Review Article - International Journal of Clinical Rheumatology (2018)
Epidemiology and awareness of osteoporosis: a viewpoint from the Middle East and North Africa
- *Corresponding Author:
- Tamer A. Gheita
Rheumatology Department
Faculty of Medicine
Cairo University
Cairo, Egypt
E-mail: gheitamer@hotmail.com
Abstract
Background: Osteoporosis (OP) is defined by low bone mass and microstructural deterioration. It is an escalating public health problem due to increase life expectancy and the resulting bone fractures represent a significant burden for both the individual and the society in terms of morbidity, mortality and cost. Osteoporosis, a multifactorial disease, results from the interaction between genetic and environmental risk factors. Currently, the data available regarding OP epidemiology and predisposing risk factors differ greatly between regions and within population ethnicities. Proper estimation of the epidemiology of OP and its health related outcomes can help identity those at risk and permit prophylactic treatment before its occurrence. The main barrier towards disease prevention strategies is the impaired awareness of the disease and its risk. Enhanced understanding of the OP disease may influence personal behaviors and reduce its prevalence.
Objectives: This review was undertaken to wrap-up and throw-light on the published literatures related to the epidemiology of osteoporosis in the Middle East and North Africa (MENA) region, and expose the extent of awareness in the corresponding populations. Describing and discussing key points on the current state of knowledge on these hot issues are well thought-out.
Conclusion: Osteoporosis prevalence is variable among MENA populations. Limited reports regarding the established prevalence of osteoporotic fractures among those populations and therefore, lack of guidelines for prevention and management were noticed. In order to improve bone health, preventive measures against OP should be considered. Increase OP awareness and preventive practices in the societies as part of the prophylactic strategy need to be initiated.
Keywords
osteoporosis, epidemiology, Middle East and North Africa, risk factors, awareness, MENA
Introduction
Osteoporosis (OP) is a systemic disease characterized by a decrease in bone mass and microarchitectural deterioration of bone tissue related to abnormalities of bone turnover and resulting in fragility and increased risk of fracture [1,2]. Numerous criteria for the diagnosis of OP have been proposed. According to the World Health Organization (WHO), osteoporosis is defined as a bone mineral density (BMD) at the hip and/or the spine at least 2.5 standard deviations (SD) below the mean peak bone mass of young healthy adults as determined by dual energy X-ray absorptiometry (DEXA) [3]. Taking in to consideration both the BMD and fracture compared to the mean BMD value in a young adult, the WHO stratified the following definitions of 4 categories: Normal: a BMD value of -1 and above, Osteopenia: a BMD value between -1 and -2.5 SDs, Osteoporosis: a BMD value >2.5 SDs below, and Severe (established) osteoporosis: a BMD value >2.5 SDs below in the presence of one or more fragility fractures [4].
Osteoporosis is recognized as a serious health problem, with about 200 million people being affected worldwide [5]. Over 40% of women and 20% of men with OP are likely to have an osteoporotic (fragility) fracture during their lifespan [6]. Mortality associated with osteoporotic fractures ranges from 15 to 30%, a rate similar to breast cancer and stroke [7].
The prevalence of OP is rising steadily and becoming a major public health issue with the universal increasing life expectancy; in particular more rapidly in the developing countries [8]. For example, it is projected that by 2050, Egypt will be close to 130 million inhabitants, and more than 30% of its population will be aged 50 years and over. Moreover, in Lebanon, Iran and Tunisia, nearly 40% of the population will be 50 years old and over [9]. Estimating the incidence of OP is challenging as it varies significantly between countries, according to the age, sex, and ethnic distribution of the population. Little is known about the epidemiology of osteoporosis in the Middle East and North Africa (MENA) countries. MENA countries include Iran, Iraq, Turkey, Saudi Arabia, Bahrain, Kuwait, Oman, Qatar, United Arab Emirates, Yemen, Jordan, Palestine, Syria, Lebanon, Egypt, Libya, Tunis, Algeria, and Morocco. Thus in order to obtain a clearer picture of regionally evaluated OP, especially about its epidemiology and extent of disease awareness, the current review has been conducted.
This review is to present the geographic variability in the prevalence of osteoporosis and risk factor in the MENA region. Common osteoporosis associated medical conditions and co morbidities are also addressed in this article. The level of awareness and knowledge of osteoporosis among the population from this region will be taken into consideration.
Epidemiology of osteoporosis in MENA
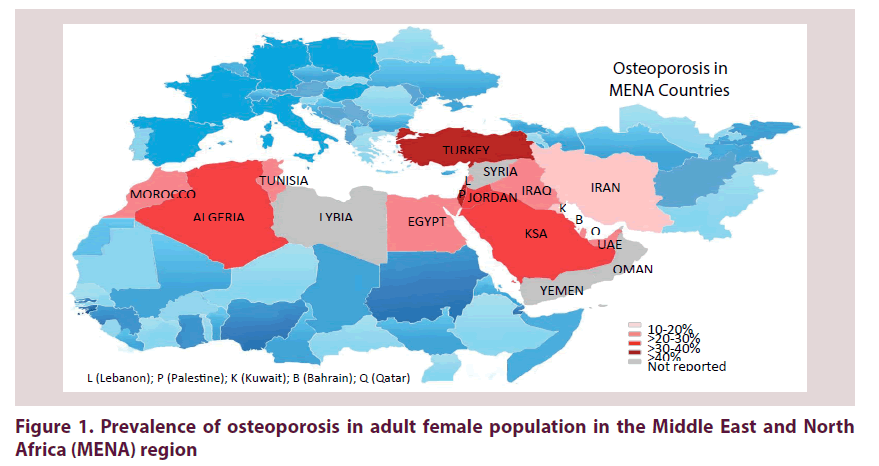
The prevalence of OP is best measured either by the frequency of reduced BMD, in spite of the well-known ethnic differences, or by frequencies of osteoporotic people based on the WHO classification. Prevalences of OP from MENA countries are highlighted in Figure 1 and findings listed in Table 1. It has to be emphasized that there is a heterogeneous prevalence of OP in the listed studies and are not comparable even within the same country. These variances can be explained by differences in populations studied (genetic background, age categories, sample sizes, style of nutrition, physical activity and reference norms), study locations (cities versus rural area), epidemiologic characteristics (ranging from hospital-based to nationwide studies), and varying investigative modalities employed.
