Research Paper - Clinical Investigation (2018) Volume 8, Issue 1
Evaluation of Edible Oyster Mushroom (Pleurotus ostreatus) on Oxidative Stress and Neurological Cognitive Disorder in Streptozotocin-Diabetic Rats
- Corresponding Author:d
- Bindhu J
Department of Biotechnology, Molecular Diagnostics and Bacterial Pathogenomics Research Laboratory
Faculty of Biotechnology, Bannari Amman Institute of Technology, Sathyamangalam, India
E-mail: bindhuravi.edu@gmail.co`m
Submitted Date: 20 December 2017; Accepted Date: 03 January 2018; Published Date: 08 January 2018
Abstract
Pleurotus ostreatus is drawn into lime light for the treatment of Diabetes mellitus for the last few years, but now it is evidently proven for the recovery of the diabetes mellitus-induced memory impairments in streptozoticin induced diabetic rats. Oxidative stress, neurological disorders, cognitive and spatial learning associated to Diabetic-Alzheimer’s is irreversibly inhibited by P. ostreatus. The availability and cost effectively makes a stepping stone for the middle to low income people to rely on P. ostreatus rather than of costly medications for the treatment of neurological and cognitive disorders associated with Diabetes. The research was done to identify a potent free radical bioactive compound against cognitive impairment in streptozotocin –diabetic rats. Five weeks after diabetic induction, P. ostreatus extract was administered orally (2 mg/lt). The cognitive, cerebral and perceptive behaviour was examined with T Maze (Radial Arm Maze), Morris Water Maze (MWM), Novel object recognition task in wistar rats (male). Besides oxidative stress parameters like Lipid peroxidation (LPO), FRAP (Ferric reducing ability of plasma), antioxidant assay and Thiol assay to determine total Thiol group in the blood was carried out to determine the neurological disorders associated with brain. P. ostreatus showed significant improvements in Spatial and Cognitive disorders when compared to the diabetic and healthy control. Also the FRAP and thiol group in blood showed tremendous increase due to the P. ostreatus proving the effectiveness of Pleurotus extract on Cognitive impairment and Oxidative stress in Diabetic mellitus.
Graphical Abstract
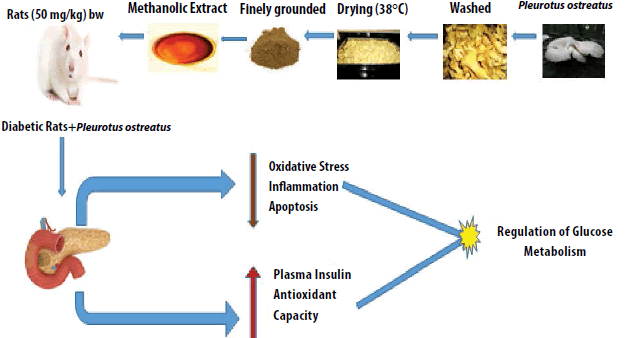
Keywords
P. ostreatus; Oxidative stress; Diabetic; Streptozotocin and Histopathology
Introduction
Diabetes mellitus a blooming disorder linked with Cognitive disorder related neurological inefficiency [1- 3]. Cellular machinery oxidation in human body is by the continuous exposure to different types of molecules that enumerates reactive species known as free radicals (ROS/RNS) [4]. These Free radical arbitrated imbalances in systemic brain manifestation with oxidative stress share a foremost part in septohippocampial dysfunction in diabetes [5,6]. Autoxidation of glucose, glycation of protein and polyol pathway are the cellular mechanisms that seem to be complicated in diabetes [1]. In some cases this free radicals causes necrosis and ultimately to the cell death [7]. Cellular functions are protected by antioxidant enzymes which maintain in vivo homeostasis throughout oxidative stress [6]. Brain is extremely prone to overproduction of ROS and faulty antioxidant and leading to neurodegeneration and association cognitive decline in aging and Alzheimer’s disease [7-9]. AD is allied to pathogenetic mechanisms with multiple aetiologies. Free radical prompted oxidative stress, diminishing of cerebral energy metabolism along with excitotoxic events are most promising events for cognitive disorder studies [10,11]. Ageing and age associated neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease stay concomitant with varying degrees of cognitive impairment leading to indisposition [12] and neuronal oxidative stress which leads to behavioural effects [13]. Patients by diabetes mellitus are reported with trifling enhancements of spatial impairment functioning [14,15]. Previous evidence suggests that the hyperglycemia as a “toxic” effect prominent to diabetic end-organ damage to brain in Type I and Type II diabetes [16]. Diabetic patients may express cognitive deficits even at their young age even more worse during elderly [17]. Recent epidemiological studies even report relation flanked by diabetes and dementia. Cognitive discrepancies are also reported in rat replicas of diabetes. Learning deficits are seen in streptozotocin convinced diabetic rats which is preventable, but not fully reversible with insulin treatment [16]. Cerebral dysfunction pathogenesis in diabetes is yet to be explicated [17]. Age-associated Perceptive debility and AD can be initially treated with