Review Article - Interventional Cardiology (2012) Volume 4, Issue 2
Evaluation and treatment of coronary bifurcation disease: current strategies and new technologies
- Corresponding Author:
- James B Hermiller
St Vincent Heart Center of Indiana, 10590 North Meridian Street
Suite 300, Indianapolis, IN 46290, USA
E-mail: herms2@gmail.com
Abstract
Keywords
Axxess Plus, coronary bifurcation, drug-eluting stent, FFR, final kissing inflation, keep it open, OCT, provisional strategy, Tryton, Xience™ SBA
Coronary bifurcations remain a challenging lesion subset, representing 15–20% of all percutaneous coronary interventions [1–3]. Because of their unique pathological geometries, bifurcations have been the center of recent bench and clinical research [4–6]. These lesions often supply large areas of myocardium, suffer from higher risks of stent thrombosis and symptomatic restenosis, and are often the reason why bypass surgery is chosen over percutaneous coronary interventions [7–9]. The predominant challenge of unprotected left main stenting has been that the bifurcation is involved in 80–90% of left main lesions. Furthermore, procedural obstacles are considerable. There have been numerous techniques that have been proposed for treating bifurcations, none completely avoiding the limitations of these slotted- tube stent platforms, which were not designed for complex, variable branching geometries [10–12]. New technologies have developed to address these issues of complex lesion anatomy [13–16]. Emerging use of optical coherence tomography (OCT), intravascular ultrasound (IVUS) and fractional flow reserve (FFR) is helping to provide a better understanding of bifurcation disease pathology and stent deployment in these lesions [5,17–19]. This review will focus on the current treatment of bifurcation disease with the available stents, discuss emerging technologies and examine the use of IVUS, OCT and FFR in optimizing bifurcation management.
Drug-eluting stents versus bare metal stents
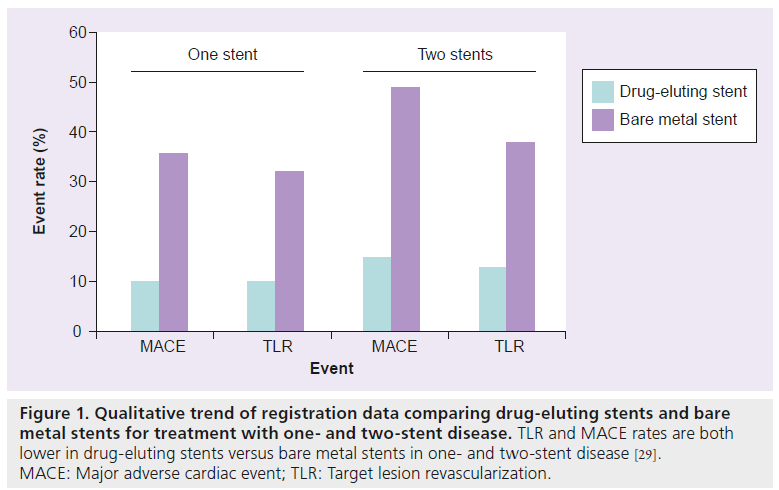
Coronary bifurcation stenting outcomes have improved substantially, primarily a result of potent drug-eluting stents (DES) that inhibit neointimal accumulation [20]. Registry data from bare metal stents (BMS) and DES studies of bifurcations illustrate the marked improvements in major adverse cardiac events (MACE) and target lesion revascularization (TLR) [21–24]. There are no large bifurcation-specific trials of DES versus BMS, although the SCANDSTENT trial of BMS versus sirolimus-eluting stents did examine a subset of 126 patients with bifurcations [25]. Sirolimuseluting stent implantation reduced restenosis rates significantly at both the main branch (MB) (4.9 vs 28.3%; p < 0.001) and the side branch (SB) (14.8 vs 43.4%; p < 0.001). MACE was also significantly reduced (9 vs 28%; p = 0.009) (Figure 1) [15]. Given these data, DES have become the default strategy for bifurcation lesions [2,15,20,26–29].
Figure 1: Qualitative trend of registration data comparing drug-eluting stents and bare
metal stents for treatment with one- and two-stent disease. TLR and MACE rates are both
lower in drug-eluting stents versus bare metal stents in one- and two-stent disease [29].
MACE: Major adverse cardiac event; TLR: Target lesion revascularization.
Single MB stent versus two-stent approach
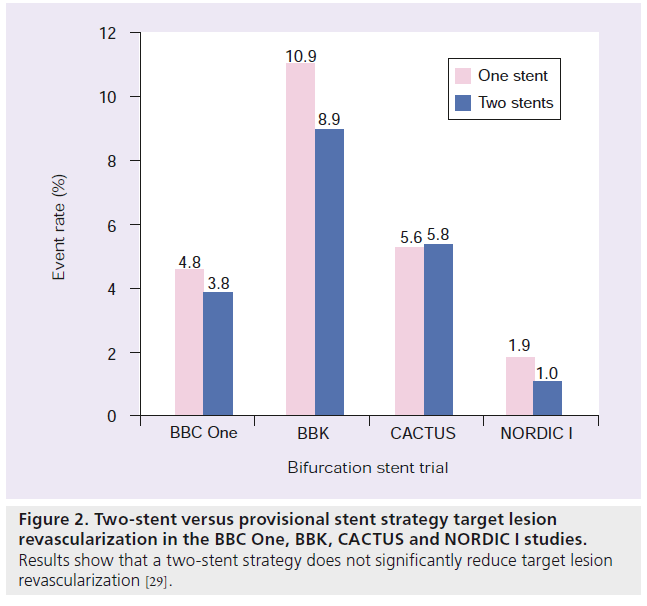
Although in the past there had been considerable debate about whether a single MB stent was preferred over a two-stent approach (SB and MB stents), multiple randomized trials have confirmed that a provisional strategy is appropriate and should be the default strategy for the majority of bifurcations. The first trial to examine this issue in a randomized fashion was the sirolimus-eluting stents implanted at coronary bifurcation lesions reported in 2004 [30]. Since, there have been four large contemporary randomized studies examining this issue [31–34]. These studies confirm that a two-stent strategy does not reduce TLR, compared with a single MB stent, but does increase contrast, fluoroscopy time and periprocedural myocardial infarction rates (Figure 2) [31,32].
Figure 2: Two-stent versus provisional stent strategy target lesion revascularization in the BBC One, BBK, CACTUS and NORDIC I studies. Results show that a two-stent strategy does not significantly reduce target lesion revascularization [29].
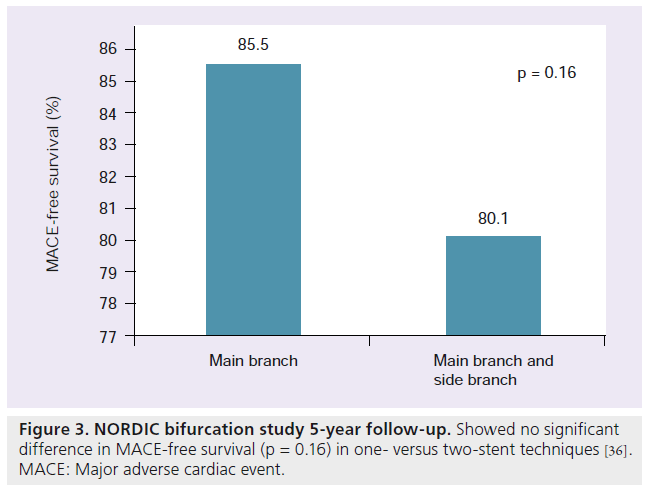
The NORDIC study was a randomized trial designed to compare MB + SB and SB stenting [32]. Four hundred and thirteen patients presenting with true bifurcation disease were enrolled. The patients were then randomized to any two-stent MB + SB stenting or MB only stenting. Only 5% of the patients in each randomized group crossed over to the unassigned therapy. For the two-stent treatment group, any two-stent technique could be employed. Evaluating the outcomes of NORDIC, fluoroscopy and procedural times were significantly longer in the MB + SB stenting group. Also the MB + SB stenting group required more contrast and showed a creatine kinase‑MB elevation (>3‑times upper limit of normal) to be significantly greater (18 vs 8%; p = 0.011). At 8 months the MB angiographically showed low rates of restenosis in both groups (MB = 4.6 vs MB + SB = 5.1%; p = 0.84). The SB restenosis rate was acceptable in both groups as well (MB = 19.2% vs MB + SB = 11.5%; p = 0.062). There was no significant difference in composite MACE, TLR, death or myocardial infarction (MI). Stent thrombosis after 16 months was low in both groups (MB = 1.0% vs MB + SB = 0.5%; p = ns) [35]. At 5‑year follow-up, the NORDIC Bifurcation study continued to show low rates of MACE between both study groups (MACE-free survival 85.5% MB vs 80.1% MB + SB; p = 0.16) (Figure 3). These rates did not differ significantly between the simple versus complex stenting groups. Stent thrombosis rates were not increased in the two-stent technique [36].
