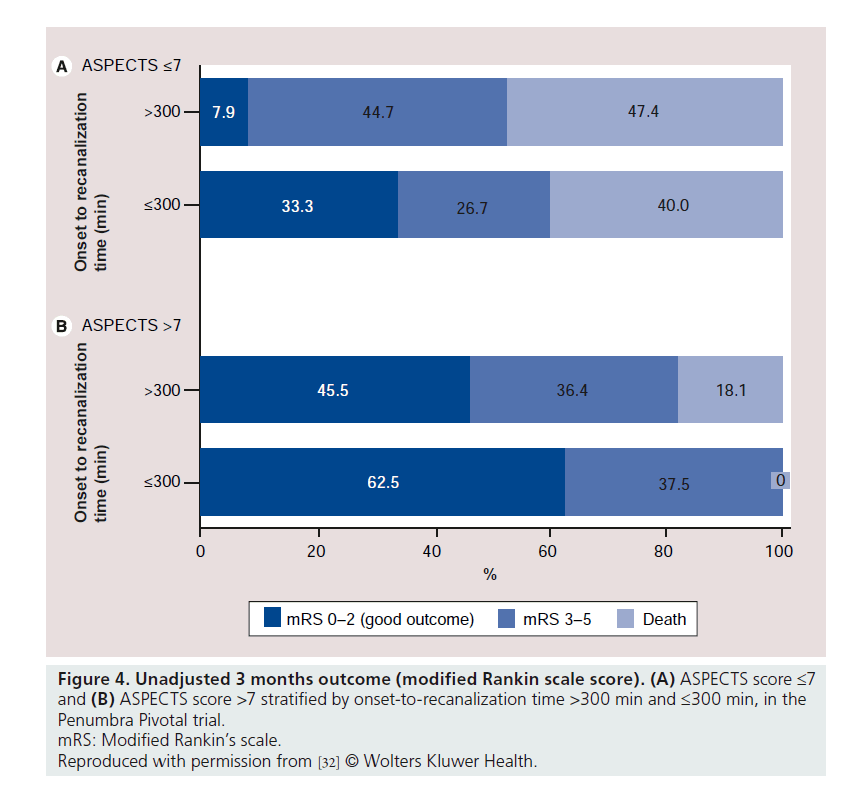
The notable dissociation between successful recanalization rate and favorable outcome in the Penumbra Pivotal trial triggered further analysis of the pivotal trial data. Our center reanalyzed the pivotal trial data investigating an association between short time to recanalization and the extent of early ischemic changes (using the semiquantitative ASPECTS score [31]) in predicting good outcome [32]. Good clinical outcome was significantly higher in the group with ASPECTS score >7 when compared with the group with ASPECTS score of 7 or less (50 vs 15%; relative risk: 3.3; 95% CI: 1.6–6.8; p = 0.0001). Widespread early ischemic changes, defined as ASPECTS score ≤4, were noted in a third (28 out of 85) of the patients; none of them had a good outcome. In addition, no patient without recanalization (16 patients) had a good outcome. There was an interaction between baseline ASPECTS score (>7 and ≤7) and onset to recanalization time (≤300 min and >300 min) in predicting good outcome (p = 0.06) (Figure 4). The study concluded that patients benefit the most with early recanalization and a favorable baseline CT scan (defined as ASPECTS score more than 7). Others suspected that the high recanalization rate might be related to the method of applying the TIMI score in the Penumbra Pivotal trial [33]. Another potential explanation is the incidence of distal emboli reported in some case series describing experience with the PS. In our experience it is unclear if some of these emboli were present at onset, rather than as a complication of device use [34].
Figure 4. Unadjusted 3 months outcome (modified Rankin scale score). (A) ASPECTS score ≤7 and (B) ASPECTS score >7 stratified by onset-to-recanalization time >300 min and ≤300 min, in the Penumbra Pivotal trial.
mRS: Modified Rankin’s scale.
Reproduced with permission from [32] © Wolters Kluwer Health.