antioxidant drugs [18]. Mild to modest diminishing of intellectual functioning (DM) are seen in pateints with diabetes mellitus (DM) [12,13]. Hyperglycemia is considered as the major precursor for End organ damage to brain in Type I and Type II diabetes [14]. Elderly people are marked with intellectual deficits associated to young diabetic adults [15]. Diabetes and Dementia are even correlated in current research for example perceptive scarcities are also reported in rat models of diabetes [16]. Diabetic patients with Cerebral dysfunction pathogenesis is an emerging era for study of Intellectual and perceptive shortages [17]. Thus it is estimated that age-associated cognitive decline such as AD could be prevented or treated using antioxidant drugs [18]. Edible mushrooms is considered equally similar to meat for vegan in the modern world [18]. P. ostreatus is primarily consumed for its taste, fiberness and nutritive value, besides it is also used industrially as a bioremediator [19,20]. The active principles of P. ostreatus are isolated for direct use as drugs, lead compounds or pharmacological agents [21], Interleukin-12 production, nitric oxide synthase activation and iron chelating properties are mediated by the bioactive compounds derived from mushrooms [22,23]. Great threat to human life by AD lingers to escalation and thus the pursuit for AD drugs takes a compelling urgency [24]. Studies are still being conducted in lots of countries to explore the utilization and consuming strategy of mushrooms and their metabolites for human ailments [25]. The antitumor, antidiabetic, anti-mutagenic, anti-proliferative, anticarcinogenic properties and neuroprotective in models of degenerative are the most attractive features of edible oyster mushrooms. These properties are thought to be mediated by the phenols by means of b-carotene, tocopherols, and total polyphenols [26]. Therefore the current research investigated the consequences of P. ostreatus on oxidative damage and spatial cognition in streptozotocin-convinced diabetic rats.
Methodology
Identification and handling of sample
P. ostreatus was purchase from Green Grow Cultivation Unit, Big Shop, Ooty, Tamilnadu and authentication was done by Dr. Annamalai, Plant Biologist, and Coimbatore. It was reserved in a hygenic, sterilized pliable container and sealed. The fruiting hyphae was washed and fragmented and finally dried in incubator at 38°C.
Preparation of extract
P. ostreatus extract was prepared by drying at 38°C for 36 h. The dehydrated sections was weighed and grounded prior to extraction. Dried sample (50 gm) was extracted with 400 ml of 100% ethanol for 9 h using soxhlet apparatus. Rotary evaporator was used in vacuo to at 40°C [27].
Refinement of bioactive compound
HPTLC: Bioactive compounds were determined qualitatively by High Performance Thin Layer Chromatography (HPTLC). The presence of Alkaloids, Flavonoids, Steroids was established with Dragendorff’s reagent by Keller-Killani test (Table 1 and Figure 1) [28].
| TEST FOR EXTRACT INFERENCE |
|---|
| Alkaloid + |
| Flavanoid + |
| Steroid + |
| Terpenoid + |
Table 1: Preliminary Phytochemical Screening of Pleurotus ostreatus Extract by HPTLC
Figure 1: TLC plates showing the presence of Alkaloid, Flavanoid, Steroid and Terpenoid after derivitization process
Chemicals and drugs
Dithiononitrobenzoic acid (DTNB), Tris base, 1,1,3,3’-tetraethoxypropane (MDA), 2-thiobarbituric acid (TBA), trichloroacetic acid (TCA), n-butanol, 2,4,6-tripyridyl-s-triazine (TPTZ), from Merck Chemical Co. (Tehran), Streptozotocin Sigma Chemical Co and P. ostreatus were used in this study.
Animals and experimental design
Male Wistar rats weighing approximately 180±10 g acquired from the Pasteur Institute Coonoor, India. Temperature maintenance was about of 25±2°C and 12/12 h of light– dark cycle. Experiments were conducted with guidelines and norms of Animal Ethics Committee. The experimental rats were assigned into five groups; each group contained 8-10 animals:
(i) Control rats (C); (ii) Control with Streptozotocin (C+D); (iii) P. ostreatus treated control rats (C+E); (iii) diabetic rats treated with P. ostreatus (D+E); (iv) diabetic rats treate3d with Std Glibenciclamide drug).
A single intraperitoneal dose of sreptozotocin (STZ) (50 mg (kg body weight)−1) dissolved in 0.2 mL of normal saline was used to induce diabetes in rats. After few days the stasis of confirmation of diabetes was done by tail vein identifying the amount of glucose in the tail vein. A rat with blood glucose levels of 250 mg/dL was set to be diabetic. After diabetes induction, P. ostreatus extract was directed orally to rats for 8 weeks (3 mg /L) [29].