Figure 3: NORDIC bifurcation study 5-year follow-up. Showed no significant difference in MACE-free survival (p = 0.16) in one- versus two-stent techniques [36]. MACE: Major adverse cardiac event.
In the CACTUS study, 350 patients were randomized to a single MB stent or the standard- crush technique. The authors found that 6‑month mortality, MI, TLR and overall MACE were not different. Stent thrombosis was 1.7 and 1.1% in the crush and provisional group, respectively, and restenosis rates were equivalent (binary restenosis of 4.6 vs 6.7% in the MB and 13.2 vs 14.7% in the SB for crush vs provisional) [31]. Although the twostent crush approach offered no advantage to a single MB stent, it was also true that if two stents were necessary, there was not a penalty in terms of higher incidence of adverse events at 6 months. Unlike the NORDIC trial, the CACTUS study was notable as 31% of the provisional stent group had previously undergone SB stenting. The criterion for crossing over from a one- to a two-stent approach was less stringent than NORDIC: two stents being allowed with >50% residual SB stenosis, grade C or greater dissection, or less than Thrombolysis in Myocardial Infarction (TIMI) 3 flow. In the BBK study the crossover rate from provisional to two stents was nearly 19% and for the BBC One study, much like NORDIC, was only 3%. The periprocedural MI rate in the BBC One study for the complex lesion group however was high at 11.2% [29,33,34].
Another explanation for why the provisional approach was so effective, but a potential deficiency of these trials, may have been based on lesion selection. In general, studies of provisional stenting include focal SB stenoses [32,37,38]. This lesion selection is a major shortcoming of these studies. It is known that SB lesions with more than focal lesion length are much less likely to respond to balloon dilation. Also, if the SB is large a two-stent strategy will often be required [29].
There are two provisional techniques for true bifurcation lesions and a recent trial suggests a third approach [1–4,27,29,39]. The standard approach includes wiring both branches, predilating the MB and then stenting the MB while jailing the SB wire. Many have suggested it is preferable to not predilate the SB, because predilatation would further distort the carina (carinal shift) that occurred after MB dilatation. Furthermore, predilatation may cause dissection, increasing the probability of tracking subintimally when reaccessing the SB. Also, predilatation increases the probability that a more proximal MB cell will be crossed, rather than recrossing into the SB distally, which enhances ostial SB support [10]. If the SB is not compromised, a final high-pressure dilation of the MB stent should be performed in most cases. The size of the balloon and inflation is based on the desired ending size of the MB. If after stent deployment or high-pressure dilatation SB compromise occurs, the SB is rewired through the struts of the stent and dilated, followed by a final kissing inflation (FKI). The proximal optimization technique can be undertaken if the SB is difficult to reaccess. If a threat of SB closure persists, then bailout stenting using a T‑stent approach of the SB is appropriate. A culotte or reverse crush technique can also be utilized for threatened SB closure as well [27,29].
The second provisional technique is the keep it open (KIO) approach. The KIO’s main indication is to employ it when the SB is diffusely diseased with the goal being to simply maintain its patency, avoiding a periprocedural MI. The KIO technique includes wiring both the MB and SB and then completely treating the MB, without recrossing into the SB or dilating it, thereby avoiding dissection and vessel closure [1,2,27,29].
Costa recently reported the INSIDE II trial, a prospective, randomized, single center trial comparing single versus double stenting techniques in more complex bifurcations with long SB plaque. In this trial the SB was first predilated. If the dilation was successful, patients were randomized to a one- versus two-stent technique (majority of two-stent techniques were T‑stenting). The study showed that a strategy of SB predilatation prior to a decision on singleversus two-stent technique was acceptable even in very complex disease [39].
How necessary is the FKI with the provisional approach? The need for a FKI is indisputable when the SB has been dilated through the struts of the MB stent, a maneuver that invariably distorts the MB stent, causing a reduction in stent minimal area [40]. Less certain is whether a kissing inflation should be performed in all bifurcations when the SB has not been dilated through the struts of the MB stent. The NORDIC III trial examined this issue [41]. Four hundred patients treated with a provisional stent approach were randomized to routine/uniform versus a selective kissing inflation strategy. There was no difference in clinical outcomes; however, restenosis of the SB was lower when a FKI was employed. In particular, patients with true bifurcation disease showed a reduced rate of restenosis of the SB when FKI was undertaken (7.6% FKI vs 20.0% no FKI; p = 0.024). Furthermore, FFR can be of use to quantify the need to postdilate the SB lesion. If the result of the FFR of the SB lesion is >0.80, the intervention can be considered to not need further dilatation of the SB. For a FFR <0.80 dilatation with a balloon can be considered [42–44].
Another practical consideration is the jailing of the SB wire. In the TULIPE study, trapping the SB wire was an independent predictor of lower TLR (odds ratio: 4.2) [37]. Jailing the guidewire behind the MB stent may facilitate rewiring the SB by reducing the angle between the SB and MB, helping to maintain SB patency and serving as a target for rewiring; practical considerations include the type of wire to jail. In general any nonhydrophilic workhorse wire is acceptable, particularly those with a core to tip design. Hydrophilic wires are not utilized, given the risk of stripping the lubricious coating/jacket during retraction of the wire. Although there are rare case reports of the guidewire being retained during the jailing maneuver, successful utilization of the technique is simplified by trapping only a small amount of wire just proximal to the radiopaque tip; moreover, if substantial resistance does occur during withdrawal, threading a rapid exchange balloon over the jailed wire up to the proximal end of the MB stent can buttress the wire, allowing for easy removal [29]. Attention to the guiding catheter should also take place ensuring dissection of the proximal vessel does not occur.
Two-stent approaches
In many cases, including during SB abrupt closure, a two-stent strategy is unavoidable in order to avoid a MI periprocedurally by maintaining SB patency. At times, it is also difficult to achieve acceptable angiographic results when the SB disease is extensive and this issue is particularly important when the SB size itself is significant (usually >2.5 mm). When these issues of large SB size and heavy burden of SB plaque length is present, an elective two-stent strategy may be best. Additionally, the SB angle may be such that rewiring the branch may be difficult. When this issue arises, two-stent approaches may provide the extra benefit of not sacrificing SB access until the SB is secured. The two-stent approaches utilized with currently available include: T‑stent, mini-crush, V‑stent/simultaneous kissing stents (SKS) and culotte stenting. FKI is considered important in all approaches and recent bench and in vivo research is working to explain the mechanisms [4,45–48]. An article by Latib et al. in 2010 visually outlines many of the techniques described below [49].
▪ T‑stent
The T‑stent without crush is the most conventional of the two-stent strategies [1,7,8,37,50–55]. The classic T‑stent approach, used in an elective manner, involves placing the SB stent first. When the SB stent is placed, care should be taken to avoid stent protrusion into the MB. After SB stent placement, the SB wire is removed, the MB is stented. FKI takes place once the SB is reaccessed. The T‑stent technique advantage is its simplicity. A disadvantage of the standard T‑stenting technique is that in some cases the ostium of the SB will not be fully covered. Also, excess stent can be seen to extend into the MB. One variation of the standard T‑stent was reported by Rizik and colleagues who described a self-aligning T‑stent approach, a technique which leverages the concept of the blocking balloon first reported by Dardas et al. and Schwartz et al. for isolated ostial SB disease [12,56,57]. Twenty six patients were described in the study. In these patients further ostial stenting during the initial procedure was required in only 15%. Two patients angiographically had restenosis in SB ostia, which led to TLR [12].
In the reverse T approach, the MB is initially stented followed by stenting of the SB [1]. The most frequently utilized reverse T technique employed is the T‑stent and small protrusion (TAP). In this approach, the MB is stented, the SB is dilated through the struts of the MB stent and then the SB stent is positioned such that only 1–2 mm of the proximal portion of the SB stent is positioned within the MB stent [49,58,59]. A MB balloon is left uninflated during SB-stent deployment. The deployment balloon is retracted slightly and kissing inflation is performed. A FKI may be performed with two noncompliant balloons. The TAP technique was reported to have a high success and long-term outcome in the report by Burzotta and colleagues who reported a TLR of 6.8% in 73 patients with very complex bifurcation disease [58]. Al Rashdan reported similar excellent results in 156 patients using the TAP [50].