Given the findings of the pivotal trial imaging subgroup analysis, an imaging-based, single- arm, prospective registry was performed to assess the safety and effectiveness of the PS in a cohort of stroke patients presenting within 8 h of symptom onset with a known core infarct volume at admission. The START Trial aimed to investigate whether a correlation exists between infarct volume and functional outcome at 90 days postprocedure. The core infarct volume at admission was assessed by computed tomography (CT) perfusion, CT angiography or magnetic resonance DWI scans and subgrouped by <50 cc, 50–100 cc and >100 cc. The registry – which stopped recruiting – aimed to enrol 200 patients from approximately 45 centers in North America. The results of the START Trial have not been published yet.
The results of the pivotal trial were replicated in the initial post marketing experience of the PS (POST trial). This was a retrospective review of 157 patients with a comparable age and stroke severity to those enrolled in the pivotal trial. However, despite a similar successful recanalization rate between POST (87%) compared to the pivotal trial (82%), more patients achieved favorable outcome (mRS ≤2) at 90 days in POST (41%) compared with the pivotal trial (25%). Nine patients (5.7%) experienced procedural complications. These included dissection (two patients), perforation (two), ICH (one), access site hematoma (one), peripheral hemorrhage (one) and cardiac arrest (one). The median aspiration time was 41 min. Two incidents of fractures of the 032 reperfusion catheter and one breakage of a 041 separator were reported but caused no serious adverse events or death. While the results are promising, the retrospective nature of the study requires caution when interpreting the results.
The safety and effectiveness of the PS as an adjunctive treatment to iv. tPA will be further assessed in the THERAPY trial, an open label, randomized, Phase IV clinical trial. Acute ischemic stroke patients presenting with evidence of a large clot burden (clot length >8 mm) in the anterior circulation will be randomized to either iv. recombinant tPA therapy alone (0.9 mg/kg to a maximum of 90 mg) or a combined iv. recombinant tPA therapy (0.9 mg/kg to a maximum of 90 mg) and IA adjunctive treatment with the PS. The primary outcomes of the study are the 90 day independent functional outcome (mRS ≤2) rate and the 90 day incidence of all serious adverse events. Secondary outcomes include the 30 day good clinical outcome rate defined by an improvement of 10 points or more in the NIHSS score at discharge, NIHSS score of 0–1 at discharge; or a 30 day mRS ≤2. In addition, the 90 day incidence of symptomatic and asymptomatic ICH will be investigated as secondary outcomes.
Another advantage of using the PS is achieving a shorter procedural time to recanalization. In the pivotal trial, the median aspiration time was 45 min, while the median time from groin puncture to end of aspiration was 97 min. However, these times were significantly reduced when the reperfusion catheter with the larger inner diameter (054) is used as compared to those used in the pivotal trial (026 to 041). In a proof-of-concept retrospective study, results of 53 consecutive patients treated with the PS 054 in nine US centers were analyzed [35]. The median time required for aspiration was 14 min (45 min in the pivotal trial and 41 min in the POST trial) while the median puncture to end of aspiration time was 52 min (97 min in the pivotal trial). The rate of symptomatic ICH was not higher than that of the pivotal trial (9.4 and 11.2%, respectively). MCA occlusions comprised 76% of cases while ICA occlusions made up 20% and vertebrobasilar 4%. These results are encouraging that increasing the reperfusion catheter diameter can enhance aspiration and reduce procedure time without affecting safety.
A number of other reports described experience with PS confirming the safety and efficacy of its use [34–37]. We have published our center’s initial experience with the use of PS in 27 patients (mean age: 61 years, median NIHSS score: 18). Successful recanalization was achieved in 85% in this cohort (67% of cases where the PS was used alone). Independent outcome was achieved in 48%. This high rate – compared with the Penumbra Pivotal trial – is likely related to a number of factors. Patients are selected for IA thrombolysis at our center based on the extent of early ischemic changes on baseline noncontrast CT and the presence of a proximal arterial occlusion. In addition, while the mean onset to IA procedure was just over 3 h, our mean puncture to recanalization time was only 80 min. Finally, general anesthesia was only used in 37% of patients. The rate of complication was also relatively low. ICH occurred in 33.3%. Among those the combined rate of parenchymal hemorrhage (PH1 and PH2) was only 7.4%, while all cause mortality rate was 19%. These relatively high rates of symptomatic ICH could be attributed to the extent of early ischemic changes in some of these treated patients. Extensive early ischemic changes in baseline imaging assessed by the ASPECTS score have been shown to predict symptomatic ICH after thrombolysis [31,38]. Another important factor is the inclusion of patients up to 8 h from the onset of stroke symptoms in some of these studies. Recanalization at a late time window has been shown to correlate with the rate of symptomatic and asymptomatic ICH in studies that monitored recanalization rates over time using transcranial Doppler [39,40].