Behavioral tests
Wisdom and recollection: Morris water maze (MWM) Neurological disorders with cognitive components like AD and Schizophrenia are studied by knowledge and recollecting pattern in animal models [13-15]. MWM is one among them in identifying the cognitive ailments allied with neuron stimulated brain reactions. In this research 5-day training was conducted for animals. After every 60 hr 4 dimensional memory retaining capability was tested. It was conducted in square shaped tank with the dimension of 60 × 120 cm (L X B). This tank was with water at a depth of 25 cm. Extra cues were also attached. The acrylic sheet platform was submerged in the water tank at a deepness of 2 cm as an escalator for the rats to come out after the trial of escape latency test. The four quadrants were marked and to the each quadrant rats were allowed to swim to find the sheet for escape latency. The time taken by each rats were noted down along with the error time taken when the rats exploited the same quadrant. The animal which remained exploring even after 1 minute was challenged physically to escape by the experimenter. This procedure was repeated with each rat from starting positions altogether in all quadrants after a small interval time between each trials conducted [30].
T maze or radial arm maze: T maze was an ideal method to identify the cognitive learning and working memory of rat models. Spatial Cues was the trick used by the animals to identify the position of the reward. This strategy contains a platform covered completely by radial arms .So each time when the rat move in the platform comes across this radial arm. Once it’s identified the rat next time try not to hit the radial arm and also remembers the exact location of it. This test denotes the complete memory and 4 dimensional movements of mice when they come across objects in their pathway. 60 hr. training was given for each group prior to the tests conducted. Encouraging was carried out by placing food rewards in each arm. During initial performance after trial time taken by the rat to identify the reward, the amount of times the cues baited and the rats visiting the cues visited before (entries into an arm visited already were calculate manually [31].
Novel and Innovative object recognition: Novel object recognition is a task for memory that does not rely on spatial cues. For this task an exploratory period for 48hr was carried out using food trays, water bottles, dust tray etc. In the test course all those trial objects (food trays, water bottles, dust tray) was switched with a novel object. The time taken by the normal animal to recognize a new object was recorded. Besides this entity animals also skilled to spot a new food with different odour, tastes and even other breed social partners [31,32].
Sociability: Sociability is a novel method to study the dejection and violent actions. Besides these there is a detail platform to study autism. In this basic test of sociability the repeated time the rat applies exploring a new impetus and the error time for an empty box was noted by the experimenter. No trial period was performed since sociability relies directly on cognitive disorders. This assessment was conducted for the animals to identify the empty boxes and the boxes with a social partner of the same breed or different breed. The time taken by the each animal to recognize the empty box was noted as well as error time was also noted when the same empty boxes was rechecked again and again [33,34].
Simple visual task: On week 8 after STZadministration, performance on a ‘simple visual task’ was evaluated. In this task the performance of identification, behavior, recognition with same breed was identified for the animals with diabetic and normal healthy control rats. This comparative study elucidates the memory errors, working memory errors, comparative errors and so on with the help of longitudinal study pattern.
Biochemical analysis
Estimation of oxidative stress parameters: The superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (TAC) activities, and glutathione (GSH) level in hepatic and pancreatic tissue were measured using commercially available kits (Sigma Aldrich, Chennai)Lipid peroxidation was measured as malondialdehyde (MDA) level in hepatic and pancreatic tissue according to Jain’s method [35].
Assay of cellular lipid peroxidation (LPO): Lipid peroxidation was measured using TBA. The rat plasma sections were mixed with TCA (20) and the precipitate was collected and dispersed in Sulphuric acid (0.05 M). Sodium sulfate 2 M was added and heated for 30 minutes in boiling water bath. The absorbance of the pink complex was measured at 532 nm [36].
Assay of total antioxidant capacity (TAC): TAC was measured by ferric reducing ability of plasma (FRAP) method. This method is based on the ability of plasma in reducing Fe3+ to Fe2+ in the presence of TPTZ. The reaction of Fe2+ and TPTZ gives a complex with blue color and maximum absorbance in 593 nm [37].
Statistical analysis
Readings was observed in Standard deviation using ANOVA table linked to Dunkan test. Statistically significant variations are compared as follows: control versus P. ostreatus canned control; control versus diabetic and diabetic versus P. ostreatus treated diabetic rats. Results were considered significantly different if p<0.05.
