Device Evaluations - Interventional Cardiology (2011) Volume 3, Issue 3
Evidence for mesh-covered stent implantation in ST-segment elevation myocardial infarction
- Corresponding Author:
- Dariusz Dudek
Department of Interventional Cardiology
Jagiellonian University Medical College, Krakow, Poland
Tel: +48 124 247 181
Fax: +48 124 247 184
E-mail: mcdudek@cyf-kr.edu.pl
Abstract
Keywords
embolization, mesh, myocardial infarction, primary angioplasty, stent, thrombus
Primary percutaneous coronary intervention (PCI) is the preferred method for reperfusion in ST-segment elevation myocardial infarction (STEMI) when logistically feasible [1]. Despite stent implantation during primary PCI being effective in epicardial vessel flow restoration, as mirrored by the high incidence of Thrombolysis In Myocardial Infarction (TIMI) grade 3 in the infarct-related artery, impaired myocardial perfusion still occurs, according to low final myocardial blush grade (MBG) and poor complete (>70%) ST-segment resolution achieved in twothirds of patients undergoing urgent PCI [2,3]. Impaired myocardial reperfusion may be caused by distal embolization of thrombus or plaque debris [4,5]. Importantly, patients with impaired myocardial reperfusion are at higher risk of larger irreversible myocardial injury, higher incidence of adverse remodeling of the left ventricle leading to consequent heart failure, as well as at higher risk of death during short- and long-term follow-up [3,4].
Overview of the market
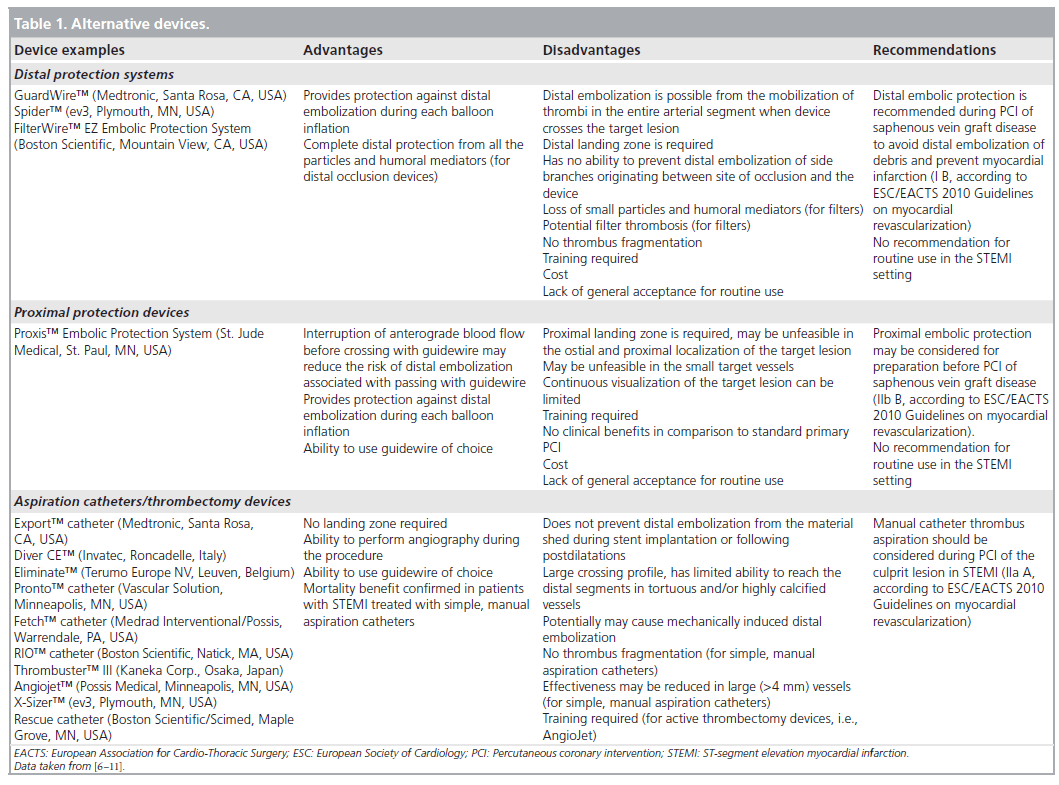
Reduction of the risk of distal embolization during primary PCI for STEMI and improvement of myocardial reperfusion is an issue of special importance. The risk of distal embolization during primary PCI may be reduced by the use of thrombectomy/aspiration catheters and distal protection devices, especially in the setting of high-thrombus burden [6,7]. However, two large randomized trials (the EMERALD [8] and the ASPARAGUS [9] trials) with a distal occlusive device – PercuSurge GuardWire™ (Medtronic) – have failed to show any benefit of distal protection during primary PCI for STEMI. In the PROMISE trial the use of filters (FilterWire EZ™ [Boston Scientific]) was not associated with improvement in myocardial reperfusion and reduction of infarct size (as evaluated by cardiac magnetic resonance) in comparison to conventional PCI [10]. In the pooled analysis of seven trials with distal protection devices, despite benefits in terms of myocardial perfusion, no mortality reduction was observed [7]. The routine use of proximal (e.g., Proxis™ Embolic Protection System [St. Jude Medical]) and distal (e.g., PercuSurge GuardWire and FilterWire EZ) protection is not currently recommended during primary PCI for STEMI [11]. In contemporary clinical practice the usage rate of embolic protection devices during PCIs, even during saphenous vein graft interventions, is low (less than 25% of cases) [12].
