Device Evaluations - Interventional Cardiology (2013) Volume 5, Issue 2
Evia HF (-T): the worlds first magnetic resonance approved pace-maker for resynchronization therapy
- Corresponding Author:
- Valeria Calvi
Cardiology Department, Ferrarotto
Hospital, University of Catania
Catania, Italy
Tel: +39 059 7436206
Fax: +39 095 362429
E-mail: valcalvi@unict.it
Abstract
Keywords
cardiac implantable electronic devices, cardiac resynchronization therapy, heart failure, MR-conditional, MRI
The use of MRI for diagnostic purposes has dramatically increased in many clinical applications. The technique makes use of intense static magnetic fields and is based on absorption and emission of nonionizing radiations in the radio wave frequency range. The main strengths of magnetic resonance (MR) as a particularly appealing noninvasive diagnostic tool, which has been precluded to patients with cardiac implantable electronic devices (CIED) up to date, are high resolution images and that there is no need for contrast medium. CIED patients and subjects indicated for MRI or for whom it may be helpful are two growing and overlapping populations raising an increasingly evident medical, organizational and financial issue to healthcare providers.
The first implantable pacemaker (PM) and lead system certified for MRI under particular operating conditions, including body area restraints, was placed on the market in 2008. Since that date, technological development has been driven towards two main directions: reducing the limitations for MR operations in the presence of a PM system; and expanding the portfolio of MR-conditional CIED models, while refining material and technical characteristics so as to make them indistinguishable from conventional devices. On one hand, within 4 years, PM systems allowing total-body MRI scans were made available; while on the other hand, MR-conditional single-, dual and triple-chamber implantable cardioverter defibrillators (ICD) have recently been brought to the market. The latest CIED model completing the portfolio of MR-conditional CIEDs is a triple-chamber PM system, allowing cardiac resynchronization therapy (CRT) with a PM option (CRT-P) for the treatment of symptoms of heart failure (Evia HF[-T], BIOTRONIK SE & Co. KG, Berlin, Germany). This device received a European Conformity Mark on the 29 March 2012. Furthermore, clinical data are currently being collected to confirm safety and efficacy in an ongoing clinical investigation sponsored by BIOTRONIK [101]. Adverse events that occur during standard clinical practice, including those occurring during clinically driven MRI examinations, are the primary end point of this study, whose results will be used to obtain approval outside of the European Conformity countries.
Overview of the market & clinical utility of MR-conditional devices
The high-magnitude static fields of current superconductive magnets (generally 1.5 T) and the computing capacity of modern computers have largely overcome the initial limits of MRI, which provides superior soft-tissue contrast with 3D high-resolution images, not impaired by bones and without the need to expose subjects to ionizing radiation or contrast agents. These unquestionable benefits have made MRI one of the most widespread methods of imaging, and the first-line choice in several neural and musculoskeletal disorders, within oncology and, more recently, cardiology. As a result, according to data sets of the Organization for Economic Cooperation and Development (OECD), 51.1 MR exams were performed on average per 1000 population in 2010. In the USA, the frequency of MRI scans nearly tripled from 34.2 per 1000 population in 1995 to 97.7 in 2010, which equals approximately 30 million MRI scans each year [102].
On the other hand, the prevalence of patients with CIED has constantly increased over the last few decades [1,2]. In the USA, the estimated prevalence of PM and ICD implants in 2009 was approximately 1.2 per 1000 population. CIED patients often share morbidities and manufacturers have estimated a 50–75% probability of a CIED patient being indicated for a MRI scan over the lifetime of their device. Further estimations suggested that in the USA, approximately 200,000 patients could have benefited from MRI in 2004 [3].
The growing epidemiological and clinical issue of MR compatibility with CIEDs has led many authors to question whether MR is also generally safe in patients with conventional devices. This is reflected by the numerous reports, publications, editorials and tutorials available on this topic. A simple PubMed search, performed in August 2012, returned 616 articles containing the words ‘pacemaker(s) MR imaging’. This is quite a surprising number considering that MRI is normally contraindicated in patients with implanted cardiac PMs. There are reports of patients with a conventional CIED who were exposed to MRI, either intentionally or inadvertently. In the former case, humanitarian reasons or ethical aspects are often behind the dilemma for a physician of whether or not to intentionally expose a patient with a conventional CIED to the risks of MR; in the latter case, serious questions are raised on the safety of current referral processes of patients undergoing MR examinations [4]. To date, severe adverse outcomes have not been documented for conventional CIED patients scanned in an MRI unit while being appropriately monitored with cardiac supervision. However, caution is required; device function changes may occur and have actually been documented, even in relatively small populations. In addition, several deaths of patients with conventional CIEDs (some of whom were not PM-dependent) have been reported during MR examinations or immediately thereafter [5], supposedly due to arrhythmias or other causes related to interactions between the implanted device-lead system and the electromagnetic fields used during the MRI process. Therefore, skepticism towards unrestricted MRI of patients with conventional CIEDs is reasonable. The American College of Radiology confirmed in 2004 [6], and reiterated in 2007 [7], the contraindication of routine MR in implanted cardiac PMs and/or ICD. As Gimbel agreeably argued, the correct answer to the question ‘what are the critical elements of safe scanning?’ is still ‘ask me at 10,000’ scans of conventional CIEDs [8].