Results
Effect of P. ostreatus extracts on the behaviour patterns and antioxidant assay
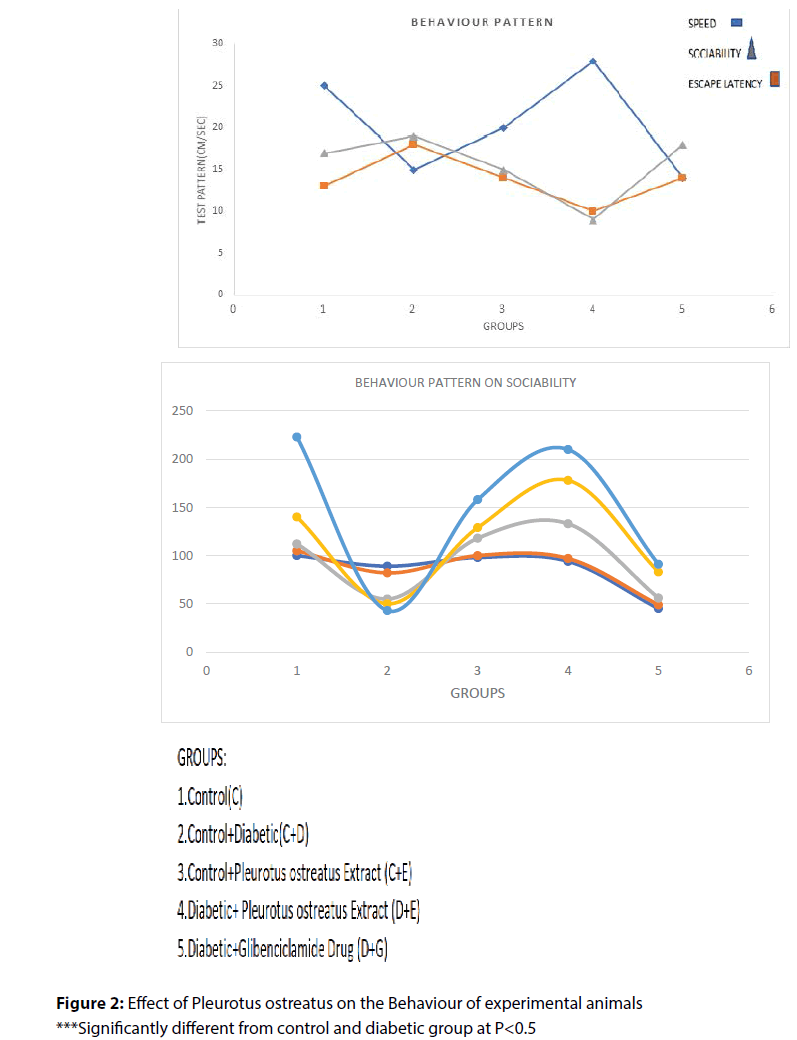
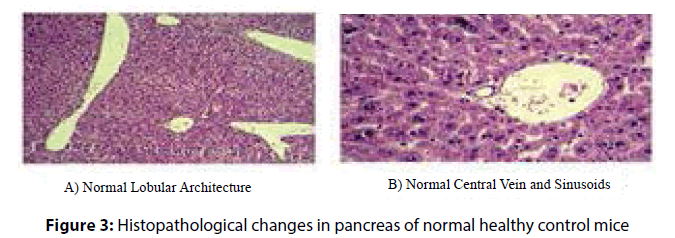
Administration of P. ostreatus decreased escape latency, travelled distance and speed in P. ostreatus control and diabetic treated P. ostreatus vs. control and diabetic groups (Table 2) and (Figure 1 and 2).
| Factors Analyzed | 50 mg/kg |
100 mg/kg |
150 mg/kg |
200 mg/kg |
250 mg/kg |
300 mg/kg |
350 mg/kg |
400 mg/kg |
450 mg/kg |
500 mg/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| Changes on skin | _ | _ | _ | _ | _ | + | + | + | + | + |
| Changes on Fur | _ | _ | _ | _ | _ | + | + | + | + | + |
| Changes on Eyes | _ | _ | _ | _ | _ | _ | + | + | ||
| Changes on Mucous membrane | _ | _ | _ | _ | _ | _ | _ | + | + | + |
| Behavior Pattern | _ | _ | _ | _ | _ | _ | + | + | + | + |
| Tremors | _ | _ | _ | _ | _ | _ | _ | _ | + | + |
| Sleep | _ | _ | _ | _ | _ | + | + | + | + | |
| Coma | _ | _ | _ | _ | _ | _ | _ | _ | _ | + |
| Death | _ | _ | _ | _ | _ | _ | _ | _ | + | + |
Table 2: Acute toxicity Studies of Pleurotus ostreatus Extract
Effect of P. ostreatus extracts on blood glucose levels of rats
Induction of diabetes mellitus was confirmed by blood glucose value above 250 mg/dL. The streptozotocin-induced diabetic rats showed consistent fasting hyperglycemia throughout the study. As shown in result section, P. ostreatus treatment to diabetic rats significantly reduced the blood glucose level. Diabetic animals also showed signs of polyuria, polydipsia and polyphagia
Impression of P. ostreatus extracts on the oxidative stress parameters in hepatic and pancreatic tissue
Malondialdehyde (MDA) level in hepatic and pancreatic tissues showed markable increase in the Diabetic group whereas at the same time there was tremendous decrease in antioxidant level (GSH) and antioxidant enzyme activity (SOD, CAT, GSH-Px) when compared to healthy control (P<0.01). P. ostreatus treatment and standard glibenciclamide treatment inhibited the formation of MDA and raised antioxidant hormone (GSH) and antioxidant enzyme activity ((SOD, CAT, GSH-Px) (Table 3).