Finally, a ‘cone crush’ modified T‑stent technique has been described by Rajdev and colleagues. For the ‘cone crush’ technique, the SB stent is aligned with a MB device (stent or MB blocking balloon). The SB stent is deployed while the MB device stays uninflated. The SB deployment balloon is then taken back several millimeters and again high pressure inflation takes place. This second inflation works to create an ostial flare or cone [60]. Afterwards, a stent is deployed in the MB and the SB is reaccesssed for the FKI.
▪ Stent crush
To prevent the incomplete coverage of the SB ostium associated with standard T‑stenting, Colombo described the crush technique [22,59]. In the original description, the approach involved crushing 5–10 mm of the proximal SB stent within the MB artery. This led to substantial distortion within the SB ostium and the MB as well. A modification of the technique, the mini-crush involves crushing only 1–2 mm of stent within the MB [22]. Ormiston demonstrated in an in vitro model that the mini-crush compared with the standard crush substantially enhanced ostial SB expansion and minimized MB distortion and underdilataion [61,62]. In the mini-crush technique, the SB stent is positioned with 1–2 mm of stent protruding into the MB, such that the proximal portion of the SB osmium is barely covered. Following, deployment of the SB stent, angiography is performed to ensure no distal dissections within the SB followed by withdrawal of the SB wire. In the classic crush manner, the SB stent is crushed with the MB stent. Following stenting of both branches, the SB is rewired, dilated to high pressure with a noncompliant balloon, followed by a FKI. This two-step inflation technique significantly improves ostial expansion compared with a single kissing inflation [62]. In addition, successfully recrossing and dilating the crushed SB stent is essential. Restenosis and stent thrombosis are much higher when a FKI is not performed [63]. IVUS has demonstrated significant underdilation of both branches when a FKI is not performed [63,64]. In the CACTUS trial, the performance of FKI compared with no FKI was associated with a lower incidence of in-hospital and follow-up MI (7.5% with FKI vs 29.0% without; p = 0.001), TLR (6.3% with FKI vs 12.9% without; p = 0.25) and angiographic restenosis in the MB (4.7% with FKI vs 16.0% without; p = 0.03) and the SB (11.9% with FKI vs 36.0% without; p = 0.001), as well as a lower incidence of stent thrombosis (0.9% with FKI vs 6.5% without; p = 0.06) [30].
The crush technique may have several advantages including that access to either branch is not surrendered until stents are deployed and complete coverage of the SB ostium is ensured. The greatest challenge involves rewiring and redilating the crushed SB stent. If a standard workhorse wire does not cross, hydrophilic wires can be helpful. Often an appropriately sized workhorse balloon will not cross into the SB, but a rapid exchange 1.5 mm balloon nearly always does, allowing for subsequent delivery of larger balloons. Also, after leaving the initial wire in place, a second buddy wire can be placed in the SB and may provide adequate support to cross into the SB. Rarely, a fixed- wire balloon is necessary. Finally, using balloons sized to each branch is essential as under sizing the MB balloon during the FKI leads to distortion and suboptimal expansion and apposition of the MB stent.
There are many variations on the crush theme. In the standard crush, the SB is crushed with the MB stent, whereas in the ‘step crush’ the SB stent is crushed by a MB balloon, which is then followed by the MB stent [65]. Except for not inflating the MB balloon with SB stent deployment, the mini-crush technique is much like the self-aligning T‑stent approach described above [12]. The DK crush or ‘sleeve technique’ was described by Jim as a variation to the step crush. In the sleeve technique, after the SB crush with the MB balloon, SB reaccess occurs followed by a kissing inflation [66–68]. The next steps in the technique are identical to step crush. The reverse crush or internal crush, a technique not often employed in contemporary practice, involves stenting the MB, followed by stenting of the SB, removal of the SB wire, then crushing the proximal SB stent edge with a MB balloon [69].
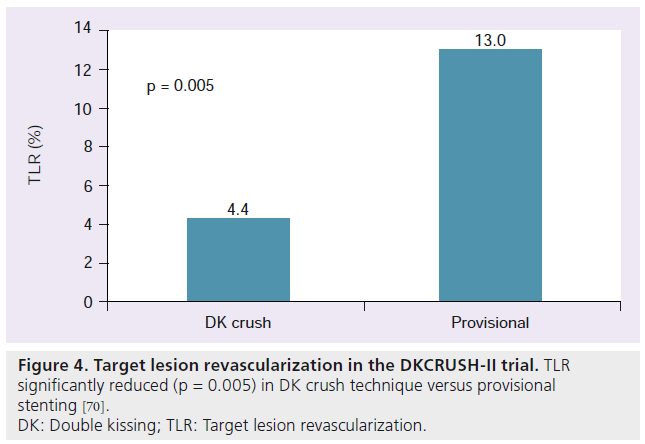
Chen et al. recently published results from the DKCRUSH‑II trial [70]. This trial compared a provisional stenting approach to the DK crush technique described above. Acute closure of the SB after provisional stenting was found to be 1.62%. Additional stenting was required in the SB in the provisional stenting group in 28.6%. Compared to provisional stenting, DK crush showed a significant reduction in TLR (4.4% DK crush vs 13.0% provisional; p = 0.005) (Figure 4) and TVR (6.5% DK crush vs 14.6% provisional; p = 0.017). No significant difference in MACE was observed between the two groups. To date, this trial is the first randomized study to demonstrate a two-stent strategy to have an advantage over provisional stenting [70].
Figure 4: Target lesion revascularization in the DKCRUSH-II trial. TLR
significantly reduced (p = 0.005) in DK crush technique versus provisional
stenting [70].
DK: Double kissing; TLR: Target lesion revascularization.
▪ V & SKS stenting
The V or SKS technique is a simple twostent approach to bifurcation disease that has the advantage of maintaining access to both branches at all times [71–73]. The technique is best for Medina 0,1,1 lesions, where the bulk of the plaque is distal to the carina. Although there are reports of treating longer proximal segments of disease with long ‘double-stent barrels’, most operators prefer to have minimal overlap of the proximal stents. The technique forms a stent double barrel in the proximal portion of the lesion by aligning the stents’ proximal edges creating a neocarina. The technique is considered the SKS approach if the neocarina extends 5 mm or more into the MB [1,74,75]. Further postdilatation can be completed as necessary. The techniques should end with a FKI. The V or SKS approach was studied in 200 consecutive patients by Sharma et al. [74]. Success during the procedure was 99% for the SB and 100% for the MB. Initial clinical success was 97%. MACE rates were low: in hospital MACE was 3% and 30‑day MACE was 5%. Practical considerations include selecting an adequately sized guiding catheter (generally 8 F allows optimal visualization) and sequentially inflating the stents rather than simultaneously deploying them. A particular challenge with the V‑stent is a proximal dissection. If this dissection does occur, a proximal bailout stent is difficult to place with an inevitable bias toward one branch. A better approach would be to extend the double barrel proximally by adding two more stents, or converting the V technique into a crush technique by compressing the SB stent with a balloon in the MB. Frequently during rewiring, the wire crosses from the lumen of one stent to the other; IVUS is essential, confirming that the correct lumen has been accessed.
▪ Culotte stent
The culotte technique may be the most challenging of the two-stent strategies, but provides superior coverage of the carina and SB ostia. In this approach, the initial stent is placed in the most angulated vessel (generally the SB). The MB is then rewired through the struts of the first stent and is then subsequently dilated and stented. Following placement of the second stent, the first stent is reaccessed and dilated. Using noncompliant balloons, separate highpressure inflations are performed in each stent followed by a FKI of 10–12 atm. This technique is typically best for large vessels. Generally the first stent should be at least 3 mm in diameter though this technique can be used for SB vessel diameters as low as 2.5 mm [76–78].