To avoid unethical and unjustified risks, specifically designed CIED systems should be used in MR, especially now that, with the Evia HF(-T), the set of MR-conditional device types has recently been completed with the addition of PMs with the biventricular-pacing option (CRT-P). There are no references in the literature to previous experiences of conventional CRT-P devices used in the MR environment. This is probably due to a lower increase in the number of CRT-P implants with respect to CRT devices with ventricular defibrillation option (CRT-D) in recent years. In Europe, the number of CRT-P implants grew from 23 per million population in 2005 to 31 per million population in 2010, compared with CRT-D implants that grew from 37 per million population to 100 per million population with the same timespan [9]. However, the latest update of the guidelines on device therapy in heart failure does not provide preferential recommendations between the CRT-P or CRT-D options to reduce morbidity and mortality in the New York Heart Association (NYHA) class III and IV heart failure, unless in secondary prevention [10]. The remarkable cost difference between CRT-P and CRT-D (particularly appealing in the economic downturn) has recently favored the former, which has more than doubled the annual growth rate in 2010 compared with 2009 in Europe (8–19%) [9].
In summary, the growing number of subjects with CIEDs and their morbidities are leading to an increasing need for a complete portfolio of CIEDs specifically developed for safe use in MR (under certain restrictive conditions), and it is desirable that they will become part of normal CIED implant practice as early as possible.
Introduction to the device
Evia HF(-T) is the first and, at present, sole CRT-P device specifically designed to be safely used in MR under specific conditions when connected with appropriate leads (MR-conditional). It is indicated to reduce mortality and morbidity in patients suffering from mild-to-severe chronic congestive heart failure according to current guidelines and to reduce related symptoms through resynchronization of both ventricles.
The technical innovations implemented in the device are esentially the same that make the entire Evia PM family (which also includes the single- and dual-chamber version) MR-conditional. Once the safe use in MR under specific conditions has been technically obtained and certified for one- or two-lead pacing systems, only a minor technological step is required to achieve the same result for a three-lead CRT system concerning the left ventricle pacing lead.
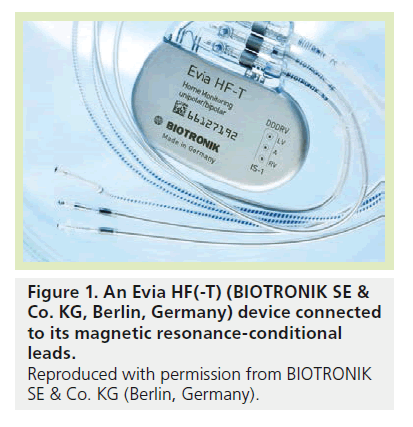
The device is apparently indistinguishable from any other conventional CRT-P system: a f lattened ellipsoidal-sealed titanium can with a volume of 14 cm3 and a mass of 27 g (Figure 1), with a battery capacity ensuring a longevity in the range of 4.6–12.2 years, according to specific operation conditions. The internal circuitry of the device was changed with respect to conventional versions to prevent MR forces from disrupting operation: ferromagnetic components were reduced to decrease susceptibility to magnetic attraction and the reed switch (which allows the device to switch to the magnet or programming status) was replaced by a Hall sensor (whose behavior in static magnetic fields is predictable). The internal conducting paths were shortened and optimized providing reduced field-coupling surfaces. In addition, the design was modified to accommodate gradient energy induced into the device and to minimize gradient field energy coupled to the lead tip, thereby reducing the potential for gradient-induced cardiac stimulation. The Evia HF(-T) is characterized by a full set of diagnostics and pacing therapy options, normally expected in a triple-chamber PM, and no functionality limitations are related to the MR-conditional feature.
As a CRT-P device, the Evia HF(-T) is normally connected with three cardiac leads conventionally positioned in the right atrium, right ventricle and left ventricle through a side branch of the coronary sinus. The lead design is crucial for a MR-conditional CIED system, as it is through lead wires that radiofrequency (RF) and passive current interfere with normal system operation. Only specific lead models can be connected to the Evia HF(-T) to make the entire system MR-conditional: the atrial and ventricular (passive or active fixation) leads are the same versions that are compatible with the single-or dual-chamber MR-conditional Evia models, already available since 2010. The Corox OTW ProMRI® left ventricle lead family has been recently introduced. These 5.8 Fr bipolar leads are characterized by a coradial internal structure with two wires per coil isolated with silicone and coated with a 0.1-mm thick polyurethane layer from the connector up to 4–7 cm from the tip (depending on the model), to reduce friction. Three different curves are available to facilitate navigation in the coronary sinus branches and fixations, as well as two length versions (75 or 85 cm). The leads are characterized by a low self-inductance coefficient to detune the lead with gradient and RF fields, including lead-tip heating. This is achieved by the internal wire geometry. No other electronic circuital elements or particular constructive materials were introduced. In our experience, the leads showed a satisfying lead maneuverability practically indistinguishable from the conventional versions.
According to the definitions provided by the American Society for Testing and Materials F2503, currently recognized by the US FDA and the European Notified Body, devices should be classified and labeled according to the following set of terms:
•• MR-safe: an item that poses no known hazards in all MR environments. Using the terminology, ‘MR-safe’ items are nonconducting, nonmetallic and nonmagnetic items;
•• MR-conditional: an item that has been demonstrated to pose no known hazards in a specified MR environment with specified conditions of use. ‘Field’ conditions that define the MR environment include static magnetic field strength, spatial gradient magnetic field, dB/dt (time rate of variation of the magnetic field), RF fields and specific absorption rate;
•• MR-unsafe: an item that is known to pose hazards in all MR environments.