| PARAMETERS | C | C+D | C+E | D+E | D+G | |
|---|---|---|---|---|---|---|
| Liver | GSH (µg/mg protein) | 512.36±91.25 | 363.72±52.3 | 529.36±100** | 451±89.44*** | 482.09±106.30 |
| CAT (U/mg Protein) | 360.6±18.36 | 318.29±61.2 | 368.23±22.8** | 322±17.55*** | 344.8±28.91 | |
| SOD(U/mg Protein) | 540±24.21 | 214.72±41.3 | 593.72±31.7** | 412±35.33*** | 523.36±36.6 | |
| GSH-PX(U/mg Protein) | 3011±200 | 2149±638 | 3162±37.23** | 2769±252*** | 2808±212 | |
| MDA(Mol/mg Protein) | 8.05±0.98 | 18.12±1.92 | 9.01±1.02** | 10.01±1.01*** | 9.93±1.52 | |
| Pancreas | GSH (µg/mg protein) | 456.03±71.36 | 313.08±52.3 | 493.12±51.3** | 360±57*** | 406.16±56.38 |
| CAT (U/mg Protein) | 278.02±17.62 | 159.13±13.32 | 286.33±28.3** | 210±23*** | 274.30±21.94 | |
| SOD(U/mg Protein) | 396±28.73 | 186.78±43.65 | 386.31±31.11** | 349±37.5*** | 364.52±40.23 | |
| GSH-PX(U/mg Protein) | 636±92.73 | 431±78 | 703.12±96** | 598±94*** | 530±68 | |
| MDA(Mol/mg Protein) | 7.33±1.08 | 16.13±1.36 | 7.50±19** | 10.01±1.61*** | 8.23±1.4 | |
Table 3: Effect of Pleurotus ostreatus extract on the oxidative stress parameters in Hepatic and Pancreatic Tissue
Effect of P. ostreatus on immunohistochemical staining of iNOS
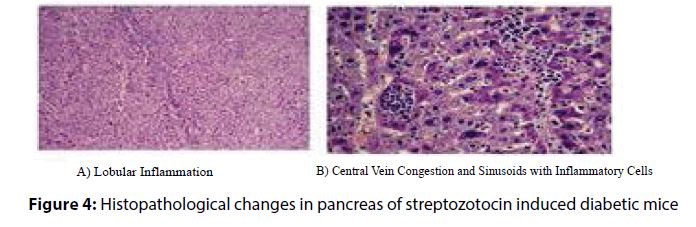
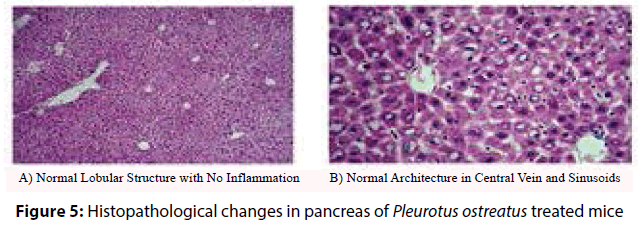
Histopathological analysis of the harvested pancreas of streptozotocin induced diabetic mice was fixed in 10% formalin for 48 h, revealed remarkable changes versus the control mice. These changes included periportal fatty infilteration with focal necrosis of hepatocytes. The normal healthy control pancreatic tissue sections showed immunoreactive B cells (Figures 3 and 4). Diabetic mice treated with P. ostreatus extract (Figure 5) revealed a remarkable improvement of hepatic tissues where reduced necrosis was observed and the cellular arrangement of hepatocytes were found to be normal. Treatment with P. ostreatus extract brought back the cellular arrangement to near normal.