| MENA Flag | Study | Year | City | No/gender or F:M/age | Study design | BMD measure mode/site | Prevalence [%] | Key findings | |
|---|---|---|---|---|---|---|---|---|---|
| Osteo-penia | OP | ||||||||
| Turkey | Keskin [10] Cankurtaran [11] Hamdi Kara [12] |
2014 2005 2007 |
Istanbul Ankara 5 cities |
620/ 4:1/54.8 ± 10.3 1247/1.7:1/65-98 724/F/57.6 ± 9.6 |
CB RS MC |
DXL/phalynx DXA/ Sp. & F Dens.M/Phal. |
- 32 27.2 |
15.3 58 30.2 |
1/4 of people >55 years are OP and only 4.9% <55 years are OP. Nutritional support and physical activity should be encouraged. Aging/illiteracy [-ve]; overweight, tea & HRT [+ve] on BMD. |
| Iran | Sharmai [13] Good [14] Dashti [15] Heidari [16] |
2008 2013 2016 2017 |
Rasht Tehran Isfahan Babol |
788/F/63.3 ± 0.4 200/F/40-65 2536/F/- 553/M/70 ± 7.7 |
PB CS-HB CS CS |
QUS-DXA DXA DXA/ Sp. & F DXA/ Sp. & F |
- 37 42.7 - |
18.5 14.5 8.2 16.2 |
Prevalence of OP increased with age. Prevalence increased with age and in peri/post-menopausal. None had OP <40 y. Frequency increased with age [1.2-26.5%]. Obesity, BMI, muscle strength & DM lower OP risk in elderly men. |
| Iraq | Gorial [17] | 2013 | Baghdad | 250/F/59 ± 6.3 | CS - MC | DXA/spine | 20 | 22.8 | OP associated with aging, menopause, low BMI & X-ray osteopenia |
| Egypt | Mahran [39] Selim [31] |
2012 2009 |
Sohag Assiut |
362/ F/35.9 ± 7.4 581/F/58.2 |
CS CS |
QUS/calcaneus QUS/calcaneus |
29 33.4 |
2.8 47.8 |
Low BMD was allied to low socioeconomic women on OCP OP prevalence in rural PM women in Upper Egypt is high |
| Saudi Arabia | Hammad [40] Tariq [18] Sadat-Ali [19] Alghamdi [20] Sadat-Ali [41] |
2017 2016 2012 2011 2017 |
Riyadh - Khobar Makkah Dammam |
101/F/21.3 ± 0.8 1205/F/20-80 5160/F/56.8 ± 2.7 257/F/19-24 455/M/ 65.3 ± 9.8 |
CS CS-HB SR CS RS |
QUS/calcaneus QUS/calcaneus DXA/QUS QUS/wrist DXA/ Sp. & F |
31.2 29.8 34 32.3 - |
2.9 27.2 36.6 7 46 |
Life style factors lead to OP and osteopenia in young adult female. Prevalence is high, occurs early and is related to age & education. Ideal screening age for low BMD among Saudis should be early PrevalenceofOP in was associated with obesity & decreased age. Prevalence in Saudi males is high & increased in the last decade. |
| Bahrain | Alsawy [23] | 2010 | Bahrain | 170/F/- | CS | QUS | 51.2 | 27.1 | Edentulism was associated with OP in PM women |
| Qatar | Bener [25] | 2007 | Qatar | 821/F/57.6 ± 9.1 | CS | DXA/ Sp. & F | - | 21.3 | The prevalence of OP in Qatari women is comparable to others. |
| Kuwait | Al-Shoumer [21] Gupta [22] |
2012 2012 |
Kuwait Kuwait |
454/F/55 ± 0.3 2296/F/59.1 ± 7.9 |
CS CS |
DXA/ Sp. & F DXA/3 sites |
38.5 45.6 |
16.2 19.3 |
Prevalence of OP is high in PM Kuwaiti women OP increased with age from 4.3% to 39.9%. |
| UAE | Fawsy [24] Al Saleh [42] |
2012 2016 |
Ajman Dubai |
444/5.3:1/59.1 ± 7.9 3985/3.4:1/42.1 ± 16 |
CS CS |
DXA/ Sp. & F QUS |
36 22.4 |
22.3 3.1 |
Prevalence was higher in females and increased with age. Prevalence of osteopenia and OP was higher in females. |
| Palestine | Abd-Alhamed [26] Aker [27] |
2010 2013 |
Jerusalem Nablus |
505/F/61.2 ± 8.5 100/0.25:1/55-67 |
CS CS |
DXA/3 sites DXA/Sp. & hip |
74 26 |
40.6 13 |
Prevalence of OP at all sites was 8%. Osteoporosis is common >50 years old and more in the spine. |
| Jordan | Hyassat [28] | 2017 | Amman | 1079/F/61.1 ± 7.2 | CS | DXA/Sp. & hip | 44.6 | 37.5 | The prevalence of OP and osteopenia is high in PM Jordanians. |
| Lebanon | Baddoura [29] | 2007 | Beirut | 432/ 1.9:1/73.6 ± 5 | CS | Dens.M/3sites | - | 28 | Prevalence of OP was higher in subjects with vertebral fractures. |
| Morocco | El Maataoui [43] Ouzzif [32] El Maghraoui [33] |
2015 2012 2015 |
Rabat Rabat Rabat |
142/M/63.3 ± 8.7 188/F/58 ± 7.6 429/F/59.5 ± 8.3 |
CS CS CS |
DXA/ Sp. & F DXA/ Sp. & F DXA/ Sp. & F |
48.6 35 58.7 |
20.4 30.8 21 |
Age, higher BMI, cigarette smoking were OP predictors in males Vitamin B12 was a risk factor for OP in healthy women. Obese PM women had a higher BMD and lower vertebral fracture. |
| Algeria | Haouichat [34] | 2014 | Algiers | 546/F/62 ± 9.42 | CS | - | - | 35.8 | PM OP is common with numerous potential risk factors detected. |
| Tunisia | Sahli [36] | 2009 | Tunisia | 1378/F/47.2 ± s15 | CS | DXA/ Sp. & F | - | 23.4 | OP in women >50 y was 23.3% at spine & 17.3% at femoral neck |
BMD: Bone mineral density, BMI: Bone mineral density, Dens.M: densitometer; DM: diabete mellitus, DXA: Dual-energy X-ray absorptiometry, DXL: Dual-energy X-ray laser absorptiometry, F: Femur, F:M: female:male ratio; HRT: Hormone therapy, MENA: Middle East and North Africa; OP: osteoporosis, Phal.: Phalangeal; QUS: Quantitative ultrasound, OCP: oral contraceptive pills; PM: post-menopausal, CS: cross sectional, PB: population-based; HB: hospital-based, CB: community-based; MC: multicentre; Sp.: Spine, SR: systematic review; RS: retrospective.
Table 1: Overview of the epidemiology characteristics of osteoporosis in Middle East and North African adults
In a community-based study involving 620 Turkish people, the prevalence of OP was 15.1% among women and 10.7% among men [10]. High frequencies of OP and osteopenia (58% and 32%, respectively) had been detected among patients admitted to the geriatric department [11]. However, lower values for OP were reported in a 2008 multi-centre study of postmenopausal women recruited from five major cities in Turkey; 30.2% had OP and 27.2% had osteopenia [12]. In North Iran, out of 788 women from a population based study, 18.5% were identified as having OP [13]. Furthermore, a cross-sectional hospital-based study in Tehran performed to assess the BMD in 200 women between 45 to 65 years found that 14.5% had OP and 37% had osteopenia [14]. While of the 2536 women who were referred to Isfahan Osteoporosis Diagnosis Center (IOSC), during 2013-2014, 49.1% had normal bone density, 42.7% had osteopenia and only 8.2% had OP [15]. Recently, a total of 16.2% elderly Iranian males had OP at either femoral neck or lumbar spine [16]. In Iraqi postmenopausal females the incidence of OP increased to reach 22.8% [17].
The prevalence of OP and osteopenia in Saudi women, aged 20-80 years, was reported to be 27.2% and 29.8 %, respectively [18]. A systematic review showed that the prevalence of low bone mass (OP and osteopenia) in Saudi Arabia is 70.5% at an average age of 56 years [19]. Alghamdi et al. showed that among female university students in KSA, OP was present in 7% while, osteopenia was found in 32.3% [20]. This suggests that the prevalence of osteopenia and OP was very high, and would occur earlier in Saudi women. The prevalence of OP in Kuwaiti postmenopausal women was estimated to be 20% and 12.4% for the spine and femur neck region, respectively [21], yet a high prevalence of low BMD was shown in more than 50% of the subjects. Furthermore, comparable findings were observed in Kuwaiti postmenopausal women with prevalence of OP increasing with age from 4.3% in women less than 50 years to 39.9% in those older than 70 years old [22]. A small sample including 170 postmenopausal Bahraini women demonstrated 51.2% had osteopenia and 27.1% had OP measured by quantitative ultrasound (QUS) [23]. On screening of healthy individuals in UAE, it was estimated that 24% had osteopenia and 2.5% had OP [9]. Another study also on a healthy population in UAE revealed that 60.8% had low BMD, of whom 4.1% had severe OP, 36.7% OP, and 59.3% osteopenia [24]. The prevalence of OP (21.3%) measured by DEXA in Qatari women was comparable to other countries [25].
Among 505 Palestinian women, OP affected the lumbar spine, femoral neck and total hip in 24%, 14% and 29.7% of subjects, respectively [26]. About 21% of elderly Palestinians had vertebral and 5% had hip OP [27]. The overall prevalence of OP and osteopenia among Jordanian postmenopausal women was 37.5% and 44.6%, respectively [28]. In Lebanon, the prevalence of OP in elderly subjects was 33% in women and 22.7% in men [29].
The prevalence of OP in Egypt was 21.9% in men and 28.4% in women [30]. Furthermore, 26% men and 53.9% women had osteopenia. The prevalence of OP in postmenopausal women in rural areas of Upper Egypt was higher reaching 47.8% [31]. Prevalence of OP in Moroccan postmenopausal women varied from 21-31% [32,33] being slightly higher (35.8%) among Algerian women [34]. In Tunis, 25% of postmenopausal women had OP [35] and a comparable prevalence (23.3%) was found in healthy Tunisian women [36]. No available data regarding the prevalence of OP in Syria, Lybia, Oman or Yemen was found.