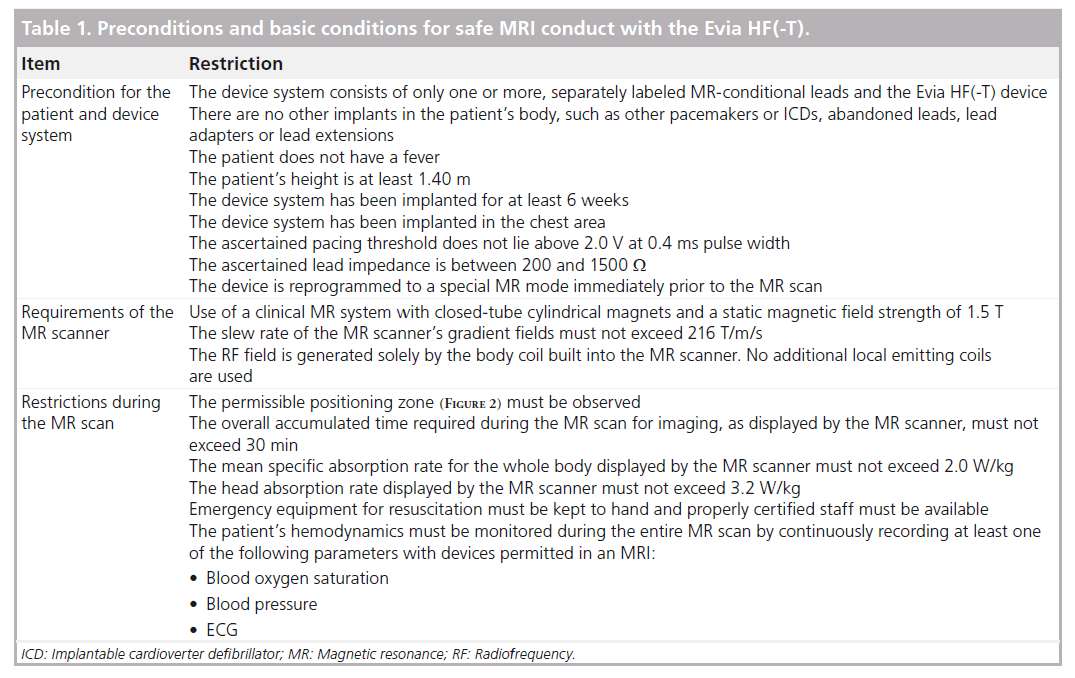
With this terminology, there are no MR-safe CIEDs available to date; all devices are ‘MR conditional’. This means that to safely perform MRI, specific conditions must be met that the manufacturers set and precisely state in the instruction-for-use manual. Certification for safe use during MRI scans holds only under these conditions; medical and legal responsibility lays entirely on the attending physician outside these conditions that apply to the patient, the MR scanner and the procedures. For the Evia HF(-T) device the following holds (Table 1):
•• Concerning the patient, the implanted device must be connected with leads provided by the Evia HF(-T) manufacturer labeled as MR conditional (of note, only one lead model for right atrium and ventricle may be connected to the device, in order to constitute an MR-conditional device system: the combination of different lead models in a single-device system has not been tested to be MR conditional); there are no other abandoned implanted systems (inactive PM/ICD leads or fractured portions of leads, adapters and extensions, among others); the patient is at least 1.40 m tall; the device implant dates back to at least 6 weeks before the MR scan; the device was implanted into the chest; the pacing thresholds have been ascertained not to be higher than 2.0 V with 0.4 ms of pulse duration, with impedances comprised between 200 and 1500 W;
•• Concerning the MR scanner, the magnetic field must be generated by closed-tube, cylindrical magnets; the field magnitude is equal to 1.5 T (neither higher nor lower); the slew rate of the MR scanner’s gradient fields must not exceed 216 T/m/s and no additional local emitting coils are used;
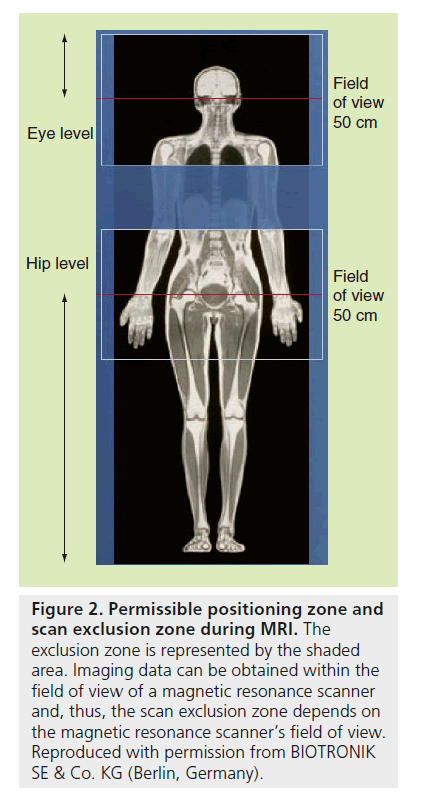
•• Concerning the MR scan procedure, it must be only performed with the patient in the dorsal position; the mark for the scanner isocenter can be positioned anywhere above the level of the eyes or below the hip bone level (Figure 2), so as to exclude the thoracic zone, approximately comprised between T1 and L4 vertebrae, from imaging; the overall accumulated time required during the MR scan for imaging must not exceed 30 min; mean specific absorption rate (SAR) must not exceed 2.0 W/kg for the whole body, and 3.2 W/kg for the head; emergency equipment for resuscitation must be available for prompt use by staff with expertise and the patients’ hemodynamics should be monitored during the MR scan.
Figure 2. Permissible positioning zone and scan exclusion zone during MRI. The exclusion zone is represented by the shaded area. Imaging data can be obtained within the field of view of a magnetic resonance scanner and, thus, the scan exclusion zone depends on the magnetic resonance scanner’s field of view. Reproduced with permission from BIOTRONIK SE & Co. KG (Berlin, Germany).