Some reports described simple successful modifications. In a paper by Kang et al, the authors described 22 cases of successful recanalization by direct wedging between the reperfusion catheter and the proximal clot, followed by forced manual suction without the use of the separator or aspiration pump [41].
Other emerging techniques
Retrievable stents (including the Solitaire™ eV3, MN, USA, and Trevo Stryker, MI, USA) are strongly emerging as the newest devices in acute ischemic therapy. These devices combine the features of removable cerebral stents and clot retriever devices. Their retrievable property overcomes the major disadvantage of permanent stents in the acute stroke setting, which is the use of strong antiplatelets agents. Initial experience with these devices has been encouraging with reports from single-center cohorts of successful recanalization rates of 85–100% [42–44]. These devices, sometimes referred to as stent retrievers, are undergoing testing in the TREVO trial. The Solitaire device is being evaluated in the SWIFT trial. The Separator™ 3D (Penumbra, CA, USA) is another new device recently approved for CE Mark in Europe. It is designed to be used with lesional aspiration, taking advantage of the benefits of the PS.
Conclusion
Endovascular stroke therapy has developed progressively over the last few years. Aspiration neurothrombectomy with the PS was developed to improve the low recanalization rates achieved with the sole use of iv. tPA and as an alternative to existing mechanical thrombectomy devices. It has been shown to be safe and effective in achieving revascularization in the acute ischemic stroke setting in multiple prospective studies. However, the rates of independent functional outcome are still lagging behind the high recanalization rates. It is planned to undergo a more definitive assessment of its safety and efficacy in a randomized clinical trial. This high-level evidence is needed to show that the high recanalization rates achieved with IA adjunctive therapies – including the PS – reflect on improved functional outcomes.
Future perspective
Acute ischemic stroke management has rapidly evolved. The ultimate goal of all therapies would be to achieve complete and sustained recanalization at the site of occlusion in a timely fashion to save as much brain tissue as possible. While endovascular devices seem to accomplish one goal of stroke therapy with their high successful recanalization rates, the evidence that they improve functional outcome is still lacking. An important aspect of this approach that needs to be better defined, is which acute ischemic stroke patients would attain the highest benefit from interventional therapy. Current evidence suggests that the ideal target would be patients presenting early after stroke symptom onset, with disabling clinical deficit, a sizable thrombus and a small ischemic core. This patient population needs to be identified and triaged rapidly, and transferred to the angiography suite for emergency revascularization. The best means of achieving recanalization are debated and will probably continue to be so as the technology continues to develop. However, the efficacy of IA stroke therapy has been shown, head-to-head comparisons of the currently used devices will be warranted to establish their perspective superiority/equivalence.
Financial & competing interests disclosure
M Goyal has received honoraria from Penumbra, Inc. for speaking engagements. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Status of endovascular stroke therapy preceding Penumbra system
▪ Acute ischemic stroke is a disabling global health problem.
▪ Timely and complete recanalization has been shown to be among the strongest predictors of good functional recovery following acute ischemic stroke.
▪ The most widely used ischemic stroke therapy (intravenous tissue plasminogen activator [iv. tPA]) is underutilized and achieves full recanalization in less than half of cases with major arterial occlusions.
The Penumbra system
▪ The Penumbra system is a novel intra-arterial (IA) device that achieves recanalization through a process of continuous aspiration and extraction.
▪ The Penumbra system is composed of a reperfusion catheter that is hooked to continuous suction and a separator wire. Gentle in and out movements of the separator break down the thrombus inside the catheter tip and allows for its easy aspiration by the reperfusion catheter.