Discussion
The current research validated that the administration of P. ostreatus improves the performance in sociability, novel object recognition, water maze tasks, simple visual task and reactive oxygen and relative stress parameters such as lipid peroxidation and antioxidant activity of the P. ostreatus extract in blood correlates with the MWM score. Thus, P. ostreatus may be involved in protecting against neuronal degenerative disorders and ROS. In this study, (30 days) administration of P. ostreatus decreased the plasma oxidative status. An increase in the production of free radicals exacerbates the neurodegenerative process by deteriorating cellular enzymes [38]. P. ostreatus extract activated the antioxidant enzymes [39] when compared to the effects of essential oil of Satureja khuzestanica on the oxidative stress in experimental hyperthyroid male rat [40]. Complete oxidative stress can be controlled by long-term intake of mushrooms [41-42]. Memory deficits contributed by ROS and cytokines released from actuated microglia and astrocytes [43,44]. Bindhu et al. reported that the P. ostreatus reduces the elevated blood glucose level in both type 1 and type 2 diabetic animals [45]. Pleurotus mushrooms enhances basal and insulin-roused glucose uptake [46], inhibit intestinal glucose uptake by sodium-dependent glucose transporter SGLT1 and mimic insulin by decreasing the countenance of genes that control gluconeogenesis [47]. Consumption of aqueous and hydroalcoholic extracts of B. vulgaris for 30 consecutive days as dose of 200 mg/kg can beneficially affect the hepatic liver enzymes, especially ALT, due to the liver regeneration and providing the source of the liver enzyme [48]. The intake of P. ostreatus caused a momentous arise in body weight and reduction in food and water intake in diabetic rats. This could possibly improve glycemic control produced by P. ostreatus in diabetic rats [49]. The increase in thio barbituric acid-reactive substances (TBARS), an index of lipid peroxidation in the diabetic rats might be due to increased levels of oxygen free radicals. In animal studies, mushroom polyphenol administration was shown to decrease serum TBARS level due to its potential antioxidant activity [50]. Similarly, T. foenum-graecum seeds are more potent than G. officinalis leaves with regards to hypoglycaemic properties, but body weight-reducing properties were similar between these two plant species. These results indicated that these two plant species can be used as an herbal treatment for DM and weight gain [51]. Behavioral protocol and increase in task complexity as accentuated due to performance deficits in diabetic rats. This protocol was depicited by study conducted in STZ-diabetic mice for normal acquisition and relatively simple tasks [52]. Oral and enema forms of hydroalcoholic extract of Calendula. G. officinalis can be offered as are potential therapeutic agents for Ulcerative colitis (UC) induced in rats and even oxidative stress parameters was also reversed by CO administration [53]. The accentuation of the deficits by variations in the behavioral protocol may reflect an inability of the diabetic rats to adapt their behavioral strategies to the MWM [54]. S. khuzestanica have a lipid-lowering and antihyperglycemic property in male rats as an animal model for human [55]. Additionally STZ did not induce toxic influences on brain. Neurodegenerative diseases for instance AD which is allied to oxidative damage and neurotoxicity could be treated with the prophylactic means of P. ostreatus. Atorvastatine can decrease the histopathological lesions especially in liver, if used alone or in combination with T. vulgaris extract [56]. The use of Carum carvi L. (caraway) hydroalcoholic extract (CHE) in topical form may be associated with reduced intensity of oral mucositis OM. This may be due to appropriate antibacterial activity and terpinene contents [57]. All these above research proves P. ostreatus plays a vital role in controlling oxidative stress.
Long-term administration of an amount of P. ostreatus that could prompt antioxidative effects might probably deal with age-related declines in memory and learning ability in humans. In the process of aging, LPO accumulates and induces disorders of cellular functions [58]. Memory–related learning ability declines with aging [59]. Therefore, the continuous intake of, P. ostreatus might promote healthy aging of the brain in older individual.
Financial & competing interest’s disclosure
This Research is a part of Ph.D work of first Author. Authors gratefully acknowledge DST SERB, New Delhi, No:SB/EMEQ-114/2014 funded facilities for the successful completion of the research work and authors would also like to acknowledge the AICTE, New Delhi, No 20/AICTE/RIFD/RPS (Policy-1) 28/2012- 13 sponsored Molecular Diagnostics and Bacterial Pathogenomics Research Laboratory, Department of Biotechnology and Bannari Amman Institute of Technology for providing an ambient environment. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- Biessels GJ, Kappelle A, Bravenboer B, Erkelens D, Gispen W. Cerebral function in diabetes mellitus. Diabetologia. 37: 643-650 (1994).
- Prescott JH, Richardson JT, Gillespie CR. Cognitive function in diabetes mellitus: the effects of duration of illness and glycaemic control. Brit J Clin Psychol. 29: 167-175 (2011).
- Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, et al. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthal Visua Sci. 54: 1325-1336 (2013).
- Sailaxmi G, Lalitha K. Impact of a stress management program on stress perception of nurses working with psychiatric patients Department of Nursing, National Institute of Mental Health & Neurosciences (INI), Hosur Road, Near Wilson Garden, Bangalore 560029, Karnataka, India
- Prickaerts J, Fahrig T, Blokland A. Cognitive performance and biochemical markers in septum, hippocampus and striatum of rats after an icv injection of streptozotocin: a correlation analysis. Behav Brain Res. 102: 73-88 (1999).