It is also mandatory that a cardiologist performs an accurate follow-up with a complete interrogation and test of the device immediately before and after the MR scan. During the initial visit, the device must be temporarily programmed in a special MR-specific mode: pulse amplitudes and widths are set at 4.8 V and 1.0 ms, respectively, the pacing rate at 80 beats per min (if it is not switched off) and all the additional automatic functions and magnet response are suspended. Pacing may be set to off or on in a single- or dual-chamber asynchronous mode, according to the patient’s specific indication. The MR mode must be terminated and the original program restored by the cardiologist once the MR examination is complete. The Evia HF(-T) version with the remote-monitoring option is provided with a useful function automatically alerting the physician that a MR mode has been activated in a device unit, allowing remote monitoring of the correct recovery of the normal operating status.
It is worth noting that in the technical manual for safe application of MR scan on BIOTRONIK MR-conditional devices (including the Evia HF[-T]), it is initially reported in the Intended Medical Use section that “there must be a clear indication for the MR scan,” meaning that “there is no doubt as to the predictable diagnostic benefit of the MR scan and that comparable results can-not be achieved with other less risky procedures. A risk/benefit analysis” must have been performed and “all of the exclusion criteria listed in this technical manual have been taken into consideration. The described restrictions and conditions for the MR scan are to be observed at all times.” These important statements clarify the manufacturer’s perspective: residual risks for device patients undergoing MRI always exist, even if, under the specified conditions, they are minimal, acceptable and the manufacturer is responsible for device noncompliance. Interestingly, similar statements are not reported in competitors’ MR-conditional technical manuals. However, risks can never be totally eliminated; therefore, if alternative diagnostic solutions are available, responding to the same need as effectively as MR would do, a careful risk/benefit analysis of the available options should be performed prior to MRI even for a patient with a MR-conditional system.
Clinical profile
Despite the impressive results of several large clinical trials on the benefit of CRT in the treatment of drug-refractory heart failure, the prevalence of responder patients is approximately 70%. MR (especially cardiac MR) is becoming an increasingly important diagnostic tool to effectively select appropriate patients who are most likely to benefit from CRT, and several specific MR techniques have been developed to study the left ventricle and interdelay in ventricular contraction [11]. These techniques may well be used in the near future to evaluate and optimize the effect of CRT postimplant and during follow-up, if MR-conditional CRT devices are made available in routine practice, allowing full-body scans and heart scans without lead-induced artefacts. The Evia HF(-T) may represent a first step towards this objective, even if it is not certified for safe use with cardiac MR and MR scan in the thoracic zone.
In general, there are limited data for randomized clinical studies on MR-conditional devices. To our knowledge, only one randomized study on MR-conditional dual-chamber PMs has been published so far, investigating MRI-related complications [12]. This study did not report sustained ventricular arrhythmias, PM inhibitions or output failures. Electrical resets, or other PM malfunctions, occurred during or after MR scans. The study randomized patients to undergo a nonclinically indicated MRI scan between 9 and 12 weeks post-implant or not to undergo MRI. No significant differences were detected between study groups in terms of MR-procedure complication-free rate and pacing capture thresholds or sensed electrogram amplitudes. These results represent a first indication that MRI-conditional systems can be safe when correctly used under the conditions specified by the manufacturers.
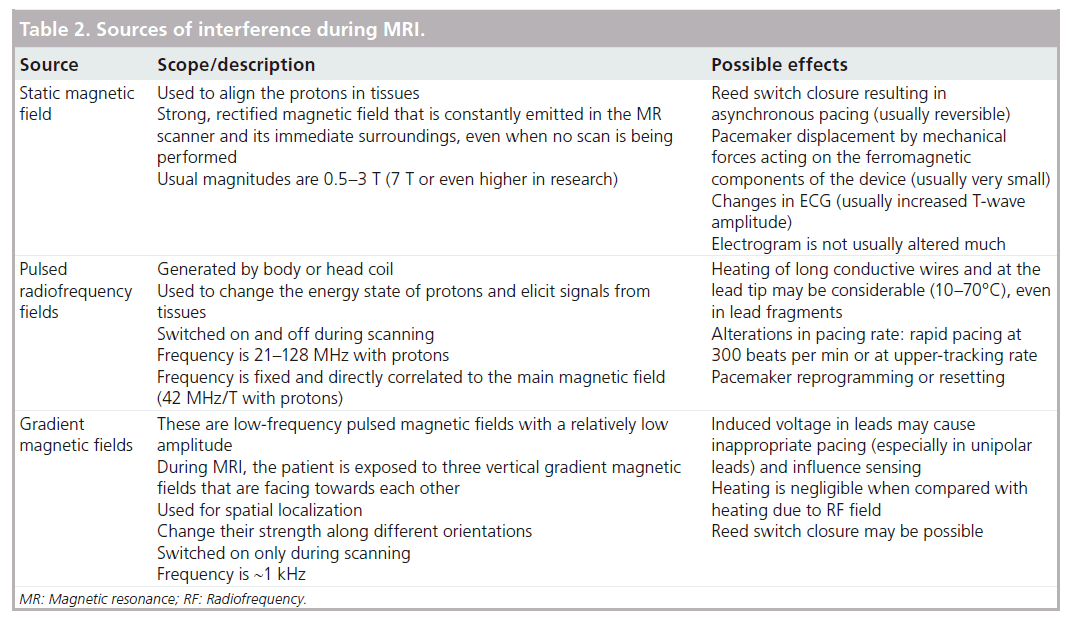
The overwhelming majority of the published in vitro or in vivo studies to investigate the MR-induced interferences included conventional devices, which represent an increasingly important topic due to broadening MRI applications, increasing CIED patient number and an aging population [13]. MR interferences with CIEDs is a very complex topic, involving a huge number of degrees of freedom resulting in largely unpredictable effects. It is now sufficiently clear that the main sources of interference are all of the principal fields normally used during MR examinations (Table 2): the main static magnetic fields, RF fields and time-varying magnetic field gradients.