A diverse prevalence rate of OP in the MENA region is obvious and is generally comparable to the corresponding postmenopausal Caucasian women in North America that ranged from 10.3-30% [3,37] but higher than that reported in Europe (20%) [38]. There is an increasing need of developing regional population-specific reference range for DEXA measurements. Reports on OP in men were limited and should not be neglected due to the associated serious complications; focus on male OP should be increased.
Risk factors of osteoporosis in MENA
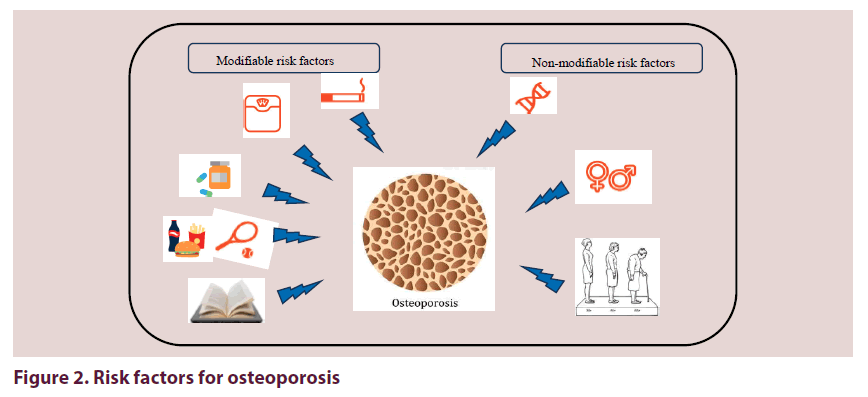
The variations in OP prevalence across MENA raise important questions about the etiology of osteoporosis. However, the reasons for this variability are unknown; a combination of genetic, environmental and lifestyle risk factors probably influence this disparity Figure 2.
Non-modifiable risk factors
Genetics: Osteoporosis is a disease caused by interaction of genetic and environmental factors by almost 70% and 30% respectively [39–44]. The most important examined gene linked to OP is vitamin D receptor gene (VDR).
There are numerous known polymorphisms in VDR, some of them are established to be associated with bone diseases as OP. In a large cohort of Iranians, Fok1 A/G (rs2228570) polymorphism was significantly associated with OP in postmenopausal women [45]. Sassi et al. [46] analyzed the association of other VDR polymorphisms (ApaI and TaqI) with BMD in postmenopausal Tunisian women and an increased risk to develop osteopenia, but not OP, was shown which strengthens the hypothesis that OP results from the interaction of genetic and environmental factors. The relationship of the 21 single nucleotide polymorphisms (SNP) in 7 selected candidate genes (LRP5, RANK, RANKL, ESR1, VDR, P2XR7, and OPG) with BMD at different body sites was evaluated in an Iranian population [47]. The overall pattern of association of these established risk SNPs were similar to those observed in other populations. Significant associations were seen with seven SNPs, one of them is VDR SNP rs731246. In consistency, analysis of LRP5 polymorphisms in postmenopausal North African (Tunisian) women showed no association with BMD at any sites [48].
Age: Osteoporosis in various age groups had different rates, although other risk factors as concomitant diseases and physical activity level may play a role. Advanced age has been identified as a significant risk factor of OP and decreased BMD [14]. The prevalence of OP was notably diverse in various age groups among Iranian population [13,15], although, no patient less than 40 years had OP [15]. The prevalence of OP in Jordanian women >60 years was significantly higher compared to those <50 years (25.5% vs. 7.4%, respectively) [49]. Advanced age was also a significant predictor of OP in Moroccan postmenopausal women [32,49]. Even though, low BMD and OP were detected in young age; among apparently healthy young Saudi women, 6% had osteopenia and 3% had OP [50,51]. Higher OP prevalence rate (7%) was detected by Alghannam et al. [52] among women ≤ 31 years old. These findings highlight that young women are not exempted from OP, which necessitates an early screening program.
Family history of osteoporosis: Family history of OP was a risk factor for postmenopausal low BMD and a negative predictor of OP [14].
Gender: Women are known to have bone loss at younger age and at a faster rate compared to men. Females aged 50 years or older have a 4 times higher rate of OP and a 2 times higher rate of osteopenia compared with males [53]. This difference may be explained by difference in other OP risk factors such as body weight, bone loss markers and smoking. Osteoporosis in men is largely a neglected condition, despite the fact that a higher incidence of fragility fractures occur in men. Studies conducted on MENA men showed higher frequency of low BMD [54–57]. For example, the prevalence of OP in Iranian men compared to postmenopausal women was 44.1% and 37%, respectively; and the difference was significant [45]. However, in another study involving 5,892 individuals from 20-91 years, there was no significant difference in BMD based on gender or menopausal status [58].
Modifiable risk factors
Socioeconomic factors: It is increasingly recognized that socioeconomic inequalities play an important role in bone health. OP was considerably prevalent among women of low social status than those living in urban areas [59]. At all BMD sites, women under absolute poverty lines had the lowest BMD [59]. According to the WHO definition, 22.4% of subjects under absolute poverty lines and 8.7% healthy postmenopausal women were considered osteoporotic [60]. Low education was associated with lower BMD and a higher prevalence of OP even after controlling for strong confounders (age, BMI, physical activity) [18,59,61]. Nevertheless, in another study, there was no significant association between OP and educational level [28].
Menopause and Parity: There is a conflict of results regarding the relationship between the menopausal status and OP [13,28]. Significant correlations were found between OP, parity, years of menstruation and menopausal status [13]. In premenopausal women, 9.9% were osteoporotic, however, the prevalence of OP raised significantly postmenopause, 39% [18]. Surprisingly, increased parity may protect women from OP and from fracture risk [62]. BMD of women with >6 children was higher than their counterparts and was sustained after prolonged lactation. Women who delivered >6 children were less osteoporotic (25.4%) compared to those who had <5 (48%). In accordance, multiparous had a lower prevalence of OP when compared to nulliparous with a notable incidence of OP-related fracture in nulliparous [63].
Nutrition and life style factors: Paying more attention to life style factors in young age may decrease bone loss later on in life. Vitamin D deficiency is high in Arab countries, in particular among females. Female gender was an independent predictor of lower vitamin D level [64]. Calcium and vitamin D intake are inversely related to the BMD [65,66], and differ between osteoporotic and non-osteoporotic subjects [14,40,45]. Dietary patterns, foods with high content of saturated fatty acids or with low nutrients are detrimental to bone health in menopausal Iranian women [67]. Vitamin B12 and high soft drink intake are reported to be independent risk factors for OP in healthy women [32,40]. These findings highlight the importance of proper food selection to maintain bone health and could be the basis for nutrition education intervention to improve lifestyle habits and promote healthy bone later in life.
Sunlight is abundant in MENA countries throughout the year. However, the extent of sunlight exposure and the influence on vitamin D level are determined much more by the dressing and clothing style [68,69]. Significant hypovitaminosis D but not OP was observed in women categorized into groups according to the longevity of dressing [70]. In Moroccan and Turkish women, wearing traditional clothing covering arms, legs and head, the risk of OP was increased [10,71]. Vitamin D inadequacy (deficiency and insufficiency) has become an epidemic with the assumption that women in Arab countries are at a higher risk due to their clothing style of wearing dark colored suits or a veil. It is a major health problem not only in elderly or women with in-door residency but also in young Saudi men [72].
Environmental exposure: The relationship between smoking exposure and OP remains controversial; smoking was not associated with OP [28,49] while others considered cigarette smoking as a predictor for OP [43,56,73]. Among battery manufacturing workers, significant elevated levels of lead concentration were accompanied with OP when compared to healthy control. In addition, lead poisoning may act as an OP risk factor in female workers [74].
Body mass index (BMI) and Physical activity: Information on the impact of BMI and physical activity on BMD is limited. The relationship between obesity and OP remains controversial. Data indicated that overweight and obesity are associated with BMD [32,58,66], and decreased the risk for OP [16]. Comparison between postmenopausal women according to their BMI showed that obese women had a higher BMD and lower prevalence of vertebral fractures [33,75]. Physical inactivity and low BMI are major risk factors of low BMD in both women and men [13,43,56,57,65,76].