The NORDIC group compared the culotte with the crush technique in a randomized trial [79]. A total of 424 patients with a bifurcation lesion were randomized to crush (n = 209) and culotte (n = 215) stenting. At 6 months there were no significant differences in MACE rates between the groups – crush 4.3% and culotte 3.7% (p = 0.87). Procedure and fluoroscopy times and contrast volumes were similar in the two groups. A total of 324 patients had a quantitative coronary assessment at the index procedure and after 8 months. The angiographic endpoints of in-segment and in-stent restenosis of main vessel and/or SB after 8 months were found to be 12.1 versus 6.6% (p = 0.10) and 10.5 versus 4.5% (p = 0.046) in the crush and culotte groups, respectively. The culotte group was more likely to have a FKI compared with the crush group. Because of the short followup and significantly less FKI in the crush group, there are insufficient clinical data to recommend one technique over another based solely on lower event rates, although angiographically, there was a trend toward less in-segment restenosis and significantly reduced in-stent restenosis following culotte stenting.
Emerging stent technologies
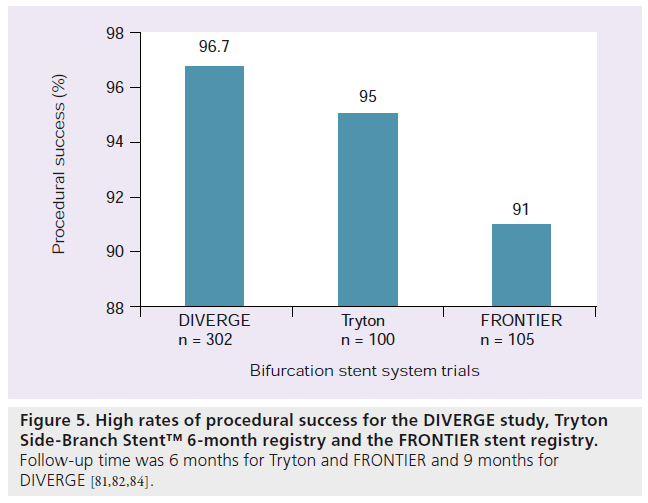
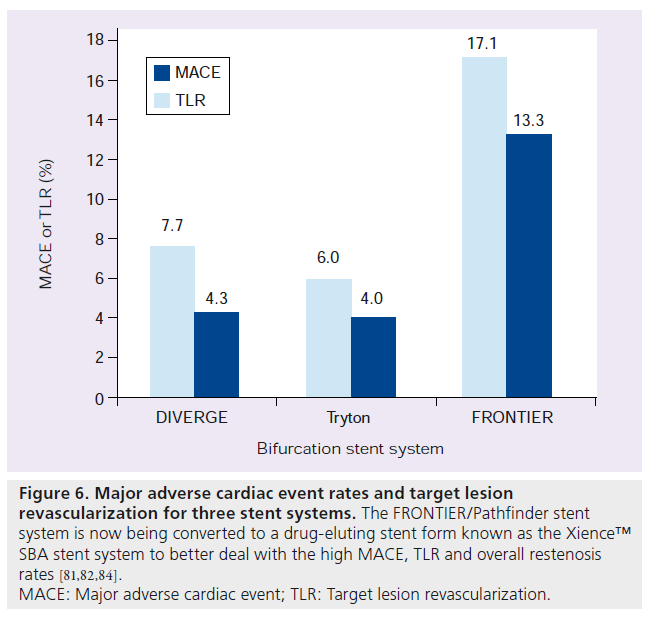
Much research and development has been undertaken to develop stent technologies unique to bifurcation disease. Currently, approximately 19 devices dedicated to bifurcation disease are available in Europe or are undergoing investigation [15]. The first generation of these dedicated bifurcation devices were difficult to use. The newer devices have shown a trend toward improvements in their deliverability. Most of the devices available have only been studied in firstin- man reports or short follow-up studies. The stent devices can be divided into three broad categories, all of which share a common goal of allowing easier SB access but differ in ostial coverage of the SB. These three categories are: stents that facilitate access to the SB after stenting the MB (e.g., Xience SBA); devices made to stent the SB first (e.g., Tryton Side-Branch Stent™, Tryton Medical, Inc., NC, USA); and devices made to scaffold the ostium of the bifurcation with stent into the MB and SB (e.g., Axxess Plus) [13–15]. See Table 1 for a summation of 11 devices currently being used clinically or undergoing trials. The three prototypical stents are reviewed below (Figures 5 & 6).
Figure 6: Major adverse cardiac event rates and target lesion
revascularization for three stent systems. The FRONTIER/Pathfinder stent
system is now being converted to a drug-eluting stent form known as the Xience™
SBA stent system to better deal with the high MACE, TLR and overall restenosis
rates [81,82,84].
MACE: Major adverse cardiac event; TLR: Target lesion revascularization.
Axxess Plus stent is a self-expanding device made from nitinol (nickel–titanium). It is a drug-coated stent eluting abluminal Biolimus A9™, which is a sirolimus analog. The strut thickness is 0.006 inches. The stent has a conical design that allows it to expand into the unique anatomies of bifurcation lesions. Three radiopaque markers facilitate placement while a fourth maker denotes the proximal end of the stent. The delivery system makes use of a single wire exchange system. The stent is designed to cover the proximal portion of the disease and usually additional stents need to be placed in the MB and the SB. The Axxess stent was evaluated in the DIVERGE study. In this study, 302 patients were treated with Axxess stent and the sent was placed successfully in 96.7% of the patients. Additional stents in one branch were needed in 21.7% of patients and additional stents were needed in both branches 64.7% of the time. During the 9-month follow-up a MACE rate of 7.7% (0.7% death, 3.3% non-Q-wave MI, 1.0% Q-wave MI and 4.3% TLR) was observed. Subacute and late stent thrombosis rates were 0.7 and 0.3%, respectively. The total restenosis rate was 6.4% (4.4% SB and 3.6% MB) [14,15,80,81].
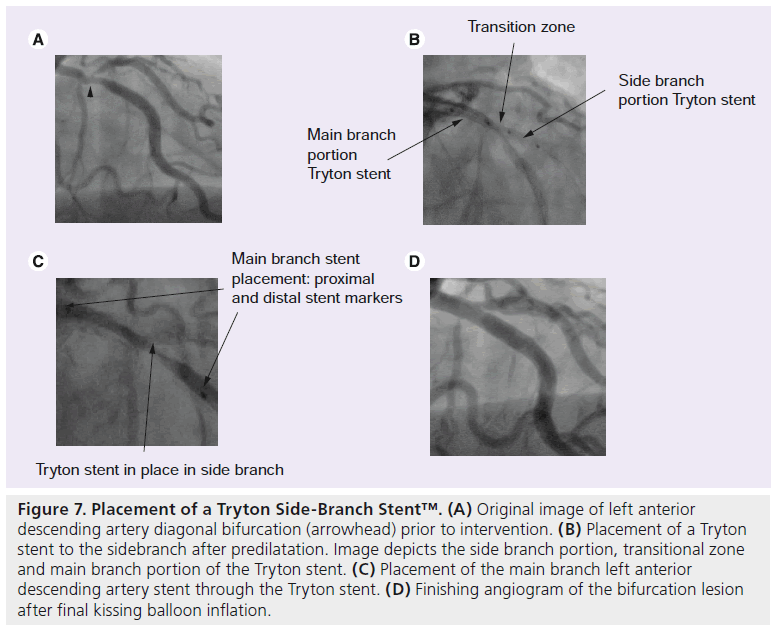
The Tryton Side-Branch Stent (Tryton Medical, Inc.) is a BMS made of cobalt chromium. It is employed in a facilitated culotte approach. It is made up of three zones. The distal SB zone is similar to a standard slotted stent tube design. The central transition zone consists of three panels; this zone has very high radial strength helping to optimally support the SB ostium. The proximal main vessel zone has a very open architecture and consists of three fronds that proximally terminate in a circumferential band. It uses a single balloon and a single rapid wire exchange system. Four radio opaque markers help guide stent placement, which is similar to the culotte method. Using a 6 F guide after optional MB and SB predilation, the stent is distally advanced over the SB wire until two middle markers span the carina. The Tryton stent is then deployed and afterwards the SB wire is moved through the transition zone of the stent into the MB. In the MB a workhorse stent is then placed. Lastly, the SB is rewired and a FKI takes place (Figure 7) [82,83].
Figure 7: Placement of a Tryton Side-Branch Stent™. (A) Original image of left anterior descending artery diagonal bifurcation (arrowhead) prior to intervention. (B) Placement of a Tryton stent to the sidebranch after predilatation. Image depicts the side branch portion, transitional zone and main branch portion of the Tryton stent. (C) Placement of the main branch left anterior descending artery stent through the Tryton stent. (D) Finishing angiogram of the bifurcation lesion after final kissing balloon inflation.