On the other hand, in several studies, the use of simple, manual aspiration catheters before stenting during primary PCI was associated with improved myocardial reperfusion in comparison to standard balloon predilatation followed by stent implantation [6,13,14]. The TAPAS study [14,15], as well as meta-analyses from De Luca et al. [6] and Burzotta et al. [16] have shown clinical benefit including mortality reduction in patients with STEMI treated with manual aspiration catheters. However, in patients treated with aspiration catheters before primary PCI with stenting, distal embolization occurs in more than 6% [17], suggesting that the use of such devices is unable to completely avoid the risk of debris protrusion or migration during stent implantation. In such clinical scenarios, the use of a mesh-covered stent – such as the MGuard stent (MGuardTM Coronary Stent System, InspireMD Ltd., Tel Aviv, Israel) [18–21] – seems a valuable option. Importantly, there is also possibility to use the MGuard stent with other devices, for example, after the use of aspiration catheters.
Introduction to the device
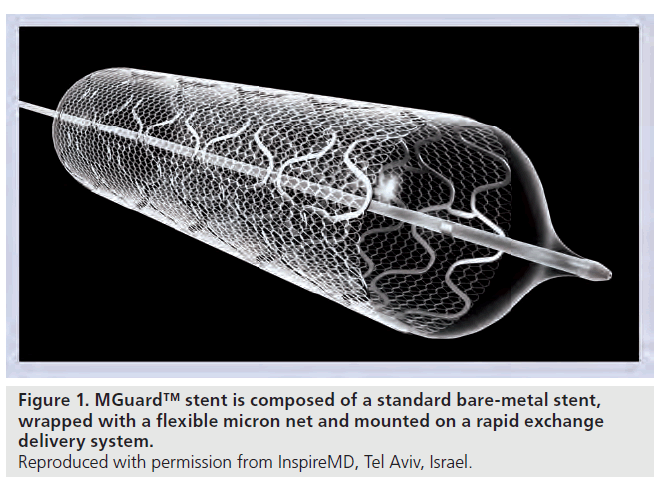
The MGuard stent is a new device intended to prevent distal embolization by thrombus and plaque fragments released during stent implantation in thrombus-containing lesions. The MGuard stent is a bare-metal stent covered with an ultra-thin, micron-level, flexible mesh (Figure 1). The mesh, secured to the coronary stent, acts like a net and locks the potentially embolic particles or thrombus material rising during the interventional treatment behind the mesh against the vessel wall. Additionally, the sleeve may reduce the impact on the arterial wall and may reduce injury, thereby possibly lowering the restenosis rate. Also, mesh may facilitate re-endothelialization by serving as a scaffold. Optimal stent upsizing or post-dilatation with less concern of embolization is possible.