Evidence for the Penumbra system
▪ The safety of the Penumbra system has been shown in a number of prospective studies leading to its approval by the US FDA for use in the acute ischemic stroke setting.
▪ The Penumbra system achieved higher recanalization rates compared to iv. tPA and the only other FDA-cleared IA device, the Merci retriever. This raised some questions about how successful recanalization was interpreted in Penumbra Pivotal trial.
Future perspective
▪ The impact the Penumbra system has on outcomes will be assessed in a randomized clinical trial (THERAPY) that compares it with the standard iv. tPA, therapy.
▪ As the field of endovascular acute ischemic stroke therapy continues to evolve, better definition is needed for the patient population with the highest likelihood of achieving complete or near complete recovery with IA therapies.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Sacco RL, Benjamin EJ, Broderick JP et al. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Risk factors. Stroke 28(7), 1507–1517 (1997).
- Roger VL, Go AS, Lloyd-Jones DM et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation 123(4), e18–e209 (2011).
- The National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333(24), 1581–1587 (1995).
- Lees KR, Bluhmki E, von Kummer R et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375(9727), 1695–1703 (2010).
- The IMS Study investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the interventional management of stroke study. Stroke 35, 904–911 (2004).
- Lee KY, Han SW, Kim SH et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by preand post-thrombolytic angiography in acute ischemic stroke patients. Stroke 38(1), 192–193 (2007).
- Jansen O, von Kummer R, Forsting M, Hacke W, Sartor K. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am. J. Neuroradiol. 16(10), 1977–1986 (1995).
- Intraarterial thrombolysis: ready for prime time? Executive Committee of the ASITN. American Society of Interventional and Therapeutic Neuroradiology. AJNR Am. J. Neuroradiol. 22(1), 55–58 (2001).
- Bhatia R, Hill MD, Shobha N et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41(10), 2254–2258 (2010).
- Tan IY, Demchuk AM, Hopyan J et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am. J. Neuroradiol. 30(3), 525–531 (2009).
- Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 59(6), 862–867 (2002).
- Rubiera M, Alvarez-Sabin J, Ribo M et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 36(7), 1452–1456 (2005).
- Alexandrov AV. Ultrasound identification and lysis of clots. Stroke 35, 2722–2725 (2004).
- Jungreis CA, Wechsler LR, Horton JA. Intracranial thrombolysis via a catheter embedded in the clot. Stroke 20(11), 1578–1580 (1989).
- del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a Phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in acute cerebral thromboembolism. Stroke 29(1), 4–11 (1998).
- Furlan A, Higashida R, Wechsler L et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 282(21), 2003–2011 (1999).
- Lewandowski CA, Frankel M, Tomsick TA et al. Combined intravenous and intra-arterial rTPA versus intra-arterial therapy of acute ischemic stroke: emergency management of stroke (EMS) bridging trial. Stroke 30(12), 2598–2605 (1999).
- The IMS II Trial investigators. The interventional management of stroke (IMS) II study. Stroke 38, 2127–2135 (2007).
- Khatri P, Hill MD, Palesch YY et al. Methodology of the interventional management of stroke III trial. Int. J. Stroke 3(2), 130–137 (2008).
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 38(3), 967–973 (2007).
- Almekhlafi MA, Hu WY, Hill MD, Auer RN. Calcification and endothelialization of thrombi in acute stroke. Ann. Neurol. 64(3), 344–348 (2008).
- Fitzgerald DJ, Wright F, FitzGerald GA. Increased thromboxane biosynthesis during coronary thrombolysis. Evidence that platelet activation and thromboxane A2 modulate the response to tissue-type plasminogen activator in vivo. Circ. Res. 65(1), 83–94 (1989).
- Gobin YP, Starkman S, Duckwiler GR et al. MERCI 1: a Phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke 35(12), 2848–2854 (2004).
- Lee W, Sitoh YY, Lim CC, Lim WE, Hui FK. The MERCI retrieval system for the management of acute ischaemic stroke-the NNI Singapore experience. Ann. Acad. Med. Singapore 38(9), 749–755 (2009).
- Smith WS, Sung G, Saver J et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke 39(4), 1205–1212 (2008).