Magro et al. recently presented a two-center registry analysis of the Tryton stent [82]. The authors looked at 6‑month follow-up of the first 100 coronary bifurcation lesions assigned to treatment with the Tryton stent. The stent was placed successfully in all but one lesion and angiographic success was 95%. A total of 9% of the lesions were in the left main artery. The TLR was 4%, MI 3% and cardiac death 1% (the cardiac death patient presented in cardiogenic shock prior to stent placement). MACE-free survival at 6 months was 94%. There were no cases of stent thrombosis. Though only a registry analysis, the real world results at 6 months were encouraging [82]. The stent is currently undergoing a large randomized trial comparing the Tryton stent to provisional stenting. A drug-eluting form of the Tryton stent is currently not available.
The Xience SBA stent (Abbott Vascular, CA, USA) is a stent system that primarily covers the MB but helps preserve access to the SB. In its earlier form, it was a BMS known as the FRONTIER or Pathfinder system. Originally, the BMS form was studied in 105 patients for the FRONTIER stent registry. In this study there was a high procedural success rate of greater than 90%. However, there was a high MACE rate driven largely by long-term TLR. MB restenosis was found to be 25.3% while SB restenosis was 29.1%. Overall, the restenosis for any branch was 44.8%. To deal with these restenosis issues, the platform has now been converted to an everolimus DES similar to the Xience V® [14,84].
The Xience SBA stent is a cobalt chromium dual-wire stent system coated with everolimus. The system uses two wires and there is a dual lumen catheter with a mandrel that helps advance the dual wires side by side to avoid wire trapping. This mandrel helps with the crossing profile of the stent system by allowing for single tip delivery of the balloons/stents. The MB balloon is attached to the rapid exchange lumen, while the SB balloon has its attachment to the over the wire lumen. The DES is attached to both of these balloons which allows easy access for the SB balloon to exit through the stent. Further helping its user profile, both balloons are also attached proximally to a common inflation lumen [14,85].
To use, the Xience SBA system is positioned just proximal to the bifurcation lesion. The mandrel is then unlocked allowing for a second wire to be placed in the SB. Next the entire system is advanced to cover the bifurcation area. With a single step inflation, the two balloons are inflated delivering the MB stent and establishing the side access for the SB. Afterwards, the clinical decision can be made for further stenting of the SB in a T‑stent technique or simply finishing with a FKI. This system was compared with standard provisional T‑stenting technique. The total time of deployment was similar for the two study groups, but time to achieve MB stenting was less in the Xience system. This decreased time led to less fluoroscopy and contrast use for the Xience system [85].
Drug-eluting balloons
Drug-eluting balloons (DEBs) have shown some promise in treating small-vessel and instent restenosis lesions [86–88]. They are particularly attractive for patients with contraindications to long-term dual antiplatelet therapy. The first generation DEB had a difficult time being delivered to complex lesions such as those in bifurcation disease. Second generation DEBs, although still rigid, have better crossing profiles. In a recent small feasibility study utilizing four different DEBs, Sgueglia et al. examined 14 patients with bifurcation disease undergoing provisional stenting. The DEB was used during FKI. All but one case were performed transradially. Procedure and angiographic success rate was 100%. At a mean follow-up of 238 days all patients were free of major cardiac events and were asymptomatic [16]. Although only a small study, these results point toward future larger studies utilizing DEB (KISSING DEBBIE study) [16]. Results from the DEBIUT study recently showed a strong favorable trend in TLR and MACE rates using DEB in the MB and SB in bifurcation disease [89].
Overall approach
The key to the therapy of bifurcations is the SB [90]. This is particularly true now that the reintervention rates in the MB with DES are quite low. There are a number of questions to ask in each bifurcation intervention and they predominantly involve the SB. Firstly, does the ostium of the SB have disease, in other words, is this a true bifurcation lesion? How clinically important is the SB? What is the SB diameter and the amount of myocardium subtended? Will the SB cause angina if restenosis occurs or if an inadequate initial result is obtained? Dauerman and colleagues found that if the SB was less than 2.5 mm in diameter, it was unlikely to cause the need for repeat revascularization [91]. Is there a high risk that the SB will close with main vessel stenting, and if it closes, will it be recrossable? Is the SB of such size and the lesion geometry of such complexity, that giving up access to the SB should be avoided? Even with todays tools, SBs close and cannot be reaccessed, potentially resulting in significant myocardial damage. How long is the SB disease? If only confined to the ostium and short in length, the likelihood that a second stent will be necessary is unlikely. Finally, is the SB dilatable and will lesion modification with a cutting balloon or rotablator be necessary for complete expansion? Does a new dedicated bifurcation stent need to be used? All these issues will establish the side-branch strategy and determine whether a second side-branch stent is necessary.
Use of imaging techniques such as IVUS can be invaluable in these complex subsets, particularly in verifying that optimal stent expansion has been achieved. Costa et al. examined 25 crush stents by IVUS, demonstrating incomplete stent apposition in the main vessel segment proximal to the carina in more than 60% of lesions. A minimum lumen area of <5 mm was found in 76% of the SB stents [64,92]. These ultrasound studies argue for meticulous two-step dilatation of the SB. Often an optimal angiographic result at the SB was frequently found to be inadequate by IVUS, necessitating further dilatation of the SB [2,93]. The physiologic severity of the SB stenosis following MB stenting is frequently overestimated, resulting in crossover to a twostent approach when a provisional strategy was initially chosen. Fractional flow measurements can be useful in these circumstances, demonstrating no significant lesion in most circumstances [94,95]. OCT, particularly as it becomes more prominent and widely adopted in clinical practice, can be useful evaluating for stent strut malaposition. OCT decreases the significant strut artifacts seen with IVUS suggesting it can provide a more accurate assessment of stent appostion [19,96]. Mario et al. recently outlined how OCT can be used for bifurcation lesions [97].
Although there are no compelling randomized data demonstrating the superiority of one of the two-stent approaches, there are data suggesting that the angle of the SB does have a significant impact on the outcome of two-stent techniques [10]. Dzavik et al. studied the outcomes of 133 patients undergoing crush stenting [98]. The patients were divided into those with a lowbifurcation angle (<50° angle) and those with a high-angle (>50°). MACE occurred more frequently in the high-angle group (22.7 vs 6.2%; p = 0.007). Bifurcation angle ≥50° (p = 0.004), no final kissing balloon inflation (p = 0.012), and creatinine clearance <40 ml/min (p = 0.031) independently predicted MACE. A subsequent study by this group extended these observations to both culotte stenting as well [99].
Conclusion
Coronary bifurcations remain a challenging lesion subset, constituting 15–20% of all coronary percutaneous interventions. Although current drug-eluting slotted tube stent platforms were not designed for bifurcating lesion geometries, their application in this lesion subset has been become very effective, particularly compared with BMS. There is now consensus that the provisional approach, with MB stenting only, is the preferred strategy for the majority of bifurcations, although when SB stenting is necessary, outcomes are acceptable. In patients having true bifurcations (Medina 1,1,1), large, stentable SBs (>2.5 mm) and nonfocal proximal/ostial plaque disease, an initial two-stent strategy is often preferred and acceptable. Which two-stent approach to employ is less certain. Use of new stent technologies can be considered at this time. Meticulous attention to optimal stent dilation is more important than the particular two-stent technique chosen. Finally, generous utilization of IVUS, OCT, and FFR may enhance decision making and optimal stent deployment.
Future perspective
Treatment of bifurcation disease is an evolving field. The stents that are currently widely adopted are made of a slotted tube platform and were not designed for the complex anatomical challenges provided by bifurcation lesions. Over the course of the next several years, we believe bifurcation treatment will evolve to use stent platforms designed specifically for bifurcations with close attention paid to the carina of the lesion. The ideal stent platform would be drug coated, easy to place and have a low TLR of both the MB and SB. OCT, as it progresses towards more routine use and availability, could provide valuable information about the bifurcation lesion prior to and after stent deployment. We foresee a future of a fusion of dedicated bifurcation stents with placement guided by intracoronary imaging.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Drug-eluting stents versus bare metal stents
▪ Drug-eluting stents are the preferred stents for bifurcation disease.
Single main branch stent versus two-stent approach
▪ NORDIC, CACTUS, BBK and BBC One showed the two-stent approach does not reduce target lesion revascularization compared with
the provisional approach but does increase contrast use, fluoroscopy time and periprocedural myocardial infarction rates.