Medications: The BMD of patients who are prescribed certain medications need to be monitored, and OP prophylactic and therapeutic strategies should be considered. For example, antipsychotic medications are associated with lower BMD [77,78]. Prevalence of OP and osteopenia detected in epileptic patients under treatment revealed that 14.2% were osteoporotic and 59.42% were osteopenic [77]. Corticosteroid-induced OP is the most common form of secondary OP and the first cause in young people. Bone loss and high rate of fractures occur early after the initiation of corticosteroids, even with inhaled steroid, and are related to dosage and therapy duration [49,79]. Cancer therapy induced OP and increased risk of fragility fracture and mortality were reported [80,81]. Chemotherapy-induced OP occurred in 25.8% of cancer survivors in Saudi Arabia [82]. Unexpected, this study indicated a high prevalence of osteopenia and OP in those patients who were ≤ 50 years and the effect on bone continued for >2 years from the last cycle of chemotherapy [82]. However, others found that hormonal replacement therapy (HRT) and oral contraceptive pills (OCP) have a protective effect on BMD, and those women were less likely to have OP [10].
Concurrent medical diseases with osteoporosis in MENA
Osteoporosis and osteopenia are frequently reported during chronic medical diseases as one of several associated comorbidities.
Rheumatic diseases
Table 2 presents studies from the MENA region on the link of OP with rheumatic diseases. Rheumatoid arthritis (RA) is a common inflammatory autoimmune disease. RA patients had a higher incidence of OP and osteopenia compared to healthy controls. Increased inflammatory markers, decreased physical activity, and medications received in RA could contribute to the general bone loss and the generation of OP in these patients [83]. Bone loss and fragility fracture are frequent in RA and related to function impairment and corticosteroids use [84]. A considerable inverse relation between age, disease duration and BMD in postmenopausal RA women was reported [85]. Impaired bone formation and uncoupling of bone turnover were more evident in postmenopausal elderly-onset Egyptian RA cases and formed a risk for hip fracture [86]. In RA, the responsibility of B-cells in the pathogenesis of postmenopausal low bone mass was also shown. Hydroxychloroquine had a potential effect in reducing bone loss and could be considered among the first line armamentarium regimens in RA management. New therapeutic options that selectively inhibit B-cells in OP should be considered especially when associated with RA [87].
| Rheumatic Disease/Study | Year | Country | City | No/gender or F:M/age | BMD measure mode/site | Frequency [%] | Key findings | |
|---|---|---|---|---|---|---|---|---|
| Osteo-penia | OP | |||||||
| Rheumatoid Arthritis [RA] | ||||||||
| Fadda [83] | 2015 | Egypt | BS/Fayoum | 50/3.7:1/20-65 | DXA/3 sites | 24 | 46 | RA patients had a high prevalence of OP |
| Hamdi [84] | 2018 | Tunisia | Tunis | 173/4.4:1/54.1 ± 11.1 | DXA/Sp.& F | 87.1 | 48 | OP and fractures were higher in RA |
| Aghaei [85] | 2013 | Iran | Gorgan | 98/F/57.8 ± 9.4 | DXA/Sp.& F | 15.3 | 13.3 | BMD was -ve related to age & duration in PM |
| Gheita [86] | 2011 | Egypt | Cairo | 60/F/62.5 ± 8.3 | DXA/3 sites | 42.2 | 45.6 | Impaired BMD & fracture risk in PM EORA |
| Gheita [87] | 2014 | Egypt | Cairo | 68/F/- | DXA/3 sites | 50 | - | Study was on patients with & without LBM |
| Ankylosing Spondylitis [AS] | ||||||||
| El Maghraoui etal.[88] | 2016 | Morocco | 67/M/40.7 ± 11 | DXA/Sp.& F | - | 16 | Men with AS had a significant reduction in BMD | |
| Hmamouchi [89] | 2013 | Morocco | Rabat | 70/M/40 ± 12 | DXA/Sp.& F | 22.9 | 50 | Hypovit.D causes OP; increasing inflammation |
| Sayed [90] | 2015 | Egypt | Cairo | 44/1:21/33 ± 8.7 | DXA/3 sites | 73 | 19 | BMD spine -ve related to disease activity & function |
| Enteropathic Arthritis/Inflammatory Bowel Disease [IBD] | ||||||||
| Azzam [91] | 2015 | KSA | Riyadh | 701/1:1/26.4 ± 11.2 | DXA/Sp.& F | 35 | 17 | CD, young age & CRP are risk factors for OP in IBD |
| Ismail [92] | 2012 | KSA | Riyadh | 95/0.7:1/30.9 ± 11.6 | DXA/Sp.& F | 44.2 | 30.5 | IBD patients are at an increased risk of low BMD |
| Mikaeli [94] | 2009 | Iran | Shariati | 50/0.7:1/35.1 ± 10.9 | DXA/Sp.& F | 60 | 8 | UC patients not on steroid need OP screening. |
| Alharbi [95] | 2014 | KSA | Riyadh | 394/1:1/30.1 ± 0.6 | DXA/Sp.& F | 31.4 | 17.1 | OP in UC due to hypovit.D more than the disease |
| Systemic Lupus Erythematosus [SLE] | ||||||||
| Hafez [96] | 2018 | Egypt | Cairo | 70/F/ 50.03 ± 5.5 | DXA/Sp.& F | 42.9 | 20 | Fractures linked to age, duration, anti-DNA, activity |
| Abdwani [97] | 2015 | Oman | Muscat | 27/2.9:1/11 ± 4 | DXA/3 sites | 85 | 15 | Jo-SLE had low BMD that worsened over follow-up |
| Systemic Sclerosis [SSC] | ||||||||
| Shahin [98] | 2013 | Egypt | Cairo | 65/F/ 39.5 ± 13.5 | DXA/3 sites | 33.8 | 52.3 | SSc have low BMD associated with hand deformity |
| Fibromyalgia Syndrome [FMS] | ||||||||
| Olama [99] | 2013 | Egypt | Mansoura | 50/F/32.3 ± 9.4 | DXA/3 sites | 30 | 8 | Hypovitaminosis D was highly prevalent |
AS: ankylosing spondylitis, CD: Crohn’s disease, CRP: C-reactive protein, BMD: bone mineral density, BMI: Bone mineral density, BS: Benisuef, DXA: DNA: deoxyribonucleic acid, Dual- X-ray absorptiometry, EORA: elderly-onset RA, F: Femur, F:M: female to male, FMS: Fibromyalgia syndrome, IBD: inflammatory bowel disease, Jo- SLE: Juvenile onset systemic lupus erythematosus, LBM: low bone mass, OP: osteoporosis, RA: Rheumatoid arthritis, SLE: Systemic Lupus Erythematosus , Sp.: spine, SSc: Systemic Sclerosis (SSc), UC: ulcerative colitis. The 3 site denote the spine, femur and distal radius.
Table 2: Rheumatic diseases associated with osteoporosis in Middle East and North Africa
In adult males with ankylosing spondylitis (AS), the prevalence of OP was 19.4% compared to 3% in healthy subjects [88]. Vitamin D deficiency in male AS may indirectly lead to OP by an increase in the inflammatory activity [89]. The BMD was remarkably decreased at all measurement sites in AS patients and at the spine was remarkably inversely related to the disease activity and physical function. Bone loss in AS patients can be explained partly by the role of inflammatory mediators and to a certain extent as a consequence of a reduction in physical activity [90]. Prevalence of osteopenia and OP in adult Saudi patients with inflammatory bowel disease (IBD) was relatively low compared to worldwide reports [91]. IBD patients are at an increased risk of low BMD and the BMI, age and calcium supplements were independent predictors [92]. OP and osteopenia are noticed with a respective frequency of 35.7% and 23.2% in Crohn’s disease (CD). Among OP risk factors in CD, the nutritional status, younger age, and high C-reactive protein (CRP) were the most important [91,93]. However, ulcerative colitis (UC) patients, after excluding OP risk factors and prolonged corticosteroid use, had a comparable BMD to the controls [94]. High prevalence of OP in UC patients may be due to vitamin D deficiency more than the disease itself [95].