No special training is required for MGuard stent use, as the technique of implantation is the same as for conventional balloon-inflated coronary stents. Unlike distal protection devices (filters), no specific distal or proximal landing zone is required (Table 1). Deliverability and crossing profile remain virtually unchanged and deployment pressures are not affected. The MGuard stent is also available as MGuard Prime with cobalt–chromium design, and improved flexibility and deliverability. The sleeve patented mechanism prevents possible sliding, folding or dislodgement. However, small balloon predilatation using low pressures before stent implantation may facilitate deliverability of the stent. MGuard stent is not recommended in vessels with extreme tortuosity or heavy calcifications, as it may impede successful passage of the system. It is not recommended to use such a stent for treatment of the lesions located distally to previously implanted coronary stents. In addition, the MGuard stent system allows the perfusion of covered side branches. However, the data concerning the use of mesh-covered stent within bifurcation lesions, including accessibility of side branches originating at the site of stent implantation and possibility of postdilatation with kissing balloons technique, are rather limited.
Clinical profile & postmarketing findings
MGuard stent was evaluated in a first-in-man trial in 29 patients with a mean age of 68.1 ± 12 years and acute coronary syndrome presentation in 72.4% [22]. Stents were implanted in both native coronary arteries (41.4%) and degenerated coronary vein grafts (58.6%). PCI with the MGuard stent was feasible and safe. The incidence of major adverse cardiac events was acceptable, and there were no incidents of PCI-related stent thrombosis, Q-wave myocardial infarction or cardiovascular death [22]. Observed rate of target vessel revascularization for the MGuard stent was 11.1%, with a mean late loss of 0.372 ± 0.23 mm and a mean percent diameter stenosis of 30.6% [18].
In the INSPIRE Study, a total of 30 patients with de novo lesions in saphenous vein graft or native vessels with angiographic evidence of instability with potential to provoke flow disturbances and/or distal embolization were included. Overall, 53.3% presented with acute coronary syndrome, and most lesions were located in the saphenous vein graft (53.3%). The MGuard stent was successfully implanted in all cases, and final TIMI grade 3 flow was achieved in all patients. There was no case of distal embolization during angioplasty [23]. At 6 months, routine control angiography was performed in all cases and reported in-stent late loss and percentage of stent obstruction were 0.99 ± 0.70 mm and 31 ± 15.6%, respectively. During 1-year follow-up there were no cases of cardiac death, two (6.6%) cases of myocardial infarction and six (20%) cases of ischemia-driven target-vessel revascularization. Importantly, there was no stent thrombosis during the long-term follow-up period [24].
Data on 100 consecutive STEMI patients (16% of patients in shock) treated with primary (68 patients) or rescue (32 patients) PCI with MGuard stent implantation were reported by Piscione et al. [21]. In this multicenter study, the MGuard stent was implanted successfully in all patients and was able to achieve optimal myocardial reperfusion (MBG 3), as well as complete ST-segment resolution 60 min after PCI in 90% of patients. Thrombus aspiration was used in 10% of patients, and direct-stenting technique in 58% of patients. Observed in-hospital mortality was quite high (7%), mainly due to inclusion of shock patients. In two patients, subacute stent thrombosis occurred (one related to type B dissection at the distal edge of the stent, and the second probably occurred as a consequence of device undersizing). No additional major cardiovascular events have been reported during 1-month follow-up [21].
Safety and feasibility of MGuard implantation in STEMI patients were also confirmed by data from 60 patients enrolled in the MAGICAL Study [25]. In this study, the MGuard stent was successfully implanted within the target lesion in 98.3% of patients. Thrombus aspiration was used in 18.3% of patients, and direct-stenting technique in 38.3% of patients. The frequency of final TIMI grade 3 flow, MBG 3 and complete (>70%) ST-segment resolution 60–90 min after PCI assessed by an independent core laboratory was 90.0, 73.3 and 61.4%, respectively. The rate of distal embolization was 5%. There was no death, reinfarction related to target vessel or ischemic target-lesion revascularization during 6-month follow-up. By protocol definition, the total major adverse cardiac events rate at 6 months was 1.7% [25].
Similarly, 6-month clinical and angiographic outcomes for 100 STEMI patients were recently reported by Apro et al. In this single center, prospective, single-arm study predilatation was performed in 77% of cases and thrombus aspiration in 18%. Final TIMI grade 3 flow was observed in 96% of patients, and distal embolization in one (1%) patient. The in-hospital rate of major adverse cardiac events was 3%. Angiographic follow-up at 6 months demonstrated 19% of binary restenosis. There was no case of clinically driven target-vessel revascularization during the 6-month follow-up period [26].