The main static magnetic field that is used to align protons is particularly intense and always present, even when the scanner is not imaging. Current MR systems normally generate 1.5 T fields (~30,000-times more intense than the Earth’s natural magnetic field), but can also reach 3 T in more modern machines. Of course, ferromagnetic components of implanted pacing systems are particularly sensitive to the static field and it has been theorized that force and torque may be generated, possibly causing device displacements. However, several studies performed at 1.5 T found that force and torque were negligible in PMs and barely perceptible in ICDs, with newer devices exerting one-fifth of the force exerted by older (before 1998) devices [14,15]. Another immediate effect of the static field on a conventional PM is the closure of the reed switch initiating asynchronous pacing, which may cause arrhythmias. The supposed permanent magnetization of the reed switch was never observed in in vitro studies and the risk may be minimized by proper reprogramming of the magnet mode or eliminated by the implementation of the Hall sensor in modern devices.
The most significant risk while conducting MRI in conventional CIED systems is the heating of the lead tips, mainly due to the modulated high-power RF pulses used during MR to excite protons to elicit signals from tissues. The technical difficulty in measuring heating in an MR environment mostly explains the controversial data reported in the literature on this aspect. Many in vitro studies reported temperature increases at the tip leads ranging from 7 (negligible) to 63°C (remarkable), depending on the scan duration, the type of electrodes, the SAR level and implant geometry [14,16–18]. In addition, in vivo studies presented conflicting data: Roguin et al. did not find any rise in lead tip temperatures, even after prolonged scan sessions (3–4 h) and high MRI energy protocol (SAR of 4 W/kg) [14]; on the other hand, Luechinger et al. recorded temperature increases of up to 20°C with thermocoupled sensors, but with only minor stimulation threshold changes (<0.5 V) and no pathological and/or histological heat-induced damage [19]. More rarely, RF pulses may cause alteration in pacing rate with high-frequency pacing or inhibitions.
Time-varying magnetic field gradients are used during MRI scans for spatial encoding. They generally change their strength along different orientations and are switched on only during scanning. Their characteristic operating frequency lies in the order of kHz and can easily be coupled to the operating frequencies of the PM, inducing voltage in pacing leads that may cause inappropriate pacing (especially in unipolar leads) and influence sensing, causing inappropriate pacing, oversensing or even asystoles.
Zikria et al. completed an accurate search of the medical literature on MRI in patients with conventional or MR-conditional PMs [20]. Their review included publications of in vivo human studies encompassing 491 MR examinations performed in patients with conventional PMs. No deaths were reported, but only 49% of cases had no significant changes in PM function after MR examinations. Serum troponin-I level alteration with pacing threshold increase was also observed in some patients, possibly due to thermal injuries at the ventricular lead tip. Reed switch closure with consequent asynchronous pacing often occurred and minor changes were observed in battery charge (temporary decreases, followed by complete recovery), lead impedance and pacing threshold (however, no critical values were found). In addition, patient symptoms were reported during scanning. These data suggest that it may well be possible to perform MR examinations on patients even with conventional cardiac PMs at least as long as guidelines were carefully defined and rigidly adhered throughout the imaging process. However, we must bare in mind that risks are never entirely eliminated and only MR-conditional devices are certified to be safely scanned under the specific conditions set by the manufacturer.
Data on the behavior of conventional devices in MR environments are precious [21], since MRI scanning of patients with conventional or even pre-existing or previously abandoned devices still represents a challenging medical decision, involving a difficult assessment of the risk/benefit ratio on an individual basis, especially when potential life-threatening scenarios have to be confirmed or excluded [22–24]. A CIED system is no longer MR conditional if it is implanted in a patient in whom other implanted operating or abandoned devices, leads or fragments are present. A MR-conditional devices cannot be simply connected to pre-existing leads and maintain the MR-conditional certification. Therefore, the decision of whether to proceed to MRI, exposing the patient to the risk of MR scan with an unsafe system, or to extract (residual parts of) pre-existing pacing systems, has to be adapted by carefully evaluating the risk/benefit balance for both options. The latter option should only be considered in the unlikely cases where the significant complications related to system extraction and replacement, including vascular damage, cardiac perforation and infection, among others, are outweighed by the expected benefit of MRI and when MRI can not be effectively replaced by other imaging techniques.