▪ Final kissing inflation is important in the provisional approach.
Two-stent approaches
▪ Two-stent strategy is unavoidable in some cases due to abrupt closure of side branch (SB), severe disease in the SB or when the SB is
large subtending a large area of myocardium.
▪ T‑stent:
– Most conventional two-stent strategy.
▪ Stent crush:
– Most used modification is mini-crush.
▪ V and simultaneous kissing stenting:
– Maintains access to both branches at all times.
▪ Culotte stent:
– May be the most challenging of the two-stent strategies.
– May provide superior coverage of the carina.
Emerging stent technologies
▪ There are three broad categories for devices currently in development for bifurcation disease:
– Stents facilitating access to SB after main branch stenting (e.g., Xience™ SBA).
– Devices made to stent the SB first (e.g., Tryton).
– Devices made to scaffold both the main branch and the ostium of the bifurcation lesion (e.g., Axxess Plus).
Drug-eluting balloons
▪ Drug-eluting balloons have recently shown some promise in small vessel disease.
▪ Drug-eluting balloons have become easier to deliver.
Overall approach
▪ Key to bifurcation disease is the side branch:
– Does the ostium of SB have disease?
– How clinically important is the SB?
– High risks for SB closure after stenting?
– Is the SB stentable?
– Does a new bifurcation device need to be considered?
▪ Consider the utility of imaging:
– Intravascular ultrasound.
– Optical coherence tomography.
References
Papers of special note have been highlighted as:
▪ of interest
- akovou I, Ge L, Colombo A. Contemporary stent treatment of coronary bifurcations. J. Am. Coll. Cardiol. 46(8), 1446–1455 (2005).
- Latib A, Colombo A. Bifurcation disease: what do we know, what should we do? JACC Cardiovasc. Interv. 1(3), 218–226 (2008).
- Rizik DG, Klassen KJ, Hermiller JB. Bifurcation coronary artery disease: current techniques and future directions (part 1). J. Invasive Cardiol. 20(2), 82–90 (2008).
- Murasato Y, Hikichi Y, Nakamura S et al. Recent perspective on coronary bifurcation intervention: statement of the ‘Bifurcation Club in KOKURA’. J. Interv. Cardiol. 23(4), 295–304 (2010).
- Nakazawa G, Yazdani SK, Finn AV, Vorphal M, Kolodgie FD, Virmani R. Pathological findings at bifurcation lesions: the impact of flow distribution on atherosclerosis and arterial healing after stent implantation. J. Am. Coll. Cardiol. 55(16), 1679–1687 (2010).
- Hu ZY, Chen SL, Zhang JJ et al. Distribution and magnitude of shear stress after coronary bifurcation lesions stenting with the classical crush technique: a new predictor for in-stent restenosis. J. Interv. Cardiol. 23(4), 330–340 (2010).
- Louvard Y, Thomas M, Dzavik V et al. Classification of coronary artery bifurcation lesions and treatments: time for a consensus! Catheter. Cardiovasc. Interv. 71(2), 175–183 (2008).
- Morice MC. Bifurcation lesions: a neverending challenge. Eur. Heart J. 29(23), 2831–2832 (2008).
- Colombo A. Innovations in bifurcations. Catheter. Cardiovasc. Interv. 71(6), E7–E8 (2008).
- Stankovic G, Darremont O, Ferenc M et al. Percutaneous coronary intervention for bifurcation lesions: 2008 consensus document from the fourth meeting of the European Bifurcation Club. EuroIntervention 5(1), 39–49 (2009).
- Hermiller JB. Bifurcation intervention: keep it simple. J. Invasive Cardiol. 18(2), 43–44 (2006).
- Rizik DG, Klassen KJ, Dowler DA, Villegas BJ. Balloon alignment T-stenting for bifurcation coronary artery disease using the sirolimus-eluting stent. J. Invasive Cardiol. 18(10), 454–460 (2006).
- Garg S, Serruys PW. Coronary stents: looking forward. J. Am. Coll. Cardiol. 56(Suppl. 10), S43–S78 (2010).
- Rizik DG, Klassen KJ. Dedicated bifurcation devices. Rev. Cardiovasc. Med. 11(Suppl. 1), S27–S37 (2010).
- Cortese B, Limbruno U. Coronary bifurcation lesions: innovative approaches and the future of bifurcation devices. Future Cardiol. 6(2), 221–230 (2010).
- Sguelia GA, Todaro D, Bisciglia A, Conte M, Stipo A, Pucci E. Kissing inflation is feasible with all second-generation drug-eluting balloons. Cardiovasc. Revasc. Med. 12(5), 280–285 (2011).
- Tyczynski P, Ferrante G, Moreno-Ambroj C et al. Simple versus complex approaches to treating coronary bifurcation lesions: direct assessment of stent strut apposition by optical coherence tomography. Rev. Esp. Cardiol. 63(8), 904–914 (2010).
- Gonzalo N, Garcia-Garcia HM, Regar E et al. In vivo assessment of high-risk coronary plaques at bifurcations with combined intravascular ultrasound and optical coherence tomography. JACC Cardiovasc. Imaging 2(4), 473–482 (2009).
- Tyczynski P, Ferrante G, Kukreja N et al. Optical coherence tomography assessment of a new dedicated bifurcation stent. EuroIntervention 5(5), 544–551 (2009).
- Colombo A, Chieffo A. Drug-eluting stent update 2007: part III: technique and unapproved/unsettled indications (left main, bifurcations, chronic total occlusions, small vessels and long lesions, saphenous vein grafts, acute myocardial infarctions, and multivessel disease). Circulation 116(12), 1424–1432 (2007).
- Al Suwaidi J, Yeh W, Cohen HA, Detre KM, Williams DO, Holmes DR Jr. Immediate and one-year outcome in patients with coronary bifurcation lesions in the modern era (NHLBI dynamic registry). Am. J. Cardiol. 87(10), 1139–1144 (2001).
- Galassi AR, Colombo A, Buchbinder M et al. Long-term outcomes of bifurcation lesions after implantation of drug-eluting stents with the ‘mini-crush technique’. Catheter. Cardiovasc. Interv. 69(7), 976–983 (2007).
- Yamashita T, Nishida T, Adamian MG et al. Bifurcation lesions: two stents versus one stent – immediate and follow-up results. J. Am. Coll. Cardiol. 35(5), 1145–1151 (2000).
- Ge L, Tsagalou E, Iakovou I et al. In-hospital and nine-month outcome of treatment of coronary bifurcational lesions with sirolimuseluting stent. Am. J. Cardiol. 95(6), 757–760 (2005).
- Thuesen L, Kelbaek H, Klovgaard L et al. Comparison of sirolimus-eluting and bare metal stents in coronary bifurcation lesions: subgroup analysis of the Stenting Coronary Arteries in Non-Stress/Benestent Disease Trial (SCANDSTENT). Am. Heart J. 152(6), 1140–1145 (2006).
- Latib A, Colombo A, Sangiorgi GM. Bifurcation stenting: current strategies and new devices. Heart 95(6), 495–504 (2009).
- Iakovou I, Foin N, Andreou A, Viceconte N, Di Mario C. New strategies in the treatment of coronary bifurcations. Herz 36(3), 198–213 (2011).
- Sharma SK, Sweeny J, Kini AS. Coronary bifurcation lesions: a current update. Cardiol. Clin. 28(1), 55–70 (2010).
- Hermiller JB. Contemporary bifurcation treatment strategies: the role of currently available slotted tube stents. Rev. Cardiovasc. Med. 11(Suppl. 1), S17–S26 (2010).
- Colombo A, Moses JW, Morice MC et al. Randomized study to evaluate sirolimuseluting stents implanted at coronary bifurcation lesions. Circulation 109(10), 1244–1249 (2004).
- Colombo A, Bramucci E, Sacca S et al. Randomized study of the crush technique versus provisional side-branch stenting in true coronary bifurcations: the CACTUS (Coronary Bifurcations: Application of the Crushing Technique Using Sirolimus-Eluting Stents) Study. Circulation 119(1), 71–78 (2009).
- Steigen TK, Maeng M, Wiseth R et al. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the NORDIC bifurcation study. Circulation 114(18), 1955–1961 (2006).
- Ferenc M, Gick M, Kienzle RP. Randomized trial on routine vs. provisional T-stenting in the treatment of de novo coronary bifurcation lesions. Eur. Heart J. 29(23), 2859–2867 (2008).