In a study on Egyptian systemic lupus erythematosus (SLE) patients, the 10-year risk of major and hip fractures was high; aging, disease duration, anti-DNA, disease activity and damage were associated with a higher fracture risk. Physicians should be aware of the high risk of future fractures in SLE [96]. Juvenile onset SLE patients had a high prevalence of OP (15%) that worsened over follow-up reaching 54% [97].
Egyptian patients with systemic sclerosis (SSc) had a low BMD at the distal radius and lumbar spine. Interestingly, OP at the distal radius was associated with hand deformity and functional disability, while at lumbar spine with age and disease duration [98].
Significant increase in the prevalence of OP and osteopenia was found in FMS patients [99].
Miscellaneous
The prevalence of OP was higher in chronic obstructive pulmonary disease (COPD) patients and associated with the severity of the disease [100]. Among Saudi patients with interstitial lung disease (ILD), 44% had OP [101]. Some studies have implicated possible linkages between OP and vascular atherosclerosis as they both share common risk factors. Increased prevalence of atherosclerosis in postmenopausal women had been demonstrated [102,103]. Among patients with type 2 diabetes in Saudi-Arabia, 29.4%- 34% had OP. Increased age, oral hypoglycemic agents, and low vitamin D was significantly increased risk of OP [104,105]. Conflict results were found regarding the BMD status in diabetic postmenopausal Iranian women. Karimifar et al. [106], found that osteopenia and osteoporosis were more common in diabetic women, however, Zakeri et al. [107], suggested that higher spine BMD in diabetic women may be attributed to a higher BMI. More than half of Egyptian patients with type 1 diabetes were OP [108]. The prevalence of OP is higher in Parkinson’s disease and multiple sclerosis patients and seems related to reduced mental, physical performance and drug therapy [109,110]. Gheita et al. [81,111] reported that BMD was notably lower in patients with different malignancies. Moreover, the severity of tumor significantly correlated with the hip and spine DXA. Prevalence of OP in chronic liver disease patients was 45.3%, and 18.2% in HCV (hepatitis C virus) patients [112,113]. Thermal burn victims have higher prevalence of OP [114,115].
Thus patients with chronic medical conditions who at high risk for OP need early proper screening; prophylactic therapies may be needed to prevent fracture risk; life style modifications to increase their mobility to protect bone health should be encouraged.
The burden of osteoporosis in MENA
The international burden of OP has been widely characterized in terms of the incidence, morbidity, mortality, and economic cost of the fragility fractures that arise [116]. Osteoporotic fractures result in serious disability, impaired quality of life and mortality. Despite their importance, the prevalence of fractures in the MENA is understudied and only few studies reported the prevalence and burden of fragility fracture in MENA.
In a recently published study conducted at the Trauma Unit of Assiut University Hospital, Egypt the prevalence of OP was high (74.9%) in patients admitted with hip fractures [117]. Among Saudi Arabians >60 years attending the primary care, fracture assessment tool (FRAX) [118] used to estimate the 10-years probability of OP reported 14.4% and 18.4% for major and hip fractures respectively [119]. A lower trend was detected in Palestinians with a FRAX of major osteoporotic and hip fractures of 3.7%, and 0.3% respectively [27]. Furthermore, OP was an independent risk factor for asymptomatic vertebral fractures in Moroccan postmenopausal women [33,120]. In addition, prevalence of vertebral fractures in apparently healthy Lebanese subjects was estimated at 19.9% in women and 12% in men. Among those, the prevalence of asymptomatic OP diagnosed by DXA was 33% in women and 22.7% in men [29]. The mortality rate of hip fracture was 7% in the Lebanese population after 1 year and increased to 18% after 5 years [54].
Most of the studies evaluating the incidence of osteoporotic fractures were not population-based and few were retrospective. Hence, evidence relating to fragility fractures is non-existing throughout the MENA region and so there is a call to action for collaborative societies to gather information on disease burden. Strategies to prevent OP and decrease fracture rates are needed.
Knowledge and practices related to osteoporosis in MENA
Although low BMD and OP are common problems, they can be prevented, which is the most cost effective strategy in fighting OP. The first step in the prevention program is the evaluation of the individuals’ knowledge about the risk factors of OP and their effect on health. Therefore, implementing an effective health education program can be designed to increase the awareness and modify the lifestyle.
Reports evaluating the knowledge of OP revealed that the majority (95.1%) of women who lived in Alexandria, Egypt, were familiar with OP and mass media was the main source of information. However, their knowledge of OP risk factors, preventive measures and consequences was considered moderate [121]. The majority (78%) of subjects in a Saudi community-based study had heard about OP. There were significant associations between the level of awareness and age, sex, education level, occupation, and income [122]. In contrast, considerable middle aged and elderly Saudi women were unaware of OP risk factors [123]. In Turkish people aged between 40 and 89 years, 19.2% of them had never heard about ‘osteoporosis,’ and 74% could not describe it. Of those who knew OP, equal percentages had heard of it from a physician, friend, and from the media [15]. The majority (81%) of Iranian females had a poor knowledge of OP and its complications [123]. Regarding the degree of awareness about various risk factors of OP, only 12% of postmenopausal Palestinian women correctly answered 70% of the knowledge questions about OP (26). Unexpectedly, female students at Damascus nursing school showed poor knowledge about OP [124]. The limited knowledge about OP and its risk factors can explain the poor practice which may put the MENA populations at an increased risk of OP and its complications. Subjects tend to avoid sun exposure and they had never done vitamin D test before [125]. Frequency of practicing preventive behaviors (adequate physical activity, calcium rich diet) correlated with the mean score of OP knowledge [122].
Strategies to increase and improve OP education are clearly needed to effectively close the current gap between the stage of knowledge of OP and the height of OP prevalence to achieve a significant impact on reducing this silent, but highly costly disease. Among these recommendations is the integration of OP in school curricula and public education efforts, suggesting that more focused programs targeting young subjects in particular are needed. Promoting a healthy lifestyle through health education is a necessary measure to prevent OP. In order to meet the challenges, health care workers have to increase the awareness about this issue through establishing educational programs in primary health care centres. Numerous suggested OP education programs including online learning, practical learning, interactive learning and feedback have to be evaluated regarding their feasibility and effectiveness in the MENA region [126]. Stages of OP prevention strategies are shown in Figure 3.
Designing appropriate intervention strategies based on the community preference and availability is mandatory. The impact of education on preventing OP and improving knowledge and health beliefs among female student has been examined in a previous interventional study; the students’ knowledge and some of the lifestyle behaviors as physical activity, but not calcium intake, changed significantly after the intervention [127]. Internet-based lectures on OP were found to increase OP knowledge in the Unites States [128], yet the effectiveness of this approach was not examined in MENA countries.
Conclusion
The prevalence of osteoporosis is high among Middle East and North Africa population and is associated with a wide range of risk factors and medical conditions. The variability in the epidemiology and risk factors of osteoporosis in MENA countries is remarkable, probably reflecting a magnitude of different factors. Limited reports regarding the established prevalence of osteoporotic fractures among those populations and therefore, lack of guidelines for prevention and management of fragility fractures were noticed. In order to improve bone health and encourage individuals to consider preventive measures against OP, the first step is to increase awareness and practices in those societies thus paving way for appropriate preventive and prophylactic managements.
Review criteria
MEDLINE, CINAHL, EMBASE, SCOPUS and Cochrane Library were searched for Englishlanguage articles with osteoporosis specific keywords and search terms in combination with the specific countries of interest. Articles published from inception up to March 2018.
References
- Managing osteoporosis in primary care: highlights from osteoporosis Canada guidelines. Can. Pharm. J. 144(Suppl.1), S5-S9.e1 (2011).
- Kraenzlin ME. Biochemical Markers of Bone Turnover and Osteoporosis Management. Bone Key. 4(7), 191-203 (2007).
- Genant HK, Cooper C, Poor G et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos. Int. 10(4), 259–64 (1999).
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos. Int. 4(6), 368–81 (1994).
- Vijayakumar R, Büsselberg D. Osteoporosis: An under-recognized public health problem: Local and global risk factors and its regional and worldwide prevalence. J. Local. Glob. Heal. Sci. 2 (2016).
- Kanis JA, McCloskey EV, Johansson H et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 24(1), 23–57 (2013).
- Cooper C, Cole ZA, Holroyd CR et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos. Int. 22(5), 1277–88 (2011).