- Kaplan M, Aviram M, Hayek T. Oxidative stress and macrophage foam cell formation during diabetes mellitus–induced atherogenesis: Role of insulin therapy. Pharm Ther. 136: 175-185 (2012).
- Weseler AR, Bast A. Oxidative stress and vascular function: implications for pharmacologic treatments.Curr Hypertens Rep. 12: 154-161 (2010).
- Price J, Verma S, Li K. Diabetic heart dysfunction: is cell transplantation a potential therapy? Heart Fail Rev. 8: 213-219 (2003).
- Butterfield DA, Howard BJ, Yatin S, Allen KL, Carney JM. Free radical oxidation of brain proteins in accelerated senescence and its modulation by N-tert-butyl-α-phenylnitrone. Proceed Nat Acad Sci. 94: 674-678 (1997).
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer›s disease brain: central role for amyloid β-peptide. Trend Mol Med. 7: 548-554 (2001).
- Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Neurobehavioral aspects of antioxidants in aging. Inter J Develop Neurosci. 18: 367-381 (2000).
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. The J Neurosci. 19: 8114-8121 (1999).
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 26: 1044-1080 (2004).
- Phillips S, Phillips A. Diabetes, dignity and cognitive impairment. Nurs Resid Care. 14: 370-373 (2012).
- Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 23: 542-549 (2000).
- Kramer L, Fasching P, Madl C, Schneider B, Damjancic P, Waldhäusl W, et al. Previous episodes of hypoglycemic coma are not associated with permanent cognitive brain dysfunction in IDDM patients on intensive insulin treatment. Diabetes. 47: 1909-1914 (1998).
- Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 16: 93-112 (2001).
- Haas EM, James P. More Vegetables, Please! Delicious Recipes for Eating Healthy Foods Each & Every Day. Oakland, California: New Harbinger Publications. p.22 (2009).
- Hall IR, Stephenson SL, Buchanan PK, Yun W, Cole ALJ. Edible and Poisonous Mushrooms of the World. Portland, Oregon: Timber Press (2003).
- Collins RA, Ng TB. Polysaccharopeptide from Coriolus versicolor has potential for use against human immunodeficiency virus type 1 infection. Life Sci. 60: 383-387 (1997).
- Nammi S, Boini MK, Lodagala SD, Behara RB. The juice of fresh leaves of Catharanthus roseus Linn. reduces blood glucose in normal and alloxan diabetic rabbits. BMC Complement Altern Med. 4: (2003).
- Han C, Liu T. A comparison of hypoglycemic activity of three species of basidiomycetes rich in vanadium. Biol Trace Elem Res. 127: 177–182 (2009).
- Georges MH. Healing Mushrooms. Square one publisher. New York, USA, 2007.
- Zaidman B, Yassin M, Mahajana J, Wasser SP. Medicinal mushrooms modulators of molecular targets as cancer therapeutics. Appl Microbiol Biotechnol. 67: 453–468 (2005).
- Jong SC, Birmingham JM. Medicinal benefits of the mushroom GanodermaAdv Appl Microbiol. 37: 101–134 (1992).
- Maua JL, Lina HC, Song SF. Antioxidant properties of several specialty mushrooms. Food Res Int. 35: 519–526 (2002).
- Cowley RC, Bennett FC. Vinca rosea. Austr J Pharm. 9: 61 (1928).
- Nair PC, Santi TN. “Lochnera rosea as a potential source of hypotensive and other remedies,” Bulletin of Research Institute of the University of Kerala 1: 51–54 (1959).
- Kaur T, Pathak C, Pandhi P, Khanduja K. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogni 67: 25-30 (2008).
- Sharifzadeh M, Tavasoli M, Naghdi N, Ghanbari A, Amini M and Roghani A. Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats. J Neurochem. 95: 1078-1090 (2005).
- Kaplan M, Aviram M and Hayek T. Oxidative stress and macrophage foam cell formation during diabetes mellitus–induced atherogenesis: Role of insulin therapy. Pharm Ther. 136: 175-185 (2012).
- Nie G, Jin C, Cao Y, Shen S, Zhao B. Distinct effects of tea catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. Arch Biochem Biophys. 397: 84-90 (2002).
- Sano M, Takahashi Y, Yoshino K, Shimoi K, Nakamura Y, Tomita I, et al. Effect of tea (Camellia sinensis L.) on lipid peroxidation in rat liver and kidney: a comparison of green and black tea feeding. Biol Pharm Bull. 18: 1006 (1995).
- Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. The J Nut Biochem. 12: 404-421 (2001).