- Hildick-Smith D, de Belder AJ, Cooter N et al. Randomized trial of simple versus complex drug-eluting stenting for bifurcation lesions: the British Bifurcation Coronary Study: old, new, and evolving strategies. Circulation 121(10), 1235–1243 (2011).
- Jensen JS, Galloe A, Lassen JF et al. Safety in simple versus complex stenting of coronary artery bifurcation lesions. The NORDIC bifurcation study 14-month follow-up results. EuroIntervention 4(2), 229–233 (2008).
- Thuesen L, Steigen TK, Erglis A et al. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: 5 year follow-up in the NORDIC bifurcation study. Presented at: The American College of Cardiology conference. New Orleans, LA, USA, 2–5 April 2011.
- Brunel P, Lefevre T, Darremont O, Louvard Y. Provisional T-stenting and kissing balloon in the treatment of coronary bifurcation lesions: results of the French multicenter ‘TULIPE’ study. Catheter. Cardiovasc. Interv. 68(1), 67–73 (2006).
- Colombo A, Moses JW, Morice MC et al. Randomized study to evaluate sirolimuseluting stents implanted at coronary bifurcation lesions. Circulation 109(10),1244–1249 (2004).
- Costa RA. IVUS imaging of the main vessel and the side branch with different stenting bifurcation treatemtn approaches (INSIDE I and II trials). Presented at: Transcatheter Cardiovascular Therapeutics. Washington, DC, USA, 20–25 October 2007.
- Ormiston JA, Webster MW, Ruygrok PN, Stewart JT, White HD, Scott DS. Stent deformation following simulated side-branch dilatation: a comparison of five stent designs. Catheter. Cardiovasc. Interv. 47(2), 258–264 (1999).
- Niemelä M, Kervinen K, Erglis A, Holm NR, Maeng M, Christiansen EH. Randomized comparison of final kissing balloon dilatation versus no final kissing balloon dilatation in patients with coronary bifurcation lesions treated with main vessel stenting: the NORDIC-Baltic Bifurcation Study III. Circulation 123(1), 79–86 (2011).
- Hildick-Smith D, Lassen JF, Albiero R et al. Consensus from the 5th European Bifurcation Club meeting. EuroIntervention 6(1), 34–38 (2010).
- Koo BK, Waseda K, Kang HJ et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 3(2), 113–119 (2010).
- Bekdash IL, Hodgson JM. The side branch ostium: understanding the Achilles heel of treating bifurcation coronary disease. Rev. Cardiovasc. Med. 11(Suppl. 1), S38–S42 (2010).
- Guérin P, Pilet P, Finet G et al. Drug-eluting stents in bifurcation: bench study of strut deformation and coating lesions. Circ. Cardiovasc. Interv. 3(2), 120–126 (2010).
- Murasato Y, Hikichi Y, Horiuchi M. Examination of stent deformation and gap formation after complex stenting of the left main coronary artery bifurcations using microfocus computed tomography. J. Interv. Cardiol. 22(2), 135–144 (2009).
- Gastaldi D, Morlacchi S, Nichetti R et al. Modeling of the provisional side-branch stenting approach for the treatment of atherosclerotic coronary bifurcations: effects of stent positioning. Biomech. Model Mechanobio. 9(5), 551–561 (2010).
- Foin N, Secco GG, Ghilencea L, Krams R, Di Mario C. Final proximal post-dilatation is necessary after kissing balloon in bifurcation stenting. EuroIntervention 7(5), 597–604 (2011).
- Latib A, Moussa I, Sheiban I, Colombo A. When are two stents needed? Which technique is best? How to Perform? Eurointervention 6(Suppl. J), J81–J87 (2010).
- Al Rashdan I, Amin H. Carina modification T stenting, a new bifurcation stenting technique: clinical and angiographic data from the first 156 consecutive patients. Catheter. Cardiovasc. Interv. 75(4), 683–690 (2009).
- Ge L, Iakovou I, Cosgrave J et al. Treatment of bifurcation lesions with two stents: one year angiographic and clinical follow up of crush versus T stenting. Heart 92(3), 371–376 (2006).
- Gunalingam B, Chan RY. A novel buddy balloon technique to recross a T-stented bifurcation. Catheter. Cardiovasc. Interv. 74(1), 103–107 (2009).
- Kaplan S, Barlis P, Dimopoulos K et al. Culotte versus T-stenting in bifurcation lesions: immediate clinical and angiographic results and midterm clinical follow-up. Am. Heart J. 154(2), 336–343 (2007).
- Routledge HC, Morice MC, Lefevre T et al. 2-year outcome of patients treated for bifurcation coronary disease with provisional side branch T-stenting using drug-eluting stents. JACC Cardiovasc. Interv. 1(4), 358–365 (2008).
- Waksman R, Bonello L. The 5 Ts of bifurcation intervention: type, technique, two stents, T-stenting, trials. JACC Cardiovasc. Interv. 1(4), 366–368 (2008).
- Dardas PS, Tsikaderis DD, Mezilis NE, Styliadis G. A technique for type 4a coronary bifurcation lesions: initial results and 6-month clinical evaluation. J. Invasive Cardiol. 15(4), 180–183 (2003).
- Schwartz L, Morsi A. The draw-back stent deployment technique: a strategy for the treatment of coronary branch ostial lesions. J. Invasive Cardiol. 14(2), 66–71 (2002).
- Burzotta F, Gwon HC, Hahn JY et al. Modified T-stenting with intentional protrusion of the side-branch stent within the main vessel stent to ensure ostial coverage and facilitate final kissing balloon: the T-stenting and small protrusion technique (TAPstenting). Report of bench testing and first clinical Italian–Korean two-centre experience. Catheter. Cardiovasc. Interv. 70(1), 75–82 (2007).
- Colombo A, Stankovic G, Orlic D et al. Modified T-stenting technique with crushing for bifurcation lesions: immediate results and 30-day outcome. Catheter. Cardiovasc. Interv. 60(2), 145–151 (2003).
- Rajdev S SA, Modi K et al. ‘Cone Crush’ a variant of modified T-stenting technique for coronary bifurcation lesions: bench testing, feasibility, and in-hospital outcomes (Abstr). J. Am. Coll. Cardiol. 49(Suppl. B), 6B (2007).
- Ormiston JA, Currie E, Webster MW et al. Drug-eluting stents for coronary bifurcations: insights into the crush technique. Catheter. Cardiovasc. Interv. 63(3), 332–336 (2004).
- Ormiston JA, Webster MW, Webber B, Stewart JT, Ruygrok PN, Hatrick RI. The ‘crush’ technique for coronary artery bifurcation stenting: insights from microcomputed tomographic imaging of bench deployments. JACC Cardiovasc. Interv. 1(4), 351–357 (2008).
- Hoye A, Iakovou I, Ge L et al. Long-term outcomes after stenting of bifurcation lesions with the ‘crush’ technique: predictors of an adverse outcome. J. Am. Coll. Cardiol. 47(10), 1949–1958 (2006).
- Costa RA, Mintz GS, Carlier SG, Lansky AJ, Moussa I, Fujii K. Bifurcation coronary lesions treated with the ‘crush’ technique: an intravascular ultrasound analysis. J. Am. Coll. Cardiol. 46(4), 599–605 (2005).
- Chen S, Tan H, Lee M, Damras, Sumitsuji, Kawajiri. More modified crush techniques for coronary bifurcation lesions: which one is better? Catheter. Cardiovasc. Interv. 68(3), 468–469; author reply 469–470 (2007).
- Chen SL, Kwan TW. Twenty-four-month update on double-kissing crush stenting of bifurcation lesions. J. Interv. Cardiol. 22(2), 121–127 (2009).
- Jim MH, Ho HH, Chan AO, Chow WH. Stenting of coronary bifurcation lesions by using modified crush technique with double kissing balloon inflation (sleeve technique): immediate procedure result and short-term clinical outcomes. Catheter. Cardiovasc. Interv. 69(7), 969–975 (2007).
- Jim MH, Ho HH, Miu R, Chow WH. Modified crush technique with double kissing balloon inflation (sleeve technique): a novel technique for coronary bifurcation lesions. Catheter. Cardiovasc. Interv. 67(3), 403–409 (2006).
- Cortese B, Limbruno U. A new technique for the treatment of bifurcation lesions: modified reverse crushing. Indian Heart J. 60(6), 605–607 (2008).