- Kinsella K, Wan H. An aging world: 2008. U.S. Census Bureau. International Population Reports. P95, 09–01 (2009).
- El-Hajj Fuleihan G, Adib G, Nauroy L. The Middle East and Africa regional audit, epidemiology, costs and burden of osteoporosis in 2011. Int. Osteoporos. Found. 102011–5000 (2011).
- Keskin Y, Çekin MD, Gündüz H et al. The Prevalence of Osteoporosis in the Thrace Region of Turkey: A Community-Based Study. Turkish. J. Phys. Med. Rehabil. Fiz. Tip. Ve. Rehabil. Derg. 60(4), (2014).
- Cankurtaran M, Yavuz BB, Halil M et al. General characteristics, clinical features and related factors of osteoporosis in a group of elderly Turkish men. Aging. Clin. Exp. Res. 17(2), 108–15 (2005).
- Hamdi Kara I, Aydin S, Gemalmaz A et al. Habitual tea drinking and bone mineral density in postmenopausal Turkish women: investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study). Int. J. Vitam. Nutr. Res. 77(6), 389–97 (2007).
- Sharmai SH, Millani F, Alizadeh A et al. Risk Factors of Osteoporosis in Women Over 50 years of Age: A Population Based Study in the North of Iran. J. Turkish-German. Gynecol Assoc. 9(1) (2008).
- Goodarzizadeh N, Shahrjerdi A, Najafi M et al. Effect of diet and lifestyle habits on bone density in postmenopausal women. J. Pharm. Res. 6(2), 309–12 (2013).
- Dashti G, Salamat M, Assi Q et al. Bone mineral density in women of different age groups in Isfahan, Iran in 2013-2014. J. Isfahan. Med. Sch. 33(365), 2271-8 (2016).
- Heidari B, Muhammadi A, Javadian Y et al. Associated Factors of Bone Mineral Density and Osteoporosis in Elderly Males. Int. J. Endocrinol. Metab. 15(1) (2017).
- Gorial FI, Aubaese ND, Husaeen NH. Prevalence and associated factors of osteoporosis in post-menopausal Iraqi women: a cross-sectional two centers study. Int. J. Mod. Biol. Med. 3(1), 41–9 (2013).
- Tariq S, Baig M, Shahzad M. Calcaneal ultrasound assessment of bone health and association of sociodemographic characteristics with bone mineral density in pre and postmenopausal females. In: Osteoporosis International, Springer London. S173–4 (2017).
- Sadat-Ali M, Al-Habdan IM, Al-Turki HA et al. An epidemiological analysis of the incidence of osteoporosis and osteoporosis-related fractures among the Saudi Arabian population. Ann. Saudi. Med. 32(6), 637–41 (2012).
- Alghamdi R, Ghabashei MA, Assas SI et al. Health knowledge and dietary intake risk factors leading to osteopenia and osteoporosis in university students. In: Osteoporosis International, Springer London. S719 (2011).
- Al-Shoumer KAS, Nair V. Prevalence of low bone mass in postmenopausal Kuwaiti women residents in the largest province of Kuwait. Arch. Osteoporos. 7, 147–53 (2012).
- Gupta R, Al-saeed O, Azizieh F et al. Evaluation of Bone Mineral Density in Postmenopausal Women in Kuwait. J. Clin. Densitom. 15(2), 211–6 (2012).
- Alsawy S. Edentulism as a predictor of osteoporosis among postmenopausal Bahraini women. J. Clin. Densitom. 12(3), 389 (2009).
- Fawsy T, Sreedharan J, Muttappallymyalil J et al. Risk factors associated with low bone mineral density in Ajman, UAE, 2009. GMJ, ASM. 1(S2), S25-S29 (2012).
- Bener A, Hammoudeh M, Zirie M. Prevalence and predictors of osteoporosis and the impact of life style factors on bone mineral density. Int. J. Rheum. Dis. 10(3), 227–33 (2007).
- Abd-Alhamed I, Saba E, Darwish HM. Prevalence and awareness of osteoporosis among postmenopausal Palestinian women. Arch. Osteoporos. 5(1–2), 111–8 (2010).
- Aker MB, Taha ASA, Sa’ed HZ et al. Estimation of 10-year probability bone fracture in a selected sample of Palestinian people using fracture risk assessment tool. BMC Musculoskelet. Disord. 14(1), 284 (2013).
- Hyassat D, Alyan T, Jaddou H et al. Prevalence and Risk Factors of Osteoporosis Among Jordanian Postmenopausal Women Attending the National Center for Diabetes, Endocrinology and Genetics in Jordan. Biores. Open. Access. 6(1), 85–93 (2017).
- Baddoura R, Arabi A, Haddad-Zebouni S et al. Vertebral fracture risk and impact of database selection on identifying elderly Lebanese with osteoporosis. Bone. 40(4), 1066–72 (2007).
- Taha M. Prevalence of osteoporosis in Middle East systemic literature review, 10th ECOO, April 2011.
- Selim MA, Mahran DG, Khalil SA et al. Prevalence and risk factors of osteoporosis in postmenopausal rural women in Upper Egypt using ultrasound densitometry. (2009).
- Ouzzif Z, Oumghar K, Sbai K et al. Relation of plasma total homocysteine, folate and vitamin B12 levels to bone mineral density in Moroccan healthy postmenopausal women. Rheumatol. Int. 32(1), 123–8 (2012).
- El Maghraoui A, Sadni S, El Maataoui A et al. Influence of obesity on vertebral fracture prevalence and vitamin D status in postmenopausal women. Nutr. Metab. (Lond). 12 (2015).
- Haouichat C, Hammoumraoui N, Lehtihet S et al. SAT0461 Prevalence of postmenopausal osteoporosis in Algerian women. Ann. Rheum. Dis. 73, 760 (2014).
- Ben Aissa R, Laatar A, Kerkeni S et al. Prévalence de Et, l’ostéoporose chez les femmes ménopausées des gouvernorats de l’Ariana 119, de la Manouba-Tunis. Tun. méd. 84(Suppl 10) (2006).
- Sahli H, Testouri N, Chihaoui M Ben et al. Bone mineral density in healthy Tunisian women. Maturitas. 63(3), 227–32 (2009).
- Wright NC, Looker AC, Saag KG et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone. Miner. Res. 29(11), 2520–6 (2014).
- Kanis JA, Johnell O, Oden A et al. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone. 27(5), 585–90 (2000).
- Mahran DG, Hussein M, Farouk O. Bone mineral density among reproductive age women in rural Upper Egypt. J. Public. Health. 20(4), 453–60 (2012).
- Hammad LF, Benajiba N. Lifestyle factors influencing bone health in young adult women in Saudi Arabia. Afr. Health. Sci. 17(2), 524–31 (2017).
- Sadat-Ali M, Almomen AW, AlOmar HK et al. The current issues on osteoporosis among male Saudi Arabians. J. Mens. Heal. 13(2), E53–9 (2017).
- Al Saleh J, Sayed MEL, Monsef N et al. The Prevalence and the determinants of musculoskeletal diseases in Emiratis attending primary health care clinics in Dubai. Oman. Med. J. 31(2), 117–23 (2016).
- El Maataoui A, Benghabrite A, El Maghraoui A et al. Relationship between sex hormone levels, bone mineral density and bone turnover markers in healthy moroccan men: a cross-sectional study. Pan. Afr. Med. J. 22 (2015).
- Pollitzer WS, Anderson JJ. Ethnic and genetic differences in bone mass: a review with a hereditary vs environmental perspective. Am. J. Clin. Nutr. 50(6), 1244–59 (1989).
- Mohammadi Z, Keshtkar A, Fayyazbakhsh F et al. Prevalence of osteoporosis and vitamin D receptor gene polymorphisms (FokI) in an Iranian general population based study (Kurdistan) (IMOS). Med. J. Islam. Repub. Iran. 29, 238 (2015).
- Sassi R, Sahli H, Souissi C et al. Polymorphisms in VDR gene in Tunisian postmenopausal women are associated with osteopenia phenotype. Climacteric. 18(4), 624–30 (2015).
- Dastgheib SA, Gartland A, Tabei SMB et al. A Candidate Gene Association Study of Bone Mineral Density in an Iranian Population. Front. Endocrinol (Lausanne). 7 (2016).