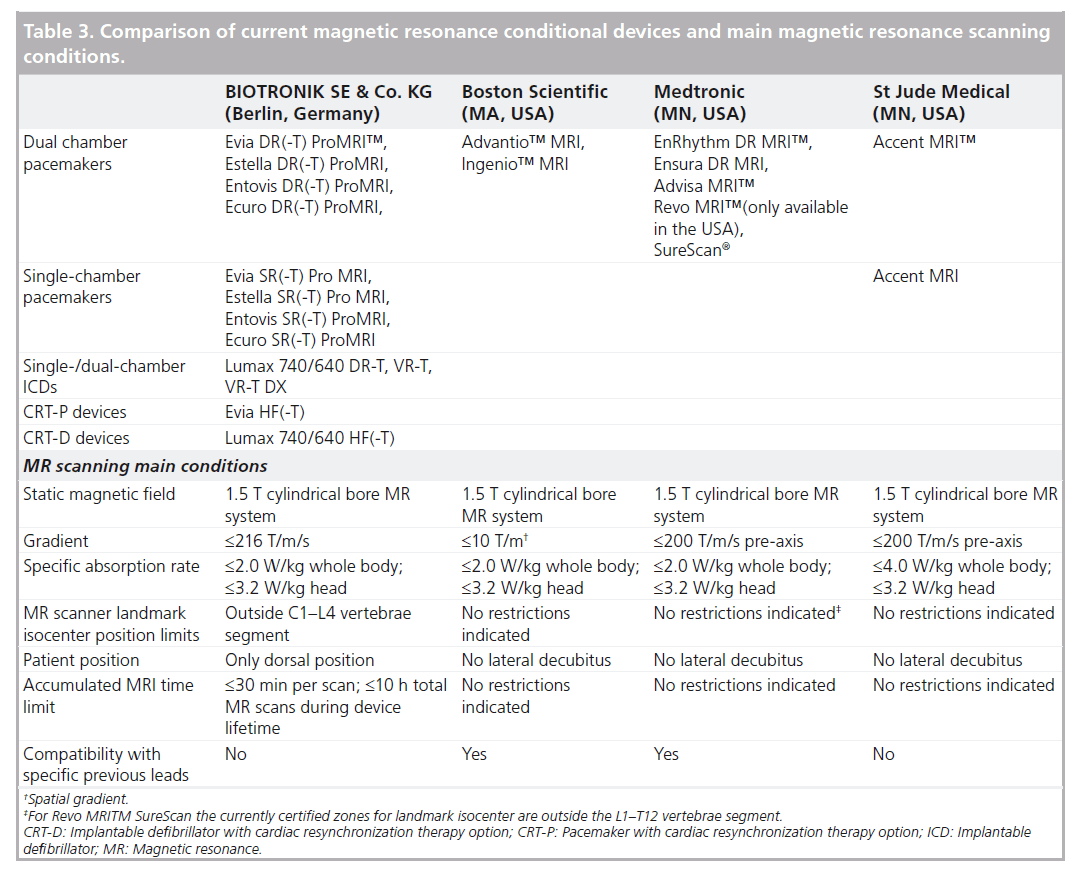
To summarize, the conditions under which MR-conditional systems can be safely scanned may partially limit the access to MRI (see Table 3 for a synoptic comparison between manufacturers). Manufacturers share most conditions, regardless of the device model. Differences do not necessarily reflect a worse performance, but may be due to a different manufacturer’s approach during tests for certifications. In particular, BIOTRONIK is the only manufacturer still not relapsing the restrain of the body-exclusion zone in MR-conditional devices, including the Evia HF(-T) PM. The competing manufacturers’ MR-conditional dual-chamber PMs allow total-body scans, but do not provide the CRT option for the treatment of congestive heart failure.
Table 3. Comparison of current magnetic resonance conditional devices and main magnetic resonance scanning conditions.
It is estimated that the most frequent anatomic locations for MR scans are the head, neck, cervical and lumbar spine, hip and extremities, accounting for more than 88% of all of the MR examinations performed in Germany in 2011 [103]. However, these estimates are based on unselected cohorts and, thus, may be inaccurate for heart failure patients with an indication for CRT. Requests for thoracic MRI are expected to increase in the near future with the growing applications of cardiac MR in the diagnosis and assessment of several cardiac diseases including myocardial infarction, myocarditis [25] and heart failure [26]. This further urges the industry to overcome the technical issues that hinder the use of cardiac MRI for those patients with heart disease requiring CRT. This may be a limit for the Evia HF(-T), which, at present, is not certified for cardiac MR, even if it is anticipated that next-generation MR-conditional CRT-P devices will shortly relapse the thoracic exclusion zone and will subsequently allow the use of cardiac MR, which is currently considered the gold standard with respect to accuracy and reproducibility of volumes, mass and wall motion. In this respect, it is also important to mention that imaging quality concerns also represent an issue for MR-conditional devices allowing total-body scans. Such devices are certified for conditional use in MR without body restriction, but they are not ‘transparent’ to MR scanners. The presence of the leads may cause variations in the surrounding magnetic field with consequent loss of resonance conditions. This generally results in image distortion, signal voids or bright areas [23]. This is a challenging objective for future technological development, especially for heart failure patients with CRT devices, limiting the steps toward the objective of developing CIEDs indistinguishable in their functionality from conventional devices and that place few safety limitations on MR (particularly cardiac MR).
Alternative devices
There are no other MR-conditional CRT-P competing systems to date. Other competing systems are MR conditional and only provide antibradycardia therapies without the CRT option.
How the technology fits into the field of medical devices
The Evia HF(-T) may be implanted in all of the indications for CRT, unless there is indication to an ICD, according to current guidelines [10]. It may, therefore, be considered to reduce mortality, morbidity, heart failure hospitalizations or prevent disease progression in several subclasses of heart failure patients, primarily in:
•• NYHA class III/IV heart failure patients on optimal therapy in sinus rhythm, with a left ventricle ejection fraction (LVEF) ≤35% and with a QRS duration of ≥120 ms and left bundle branch block–QRS morphology (class of indication I, level of evidence A) or ≥150 ms irrespective of QRS morphology (class of indication IIa, level of evidence A), who are expected to survive with good functional status for >1 year;
•• NYHA class II heart failure patients on optimal therapy in sinus rhythm, with a LVEF ≤30% and with a QRS duration of ≥130 ms and left bundle branch block–QRS morphology (class of indication I, level of evidence A) or ≥150 ms irrespective of QRS morphology (class of indication IIa, level of evidence A), who are expected to survive with good functional status for >1 year.