- Chen SL, Santoso T, Zhang JJ, Ye F, Xu YW, Fu Q. A randomized clinical study comparing double kissing crush with provisional stenting for the treatment of coronary bifurcation lesions: results from the DKCRUSH-II (Double Kissing Crush versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions) trial. J. Am. Coll. Cardiol. 57(8), 914–920 (2011).
- Morton AC, Siotia A, Arnold ND et al. Simultaneous kissing stent technique to treat left main stem bifurcation disease. Catheter. Cardiovasc. Interv. 69(2), 209–215 (2007).
- Helqvist S, Jorgensen E, Kelbaek H et al. Percutaneous treatment of coronary bifurcation lesions: a novel ‘extended Y’technique with complete lesion stent coverage. Heart 92(7), 981–982 (2006).
- Sharma SK, Choudhury A, Lee J et al. Simultaneous kissing stents (SKS) technique for treating bifurcation lesions in medium-tolarge size coronary arteries. Am. J. Cardiol. 94(7), 913–917 (2004).
- Sharma S. Simultaneous kissing drug-eluting stent technique for percutaneous treatment of bifurcation lesions in large-size vessels. Catheter. Cardiovasc. Interv. 65(1), 10–16 (2005).
- Y-Hassan S, Lindroos MC, Sylvén C. A novel stenting technique for coronary artery bifurcation stenosis. Catheter. Cardiovasc. Interv. 73(7), 903–909 (2009).
- Chevalier B, Glatt B, Royer T, Guyon P. Placement of coronary stents in bifurcation lesions by the ‘culotte’ technique. Am. J. Cardiol. 82(8), 943–949 (1998).
- Adriaenssens T, Byrne RA, Dibra A et al. Culotte stenting technique in coronary bifurcation disease: angiographic follow-up using dedicated quantitative coronary angiographic analysis and 12-month clinical outcomes. Eur. Heart J. 29(23), 2868–2876 (2008).
- Mezzapelle G, Baldari D, Baglini R. Culotte bifurcation stenting with paclitaxel drug-eluting stent. Cardiovasc. Revasc. Med. 8(1), 63–66 (2007).
- Erglis A, Kumsars I, Niemelä M et al. NORDIC PCI Study Group. Randomized comparison of coronary bifurcation stenting with the crush versus the culotte technique using sirolimus eluting stents: the NORDIC stent technique study. Circ. Cardiovasc. Interv. 2(1), 27–34 (2009).
- Grube E, Buellesfeld L, Neumann FJ et al. Six-month clinical and angiographic results of a dedicated drug-eluting stent for the treatment of coronary bifurcation narrowings. Am. J. Cardiol. 99(12), 1691–1697 (2007).
- Verheye S, Agostoni P, Dubois CL et al. 9-month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the Axxess self-expanding biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectivley) study. J. Am. Coll. Cardiol. 53(12), 1031–1039 (2009).
- Magro M, Wykrzykowska J, Serruys PW et al. Six-month clinical follow-up of the Tryton side branch stent for the treatment of bifurcation lesions: a two center registry analysis. Catheter. Cardiovasc. Interv. 77(6), 798–806 (2011).
- Onuma Y, Müller R, Ramcharitar S et al. Tryton I, first-in-man (FIM) study: six month clinical and angiographic outcome, analysis with new quantitative coronary angiography dedicated for bifurcation lesions. EuroIntervention 3(5), 546–552 (2008).
- Lefèvre T, Ormiston J, Guagliumi G et al. The FRONTIER stent registry: safety and feasibility of a novel dedicated stent for the treatment of bifurcation coronary artery lesions. J. Am. Coll. Cardiol. 46(4), 592–598 (2005).
- Rizik DG, Klag JM, Tenaglia A et al. Evaluation of a bifurcation drug-eluting stent system versus provisional T-stenting in a perfused synthetic coronary artery model. J. Interv. Cardiol. 22(6), 537–546 (2009).
- Scheller B, Hehrlein C, Bocksch W et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 355(20), 2113–2124 (2006).
- Unverdorben M, Vallbracht C, Cremers B et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation 119(23), 2986–2994 (2009).
- Unverdorben M, Kleber FX, Heuer H et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin. Res. Cardiol. 99(3), 165–174 (2010).
- Belkacemi A, Agostoni P, Voskuil M et al. Coronary bifurcation lesions treated with the drug-eluting balloon: a preliminary insight fro the DEBIUT study. EuroIntervention 7(Suppl. K), K66–K69 (2011).
- Hermiller J, Rizvi A. Commentary: Bifurcations: the problem is the side branch. J. Invasive Cardiol. 18(10), 461 (2006).
- Dauerman HL, Higgins PJ, Sparano AM et al. Mechanical debulking versus balloon angioplasty for the treatment of true bifurcation lesions. J. Am. Coll. Cardiol. 32(7), 1845–1852 (1998).
- Hahn JY, Song YB, Lee SY et al. Serial intravascular ultrasound analysis of the main and side branches in bifurcation lesions treated with the T-stenting technique. J. Am. Coll. Cardiol. 54(2), 110–117 (2009).
- Trabattoni D, Bartorelli AL. IVUS in bifurcation stenting: what have we learned? EuroIntervention 6(Suppl. J), J88–J93 (2010).
- Koo BK. Physiologic evaluation of bifurcation lesions using fractional flow reserve. J. Interv. Cardiol. 22(2), 110–113 (2009).
- Koo BK, Kang HJ, Youn TJ et al. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J. Am. Coll. 46(4), 633–637 (2005).
- Barlis P, Dimopoulos K, Tanigawa J et al. Quantitative analysis of intracoronary optical coherence tomography measurements of stent strut apposition and tissue coverage. Int. J. Cardiol. 141(2), 151–156 (2009).
- Mario CD, Lakovou L, van der Giessen WJ et al. Optical coherence tomography for guidance in bifurcation lesion treatment. EuroIntervention 6(Suppl. J), J99–J106 (2010).
- Dzavik V, Kharbanda R, Ivanov J et al. Predictors of long-term outcome after crush stenting of coronary bifurcation lesions: importance of the bifurcation angle. Am. Heart J. 152(4), 762–769 (2006).
- Collins N, Seidelin PH, Daly P et al. Long-term outcomes after percutaneous coronary intervention of bifurcation narrowings. Am. J. Cardiol. 102(4), 404–410 (2008).
- Costa RA. Preliminary resutls of the novel TMI (TriReme Medical Inc.) Antares side branch adaptive system (Antares SAS™ Stent) for the treatment of de novo coronary bifurcation. J. Am. Coll. Cardiol. 51, B51 (2008).
- Solar RJ. The Y Med SideKicK stent delivery system for the treatment of coronary bifurcation and ostial lesions. Presented at: Cardiovascular Revascularization Therapies. Washington, DC, USA, 8 March 2007.
- Lefevre T. Invatec twin rail bifurcation stent. Presented at: Transcatheter Cardiovascular Therapeutics. Washington, DC, USA, 17–21 October 2005.
- Del Blanco BG, Marti G, Bellera N et al. Clinical and procedural evaluation of the Nile Croco® dedicated stent for bifurcations: a single centre experience with the first 151 consecutive non-selected patients. EuroIntervention 7(2), 216–224 (2011).
- Ormiston JA, Lefèvre T, Grube E et al. First human use of the TAXUS Petal paclitaxeleluting bifurcation stent. EuroIntervention 6(1), 46–53 (2010).
- Verheye S, Ramcharitar S, Grube E et al. Six-month clinical and angiographic results of the STENTYS® self-apposing stent in bifurcation lesions. EuroIntervention 7(5), 580–587 (2011).
- Mamas MA, Farooq V, Latib A et al. Use of the Sideguard (Cappella) stent in bifurcation lesions: a real-world experience. EuroIntervention 7(10), 1170-1180 (2012).
- Meredith IT, Worthley S, Whitbourn R et al. First-in-human experience with the Medtronic Bifurcation Stent System. EuroIntervention 7(6), 662–669 (2011).
▪ A review of dedicated bifurcation devices.
▪ Report of SCANDSTENT trial showing reduced rates of restenosis for bifurcation lesions treated with drug-eluting stents compared with bare metal stents.
▪ A report of the NORDIC trial.
▪ A report of the BBC One trial.
▪ A review of two-stent techniques for bifurcation disease.
▪ A report of the Axxess stent.
▪ A report of the Tryton stent.
▪ A review of intravascular ultrasound in bifurcation disease.
▪ A review of the use of optical coherence tomography in bifurcation disease.