- Sassi R, Sahli H, Souissi C et al. Association of LRP5 genotypes with osteoporosis in Tunisian post-menopausal women. BMC. Musculoskelet. Disord. 15, 144 (2014).
- El-Heis MA, Al-Kamil EA, Kheirallah KA et al. Factors associated with osteoporosis among a sample of Jordanian women referred for investigation for osteoporosis. East. Mediterr. Heal. J. 19(5), 459–64 (2013).
- Zeidan ZA, Sultan IE, Guraya SS et al. Low bone mineral density among young healthy adult Saudi women Prevalence and associated factors in the age group of 20 to 36 years. Saudi. Med. J. 37(11), 1225–33 (2016).
- Al-Habdan IM, Sadat-Ali M, Al-Muhanna FA et al. Bone mass measurement using quantitative ultrasound in healthy Saudi women: a cross-sectional screening. Saudi. Med. J. 30(11), 1426–31 (2009).
- Gannagé-Yared, Chemali, Sfeir et al. Dietary Calcium and Vitamin D Intake in an adult Middle Eastern population: food sources and relation to lifestyle and PTH. Int. J. Vitam. Nutr. Res. 75(4), 281–9 (2005).
- Alswat KA. Gender Disparities in Osteoporosis. J. Clin. Med. Res. 9(5), 382–7 (2015).
- Maalouf G, Gannagé-Yared MH, Ezzedine J et al. Middle East and North Africa consensus on osteoporosis. J. Musculoskelet. Neuronal. Interact. 7(2), 131 (2007).
- El-Desouki MI, Sulimani RA. High prevalence of osteoporosis in Saudi men. Bone. 40(6), S285 (2007).
- Elgendi SS, Rashad SM, Mohamed FH et al. Risk factors of Egyptian male osteoporosis. Int. J. Rheum. Dis. 11(4), 393–9 (2008).
- Salamat MR, Salamat AH, Abedi I et al. Relationship between weight, body mass index, and bone mineral density in men referred for dual-energy X-ray absorptiometry scan in Isfahan, Iran. J. Osteoporos. 2013 (2013).
- Salamat MR, Salamat AH, Janghorbani M. Association between obesity and bone mineral density by gender and menopausal status. Endocrinol. Metab. 31(4), 547–58 (2016).
- Maddah M, Sharami SH, Karandish M. Educational difference in the prevalence of osteoporosis in postmenopausal women: a study in northern Iran. BMC. Public. Health. 11 (2011).
- Amiri M, Nabipour I, Larijani B et al. The relationship of absolute poverty and bone mineral density in postmenopausal Iranian women. Int. J. Public. Health. 53(6), 290–6 (2008).
- Allali F, Rostom S, Bennani L et al. Educational level and osteoporosis risk in postmenopausal Moroccan women: a classification tree analysis. Clin. Rheumatol. 29(11), 1269–75 (2010).
- Sadat-Ali M, Al-Habdan I, Al-Mulhim A-A et al. Effect of parity on bone mineral density among postmenopausal Saudi Arabian women. Saudi. Med. J. 26(10), 1588–90 (2005).
- Cure-Cure C, Cure-Ramirez P, Teran E et al. Bone-mass peak in multiparity and reduced risk of bone-fractures in menopause. Int. J. Gynaecol. Obstet. 76(3), 285–91 (2002).
- Elshafie DE, Al-Khashan HI, Mishriky AM. Comparison of vitamin D deficiency in Saudi married couples. Eur. J. Clin. Nutr. 66(6), 742–5 (2012).
- Hossein-nezhad A, Maghbooli Z, Bandarian F et al. Association of bone mineral density and lifestyle in men. Iran. J. Public. Health. (S), 51–6 (2007).
- Yousef FM. Associations Factors Affecting on Osteoporosis in Postmenopausal Women in Saudi Arabian, Jeddah. Int. J. Pharm. Res. Allied. Sci. 6(2), 204–12 (2017).
- Karamati M, Jessri M, Shariati-Bafghi SE et al. Dietary patterns in relation to bone mineral density among menopausal Iranian women. Calcif. Tissue. Int. 91(1), 40–9 (2012).
- el-Sonbaty MR, Abdul-Ghaffar NU. Vitamin D deficiency in veiled Kuwaiti women. Eur. J. Clin. Nutr. 50(5), 315–8 (1996).
- Rassouli A, Milanian I, Moslemi-Zadeh M. Determination of serum 25-hydroxyvitamin D(3) levels in early postmenopausal Iranian women: relationship with bone mineral density. Bone. 29(5), 428–30 (2001).
- Al Attia HM, Ibrahim MA. The high prevalence of vitamin D inadequacy and dress style of women in the sunny UAE. Arch. Osteoporos. 7(1–2), 307–10 (2012).
- Allali F, El Aichaoui S, Saoud B et al. The impact of clothing style on bone mineral density among post-menopausal women in Morocco: a case-control study. BMC. Public. Health. 6 (2006).
- Fayed HL, Saleh AH. Frequency of vitamin D inadequacy among Saudi males visiting a Rheumatology Outpatient Clinic of a tertiary hospital in Al-Qassim region: Effect of vitamin D supplementation. The Egyptian Rheumatologist. 39(4), 249-254 (2017).
- Keramat A, Patwardhan B, Larijani B et al. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC. Musculoskelet. Disord. 9, 28 (2008).
- Raafat BM, Hassan NS, Aziz SW. Bone mineral density (BMD) and osteoporosis risk factor in Egyptian male and female battery manufacturing workers. Toxicol. Ind. Health. 28(3), 245–52 (2012).
- Kharroubi A, Saba E, Smoom R. Serum 25-hydroxyvitamin D and bone turnover markers in Palestinian postmenopausal osteoporosis and normal women. Arch. Osteoporos. 12(1), 13 (2017).
- Heidari B, Hosseini R, Javadian Y et al. Factors affecting bone mineral density in postmenopausal women. Arch. Osteoporos. 10(1) (2015).
- Al-Omran AS, Abu-Madini MS, Sadat-Ali M et al. Low Bone Mass Secondary to Antipsychotic Medications. Saudi. J. Med. Med. Sci. 4(3), 202–5 (2016).
- Albaghdadi O, Alhalabi MS, Alourfi Z et al. Bone health and vitamin D status in young epilepsy patients on valproate monotherapy. Clin. Neurol. Neurosurg. 146, 52–6 (2016).
- Al-Osail AM, Sadat-Ali M, Al-Elq AH et al. Glucocorticoid-related osteoporotic fractures. SINGAPORE Med J. 51(12), 948–51 (2010).
- Hoff AO, Gagel RF. Osteoporosis in breast and prostate cancer survivors. Oncology. 19(5), 651–8 (2005).
- Gheita TA, Ezzat Y, Sayed S et al. Musculoskeletal manifestations in patients with malignant disease. Clin. Rheumatol. 29(2), 181–8 (2010).
- Al Amri A, Sadat-Ali M. Cancer chemotherapy-induced osteoporosis: How common is it among Saudi Arabian cancer survivors. Indian. J. Cancer. 46(4), 331 (2009).
- Fadda S, Hamdy A, Abulkhair E et al. Serum levels of osteoprotegerin and RANKL in patients with rheumatoid arthritis and their relation to bone mineral density and disease activity. Egypt. Rheumatol. 37(1), 1–6 (2015).
- Hamdi W, Alaya R, Kaffel D et al. Risk factors associated with bone loss and occurrence of fragility fractures in rheumatoid arthritis patients. The Egyptian Rheumatologist. (2018).
- Aghaei M, Sedighi S, Behnampour N et al. Change in bone mineral density in post menopausal women with rheumatoid arthritis. Bangladesh. J. Med. Sci. 12(2), 158 (2013).
- Gheita T, Fawzy S, Rizk A et al. Impaired bone formation and osteoporosis in postmenopausal elderly onset rheumatoid arthritis patients. The Egyptian Rheumatologist. 33(3), 155-62 (2011).
- Gheita T, Kenawy S, Gheita H. AB0362 B-Cell Activating Factor of the Tumor Necrosis Factor Family (BAFF) in Postmenopausal Rheumatoid Arthritis Patients with Low Bone Mass: A Possible Protective Role of Hydroxychloroquine. Ann. Rheum. Dis. 73, 924 (2014).