In addition, the Evia HF(-T) may also be considered in NYHA class III/IV heart failure patients with permanent atrial fibrillation, a QRS duration of ≥120 ms and a LVEF of ≤35%, in the presence of intrinsically slow ventricular rate or PM dependence as a result of atrio–ventricular node ablation and in all the current class I pacing indications in the presence of a LVEF of ≤35%, NYHA class III/IV (even NYHA class II, but with conflicting opinions).
In NYHA class II heart failure patients with LVEF ≤35% and QRS duration ≥150 ms, a CRT device with defibrillation function should be preferred.
Currently, the Evia HF(-T) is only available in European Conformity-approved countries (European Community, Switzerland) and not yet in the rest of the world.
Conclusion
In light of the available data from clinical trials it is in this author’s opinion that with the Evia HF(-T), the set of MR-conditional CIEDs is complete. Therefore, for any indication to pacing, for example sudden cardiac death prevention and cardiac resynchronization for the treatment of heart failure, an appropriate device is now available that can be safely used in an MR environment, even if under some operating limitations. On the one hand, this technological advancement is a valuable opportunity, but on the other hand, it raises some ethical issues: is it appropriate to extend this technology to all patients indiscriminately? Should careful patient selection be the most reasonable option? Who is indicated to receive a MR-conditional device?
How to select patients who are at the highest risk to undergo a MR scan in their life?
Future perspective
In our opinion, these questions will lose significance in the coming years. In other words, we believe we are experiencing a paradigm shift in the world of pacing and hopefully, within the next few years, all of the commercially available devices will be MR conditional. From this perspective, reducing the MR-operational limitations of current devices may be considered of secondary importance. In our view, it is of utmost importance that MR conditional – even with current limitations – becomes a standard feature of CIED systems.
Executive summary
Device description
•• The Evia HF(-T) (BIOTRONIK SE & Co. KG, Berlin, Germany) is the first and, at present, the sole available pacemaker system with the cardiac resynchronization therapy function specifically designed to be safely magnetic resonance (MR)-scanned under specific conditions.
•• It may be implanted to reduce mortality, morbidity or prevent disease progression in several subclasses of NYHA class II–IV heart failure patients.
•• Housing shape is a flattened ellipsoidal-sealed titanium can of 14 cm3 volume and 27 g mass; the expected longevity ranges between 4.6 and 12.2 years.
•• The internal circuitry of the device was changed with respect to conventional versions, to prevent MR forces from disrupting the operation: internal circuitry was optimized, ferromagnetic components were reduced to decrease susceptibility to magnetic attraction and the reed switch (which allows the device to switch to the magnet or programming status) was replaced by a Hall sensor (whose behavior in static magnetic fields is predictable).
Safety
•• The Evia HF(-T) device must be connected to specific MR-conditional leads and there must be no other implants in the patient’s body. For example, other pacemakers or implantable defibrillators, abandoned leads, lead adapters or extensions.
•• After which, a patient with an Evia HF(-T) device can safely undergo MR examinations, provided that specific mandatory conditions are fulfilled.
•• The main conditions are: the magnetic field is generated by closed-tube cylindrical magnets with a magnitude of 1.5 T; gradient fields do not exceed 216 T/m/s; the device implant is in the patient’s chest and dates back to at least 6 weeks before the MR scan with normal electrical performances; the mean specific absorption rate does not exceed 2.0 W/kg for the patient’s body and 3.2 W/kg for the patient’s head.
•• The cardiologist and radiologist must carefully check that all of the conditions are satisfied. The device must be prepared before the MRI procedure and emergency equipment for resuscitation must be available for prompt use by staff with expertise; patient’s hemodynamics should be monitored during the MR scan; and the device must be fully checked after the procedure, the initial programming must be resumed thereafter.
Clinical context
•• The growth and evolution of the MR technique, paralleled by the constantly increasing prevalence of patients with cardiac implantable electronic devices (CIEDs) could result in an estimated 50–75% probability of a patient being indicated for a MR scan over the lifetime of their device. This explains the need for a new technology of MR-conditional CIEDs.
•• This is especially true for patients with indications for cardiac resynchronization therapy due to both the high morbidity of these patients and to the increasing capacity of cardiac MR as a selection and evaluation tool of heart failure patients.
•• There are few data of randomized clinical studies on MR-conditional devices. The Evia HF(-T) has been made available very recently and there are still no reports in the literature. Our first direct implantation experiences did not show significant differences with a conventional cardiac resynchronization therapy with a pacemaker implant option.
•• The majority of the published studies included conventional devices, which represents an increasingly important topic due to broadening MR applications, increasing CIED patient number and the aging population.
•• The main sources of interference are the static magnetic fields, the high-power radiofrequency pulses used to elicit signals and the time-varying magnetic field gradients used for spatial encoding. These interference sources were evaluated in in vitro and in vivo studies with conventional devices, and data suggest that MR scanning may be considered in very special cases and following a strict safety protocol.
•• Complications were reported in several experiences, therefore, MR still remains a contraindication for conventional CIEDs. Only MR-conditional devices should be used in a MR environment and under mandatory conditions. It is desirable that these devices will soon become the standard platform in routine applications.
Acknowledgements
The authors would like thank X Antoniou, BIOTRONIK AG, for her critical review of the manuscript.
Financial & competing interests disclosure
A Gargaro is an employee of BIOTRONIK Italia, an affiliate of BIOTRONIK SE & Co., Berlin, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- Mond HG, Irwin M, Morillo C, Ector H. The world survey of cardiac pacing and cardioverter defibrillators: calendar year 2001. Pacing Clin. Electrophysiol. 27(7), 955–964 (2004).