- Gispen WH, Biessels G-J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 23: 542-549 (2000).
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol.108: 101-106 (1998).
- Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 239: 70-76 (1996).
- Yatin SM, Aksenov M, Butterfield DA. The antioxidant vitamin E modulates amyloid β-peptide-induced creatine kinase activity inhibition and increased protein oxidation: implications for the free radical hypothesis of Alzheimer›s disease. Neurochem Res. 24: 427-423 (1999).
- Frei B, Higdon JV. Antioxidant activity of tea polyphenols in-vivo: evidence from animal studies. The J Nut. 133: 3275-3284 (2003).
- Assaei R, Mostafavi-Pour Z, Pajouhi N, Omrani GH, Sepehrimanesh M, Zal F. Effects of essential oil of Satureja khuzestanica on the oxidative stress in experimental hyperthyroid male rat. Vet Res Form. 6: 233 (2015).
- Maua JL, Lina HC, Song SF. Antioxidant properties of several specialty mushrooms. Food Res Int. 35: 519–526 (2002).
- Huang LN. The mysterious folk medicinal fungus Inonotus obliquus. Edible Fungi China 21: 7-8 (2002).
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proceed Nat Acad Sci. 93: 4765-4769 (1996).
- Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277: 34933-34940 (2002).
- Bindhu RR, Renitta E, Lakshmi PM, Issac R, Naidu S. Evaluation of antidiabetic potential of oyster mushroom(Pleurotus ostreatus) in alloxan-induced diabetic mice. Immunopharm Immunotoxicol. 35: 101–109 (2013).
- Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 4: 18 (2004).
- Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 83: 109-116 (2002).
- Karami M, Sepehrimanesh M, Koohi-Hosseinabadi O, Fattahi M, Jahromi IR, Mokhtari M, et al. Therapeutic Effects of Hydroalcoholic and Aqueous Extracts of Berberis Vulgaris Fruits in Streptozotocin Induced Type 1 Diabetes Mellitus Rats. Rom J Diab Nutr Metabol Dis. 23: 239-245 (2016).
- Abolfathi AA, Mohajeri D, Rezaie A, Nazeri M. Protective Effects of Green Tea Extract against Hepatic Tissue Injury in Streptozotocin-Induced Diabetic Rats. Evid Based Compl Alter Med. 1-10 (2012).
- Popoviç M, Biessels GJ, Isaacson RL, Gispen WH. Learning and memory in streptozotocin-induced diabetic rats in a novel spatial/object discrimination task. Behav Brain Res. 122: 201-207 (2001).
- Shojaee SS, Vahdati A, Assaei R, Sepehrimanesh M. Effect of Galega officinalis leaf powder and Trigonella foenum-graecum seed powder on blood glucose levels and weight gain in a diabetes mellitus rat model. Compar Clin Pathol. 24: 145-148 (2015).
- Flood JF, Mooradian AD, Morley JE. Characteristics of learning and memory in streptozocin-induced diabetic mice. Diabetes. 39: 1391-1398 (1990).
- Tanideh N, Jamshidzadeh A, Sepehrimanesh M, Hosseinzadeh M, Koohi-Hosseinabadi O, Najibi A, et al. Healing acceleration of acetic acid-induced colitis by marigold (Calendula officinalis) in male rats. Saud J Gastroent: offic J Saudi Gastroent Assoc. 22: 50 (2016).
- Huang HJ, Liang KC, Chen CP, Chen CM, Li HM. Intrahippocampal administration of impairs spatial learning and memory in hyperglycemic mice. Neurobiol Learn Memory. 87: 483-494 (2007).
- Hafezi H, Vahdati A, Sepehrimanesh M. Effect of Satureja khuzestanica Jamzad extract on serum lipid profile, blood glucose level and body weight gain in diabetes mellitus: a Rattus norvegicus model. Compar Clin Pathol. 24: 1033-1037 (2015).
- Koohi-Hosseinabadi O, Moini M, Safarpoor A, Derakhshanfar A, Sepehrimanesh M. Effects of dietary Thymus vulgaris extract alone or with atorvastatin on the liver, kidney, heart, and brain histopathological features in diabetic and hyperlipidemic male rats. Compar Clin Pathol. 24: 1311-1315 (2015).
- Mardani M, Afra SM, Tanideh N, Andisheh Tadbir A, Modarresi F, Koohi‐Hosseinabadi O, et al. Hydroalcoholic extract of Carum carvi L. in oral mucositis: a clinical trial in male golden hamsters. Oral dis. 22: 39-45 (2016).
- Harman D. The aging process. Proceed Nat Academy Sci. 78: 7124-7128 (1981).
- Oler JA, Markus EJ. Age-related deficits on the radial maze and in fear conditioning: hippocampal. Hippocampus. 8: 402-415 (1998).