- Devlin TG, Baxter BW, Feintuch TA, Desbiens NA. The merci retrieval system for acute stroke: the southeast regional stroke center experience. Neurocrit. Care 6(1), 11–21 (2007).
- Smith WS, Sung G, Starkman S et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36(7), 1432–1438 (2005).
- Menon B, Davis M, Herrera C et al. P005 anesthetic considerations and the role of blood pressure management in the endovascular treatment of acute ischemic stroke. J. NeuroIntervention Surg. 2, A17 (2010).
- Bose A, Henkes H, Alfke K et al. The Penumbra system: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am. J. Neuroradiol. 29(7), 1409–1413 (2008).
- Penumbra Pivotal Stroke Trial investigators. The Penumbra Pivotal Stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 40, 2761–2768 (2009).
- Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta stroke programme early CT score. Lancet 355(9216), 1670–1674 (2000).
- Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke 42(1), 93–97 (2011).
- Saver JL, Liebeskind DS, Nogueira RG, Jahan R. Need to clarify thrombolysis in myocardial ischemia (TIMI) scale scoring method in the Penumbra Pivotal Stroke trial. Stroke 41(2), e115–e116 (2010).
- Menon BK, Hill MD, Eesa M et al. Initial experience with the Penumbra stroke system for recanalization of large vessel occlusions in acute ischemic stroke. Neuroradiology 53(4), 261–266 (2011).
- Frei D, Turk A, Heck D et al. The SPEED trial: a study of the Penumbra early evacuation device. Presented at: International Stroke Conference. Los Angeles, CA, USA, 9–11 February 2011.
- Taschner CA, Treier M, Schumacher M, Berlis A, Weber J, Niesen W. Mechanical thrombectomy with the Penumbra recanalization device in acute ischemic stroke. J. Neuroradiol. 38(1), 47–52 (2011).
- Kulcsar Z, Bonvin C, Pereira VM et al. Penumbra system: a novel mechanical thrombectomy device for large-vessel occlusions in acute stroke. AJNR Am. J. Neuroradiol. 31(4), 628–633 (2010).
- Singer OC, Kurre W, Humpich MC et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke 40(8), 2743–2748 (2009).
- Molina CA, Montaner J, Abilleira S et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 32(5), 1079–1084 (2001).
- Dinia L, Rubiera M, Ribo M et al. Reperfusion after stroke sonothrombolysis with microbubbles may predict intracranial bleeding. Neurology 73(10), 775–780 (2009).
- Kang DH, Hwang YH, Kim YS, Park J, Kwon O, Jung C. Direct thrombus retrieval using the reperfusion catheter of the Penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR Am. J. Neuroradiol. 32(2), 283–287 (2011).
- Aleu A, Dorado L, Castao C et al. Endovascular therapy for acute ischemic stroke using retrievable stents-stentrievers. Presented at: International Stroke Conference. Los Angeles, CA, USA, 9–11 February 2011.
- Pereira V, Narata A, Yilmaz H et al. Solitaire device for immediate flow restoration and revascularization in acute stroke. Geneva experience. Presented at: International Stroke Conference. Los Angeles, CA, USA, 9–11 February 2011.
- Menon BK, Kochar P, Ah-Seng A et al. Initial experience with a self-expanding retrievable stent for recanalization of large vessel occlusions in acute ischemic stroke. Neuroradiology 54(2), 147–154 (2011).
▪ Among the earliest studies to provide high-quality evidence that the intra-arterial approach is effective in the management of acute ischemic stroke.
▪▪ The first report describing the clinical experience with the Penumbra system in Europe.
▪▪ This study showed the safety and efficacy of the Penumbra system and led to its approval by the US FDA for the treatment of acute ischemic stroke.
▪▪ Identified that a short time to recanalization in patients with limited extent of early ischemic changes on baseline computed tomography derived the highest benefit in the Penumbra Pivotal trial.
▪ Showed that the use of the larger reperfusion catheter (054) resulted in faster recanalization without affecting the safety or accessability.
▪ Describes a successful modification to the use of the Penumbra system by directly applying manual suction at the reperfusion catheter instead of connecting it to the suction device.