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009 – a World Society of Arrhythmia’s project. Pacing Clin. Electrophysiol. 34(8), 1013–1027 (2011).
- Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin. Electrophysiol. 28(4), 326–328 (2005).
- Ferris NJ, Kavnoudias H, Thiel C, Stuckey S. The 2005 Australian MRI safety survey. AJR Am. J. Roentgenol. 188(5), 1388–1394 (2007).
- Martin ET, Sandler DA. MRI in patients with cardiac devices. Curr. Cardiol. Rep. 9(1), 63–71 (2007).
- Kanal E, Borgstede JP, Barkovich AJ et al.; American College of Radiology. American College of Radiology white paper on MR Safety: 2004 update and revisions. Am. J. Roentgenol. 182(5), 1111–1114 (2004).
- Kanal E, Barkovich AJ, Bell C et al. ACR Blue Ribbon Panel on MR safety. ACR guidance document for safe MR practices: 2007. AJR Am. J. Roentgenol. 188(6), 1447–1474 (2007).
- Gimbel JR. The safety of MRI scanning of pacemakers and ICDs: what are the critical elements of safe scanning? Ask me again at 10,000. Europace 12(7), 915–917 (2010).
- Dubner S, Auricchio A, Steinberg JS et al. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace 14(2), 278–293 (2012).
- McMurray JJ, Adamopoulos S, Anker SD et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 14(8), 803–869 (2012).
- Ypenburg C, Westenberg JJ, Bleeker GB et al. Noninvasive imaging in cardiac resynchronization therapy – part 1: selection of patients. Pacing Clin. Electrophysiol. 31(11), 1475–1499 (2008).
- Wilkoff BL, Bello D, Taborsky M et al.; EnRhythm MRI SureScan Pacing System Study Investigators. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm 8(1), 65–73 (2011).
- Roguin A, Schwitter J, Vahlhaus C et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace 10(3), 336–346 (2008).
- Roguin A, Zviman MM, Meininger GR et al. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation 110(5), 475–482 (2004).
- Luechinger R, Duru F, Scheidegger MB, Boesiger P, Candinas R. Force and torque effects of a 1.5-Tesla MRI scanner on cardiac pacemakers and ICDs. Pacing Clin. Electrophysiol. 24(2), 199–205 (2001).
- Sommer T, Vahlhaus C, Lauck G et al. MR imaging and cardiac pacemakers: in vitro evaluation and in vivo studies in 51 patients at 0.5 T. Radiology 215(3), 869–879 (2000).
- Achenbach S, Moshage W, Diem B, Bieberle T, Schibgilla V, Bachmann K. Effects of magnetic resonance imaging on cardiac pacemakers and electrodes. Am. Heart J. 134(3), 467–473 (1997).
- Calcagnini G, Triventi M, Censi F et al. In vitro investigation of pacemaker lead heating induced by magnetic resonance imaging: role of implant geometry. J. Magn. Reson. Imaging 28(4), 879–886 (2008).
- Luechinger R, Zeijlemaker VA, Pedersen EM et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur. Heart J. 26(4), 376–383 (2005).
- Zikria JF, Machnicki S, Rhim E, Bhatti T, Graham RE. MRI of patients with cardiac pacemakers: a review of the medical literature. AJR Am. J. Roentgenol. 196(2), 390–401 (2011).
- Shinbane JS, Colletti PM, Shellock FG. Magnetic resonance imaging in patients with cardiac pacemakers: era of ‘MR conditional’ designs. J. Cardiovasc. Magn. Reson. 13, 63 (2011).
- Wollmann C, Grude M, Tombach B et al. Safe performance of magnetic resonance imaging on a patient with an ICD. Pacing Clin. Electrophysiol. 28(4), 339–342 (2005).
- Nazarian S, Roguin A, Zviman MM et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation 114(12), 1277–1284 (2006).
- Goldsher D, Jahshan S, Roguin A. Successful cervical MR scan in a patient several hours after pacemaker implantation. Pacing Clin. Electrophysiol. 32(10), 1355–1356 (2009).
- Gallagher S, Jones DA, Anand V, Mohiddin S. Diagnosis and management of patients with acute cardiac symptoms, troponin elevation and culprit-free angiograms. Heart 98(13), 974–981 (2012).
- Partington SL, Cheng S, Lima JA. Cardiac magnetic resonance imaging for stage B heart failure. Heart Fail. Clin. 8(2), 179–190 (2012).
- Evia/Entovis HF-T Master Study. www.clinicaltrials.gov/ct2/show/ NCT01545739 (Accessed 11 March 2013)
- OECD Health Data 2012. http://stats.oecd.org/index. aspx?DataSetCode=HEALTH_STAT# (Accessed 21 August 2012)
- Barmer GEK Arztreport 2011. www.barmer-gek.de/barmer/web/Portale/ Presseportal/Subportal/Presseinformationen/Archiv/2011/110201-Arztreport-2011/Arztreport-2011-PDF,property=Data.pdf (Accessed 28 August 2012)
• Inspiring and provocative editorial on the reasons for caution in the use of MRI in conventional devices.
• First randomized study on a magnetic resonance (MR) conditional device.
•• Competent and explanatory position paper on the MR in cardiac implantable electronic devices, with a complete literature review and an interesting explanation of several technical aspects of MR and device interference.
•• Complete review of the medical literature on MR of patients with cardiac implantable electronic devices.
•• Excellent review with a complete comparison of MR conditions of different manufacturers’ devices.
▪ Websites