- El Maghraoui A, Ebo’o FB, Sadni S et al. Is there a relation between pre-sarcopenia, sarcopenia, cachexia and osteoporosis in patients with ankylosing spondylitis? BMC. Musculoskelet. Disord. 7 (2016).
- Hmamouchi I, Allali F, El Handaoui B et al. The relation between disease activity, vitamin D levels and bone mineral density in men patients with ankylosing spondylitis. Rheumatol. Reports. 5(1), 3 (2013).
- Sayed S, Darweesh H, Fathy K et al. Clinical significance of bone mineral density in Ankylosing Spondylitis patients: Relation to disease activity and physical function. The Egyptian Rheumatologist. 37(1), 35-39 (2015).
- Azzam N, Alharbi O, Aljebreen A et al. Su1289 Prevalence of low bone mineral density in adult Saudi patients with inflammatory bowel disease: a multicenter prospective clinical study. Gastroenterology. 148(4), S-462 (2015).
- Ismail MH, Al-Elq AH, Al-Jarodi ME et al. Frequency of low bone mineral density in Saudi patients with inflammatory bowel disease. Saudi. J. Gastroenterol. 18(3), 201–7 (2012).
- Cheour E, Hamdi W, Sahli H et al. Osteopenia in patients with Crohn disease. Tunis. Med. 85(11), 920–4 (2007).
- Mikaeli J, Goharifar H, Shahram F et al. Evaluation of osteoporosis in a selected group of Iranian patients with ulcerative colitis. Govaresh. 14(2), 122–6 (2009).
- Alharbi OR, Azzam NA, Almalki AS et al. Clinical epidemiology of ulcerative colitis in Arabs based on the Montreal classification. WORLD J Gastroenterol. 20(46), 17525–31 (2014).
- Hafez EA, ElBakry SA, Ibrahim SI et al. Assessment of fracture risk in a cohort of Egyptian female Systemic Lupus erythematosus patients. The Egyptian Rheumatologist. 40(2), 95-91 (2018).
- Abdwani R, Abdulla E, Yaroubi S et al. Bone mineral density in juvenile onset systemic lupus erythematosus. Indian. Pediatr. 52(1), 38–40 (2015).
- Shahin AA, Zayed HS, Sayed SS et al. Bone mineral density in patients with systemic sclerosis and its association with hand involvement. The Egyptian Rheumatologist. 35(4), 233-238 (2013).
- Olama SM, Senna MK, Elarman MM et al. Serum vitamin D level and bone mineral density in premenopausal Egyptian women with fibromyalgia. Rheumatol. Int. 33(1), 185–92 (2013).
- El-Gazzar AG, Abdalla ME, Almahdy MA. Study of Osteoporosis in chronic obstructive pulmonary disease. Egypt. J. Chest. Dis. Tuberc. 62(1), 91–5 (2013).
- Alhamad EH, Nadama R. Bone mineral density in patients with interstitial lung disease. Sarcoidosis. Vasc. Diffuse. Lung. Dis. 32(2), 151–9 (2015).
- Hmamouchi I, Allali F, Khazzani H et al. Low bone mineral density is related to atherosclerosis in postmenopausal Moroccan women. BMC Public Health. 9(1), 388 (2009).
- Shokry M, Hassan AA, Ibrahim G et al. Relation between postmenopausal osteoporosis and coronary and peripheral arterial disease. Middle East Fertil Soc J. 17(3), 181–6 (2012).
- Al-Homood IA, Sheshah I, Mohammed AGA et al. The Prevalence and Risk Factors of Osteoporosis among a Saudi Female Diabetic Population. Open. Access. Maced. J. Med. Sci. 5(2), 177 (2017).
- Alwahhabi BK. Osteoporosis in Saudi Arabia: Are we doing enough? Saudi. Med. J. 36(10), 1149 (2015).
- Karimifar M, Pasha MAP, Salari A et al. Evaluation of bone loss in diabetic postmenopausal women. J. Res. Med. Sci. J. Isfahan. Univ. Med. Sci. 17(11), 1033 (2012).
- Zakeri Z, Azizi Z, Mehrabifar H et al. Evaluation of bone mineral density in premenopausal women with type-2 diabetes mellitus in Zahedan, southeast Iran. J. Pak. Med. Assoc. 61(5), 443–5 (2011).
- Abd El Dayem SM, El-Shehaby AM, Abd El Gafar A et al. Bone density, body composition, and markers of bone remodeling in type 1 diabetic patients. Scand. J. Clin. Lab. Investig. 71(5), 387–93 (2011).
- Abou-Raya S, Helmii M, Abou-Raya A. Bone and mineral metabolism in older adults with Parkinson’s disease. Age. Ageing. 38(6), 675–80 (2009).
- Ayatollahi A, Mohajeri-Tehrani MR, Nafissi S. Factors affecting bone mineral density in multiple sclerosis patients. Iran. J. Neurol. 12(1), 19 (2013).
- Gheita T, Sayed S, Hammam W et al. Subclinical Hypovitaminosis D and Osteoporosis in Breast Cancer Patients. Middle. East. J. Intern. Med. 63(1974), 1–6 (2015).
- Wariaghli G, Mounach A, Achemlal L et al. Osteoporosis in chronic liver disease: a case-control study. Rheumatol. Int. 30(7), 893–9 (2010).
- El-Atrebi KA, El-Bassyouni HT, El-Atrebi AA. Osteoporosis in Chronic Hepatitis C Virus with Advanced Liver Fibrosis. J. Gastroenterol. Hepatol. Res. 3(12), 1392–5 (2014).
- Roshanzamir S, Partovi A, Dabbaghmanesh A. Prevalence and severity of bone loss in burned patients. Burns. 43(4), 766–70 (2017).
- Roshanzamir S, Dabbaghmanesh MH, Dabbaghmanesh A et al. Autonomic dysfunction and osteoporosis after electrical burn. Burns. 42(3), 583–8 (2016).
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 16(2), S3–7 (2005).
- Farouk O, Mahran DG, Said HG et al. Osteoporosis among hospitalized patients with proximal femoral fractures in Assiut University Trauma Unit, Egypt. Arch. Osteoporos. 12(1) (2017).
- Kanis JA, Johnell O, Odén A et al. FRAXTM and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 19(4), 385–97 (2008).
- Amin TT, Al Owaifeer A, Al-Hashim H et al. Osteoporosis among older Saudis: risk of fractures and unmet needs. Arch. Osteoporos. 8(1–2), 118 (2013).
- El Maghraoui A, Ouzzif Z, Mounach A et al. Hypovitaminosis D and prevalent asymptomatic vertebral fractures in Moroccan postmenopausal women. BMC Womens Heal. 12 (2012).
- El-Tawab SS, Saba EKA, Elweshahi HMT et al. Knowledge of osteoporosis among women in Alexandria (Egypt): A community based survey. Egypt. Rheumatol. 38(3), 225–31 (2016).
- Alamri FA, Saeedi MY, Mohamed A et al. Knowledge, attitude, and practice of osteoporosis among Saudis: a community-based study. J. Egypt. Public. Health. Assoc. 90(4), 171–7 (2015).
- Khashayar P, Qorbani M, Keshtkar A et al. Awareness of osteoporosis among female head of household: an Iranian experience. Arch. Osteoporos. 12(1) (2017).
- Sayed-Hassan R, Bashour H, Koudsi A. Osteoporosis knowledge and attitudes: a cross-sectional study among female nursing school students in Damascus. Arch. Osteoporos. 8(1–2), 149 (2013).
- Salmanpour VA, Ibrahim HS, Salameh AG et al. Vitamin D deficiency: knowledge and practices among the adult population in Sharjah, United Arab Emirates. Arch. Osteoporos. 11(1) (2016).
- Nguyen VH. Osteoporosis knowledge assessment and osteoporosis education recommendations in the health professions. Osteoporos. Sarcopenia. 2(2), 82–8 (2016).
- Sanaeinasab H, Tavakoli R, Karimizarchi A et al. The effectiveness of education using the health belief model in preventing osteoporosis among female students. East. Mediterr. Heal. J. 19(2), 1 (2013).
- Hansen KE, Rosenblatt ER, Gjerde CL et al. Can an online osteoporosis lecture increase physician knowledge and improve patient care? J. Clin. Densitom. 10(1), 10–20 (2007).