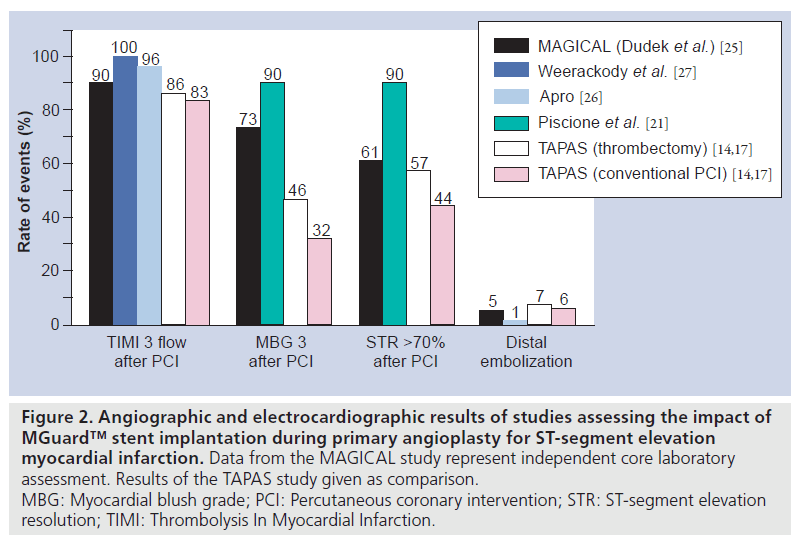
In addition, the safety and eff icacy of MGuard stent implantation during primary PCI for STEMI was confirmed by Weerackody et al. [27]. In this study, 51 patients were successfully treated with MGuard stent implantation. TIMI grade 3 flow was achieved in all cases, without procedural complications, and ST-segment elevation resolution >50% after PCI was reported for 96% of patients. There were two deaths (both in patients with cardiogenic shock) during hospital stay. Among nonshock patients, target-vessel revascularization rate at 1 year was 6%, without stent thrombosis occurrence [27]. Angiographic and electrocardiographic results of major studies assessing MGuard stent during primary PCI for STEMI are shown in Figure 2.
Figure 2: Angiographic and electrocardiographic results of studies assessing the impact of MGuard™ stent implantation during primary angioplasty for ST-segment elevation myocardial infarction. Data from the MAGICAL study represent independent core laboratory assessment. Results of the TAPAS study given as comparison. MBG: Myocardial blush grade; PCI: Percutaneous coronary intervention; STR: ST-segment elevation resolution; TIMI: Thrombolysis In Myocardial Infarction.
In the ongoing iMOS Registry more than 1000 patients will be enrolled and treated with MGuard stent in all approved indications. The interim analysis of 200 patients (77% with STEMI), presented by Danzi during EuroPCR 2010, revealed a 98% success rate, 96% rate of TIMI grade 3 flow after PCI and ST-segment elevation resolution >50% in 76% of patients [28]. Subanalysis of 157 STEMI patients from 211 patients enrolled revealed 1.9% rate of death, 1.3% rate of reinfarction and no need of repeated target vessel revascularization during 30-day follow- up. The total major adverse cardiac events rate was 2.5% [28].
The MASTER study, a large, randomized, multicentre study of 406 patients with STEMI treated with primary or rescue PCI is planned to compare MGuard stent implantation to standard bare-metal/drug-eluting stents (with or without prior thrombus aspiration). The primary end point of the study is the frequency of complete ST-segment resolution after PCI. Also, a second randomized study, called GUARDIAN [101], comparing MGuard versus thrombus aspiration with bare-metal stent implantation is ongoing.
How does the technology fit into the field of medical devices?
According to current European Society of Cardiology/European Association for Cardio- Thoracic Surgery Guidelines on myocardial revascularization, the use of mesh-based protection may be considered for PCI of highly thrombotic or coronary vein graft lesions (IIb C) [11]. The MGuard stent is a bare-metal stent approved in the European community for use in native coronaries and saphenous vein grafts – CE mark registration number 51168-23-A0 was delivered on 13 November 2007. Based on the CE approval, MGuard stent also received approval in countries including: Australia, Brazil, Argentina, Mexico, Israel, South Africa, India, Pakistan, Thailand and Taiwan. MGuard Prime (not yet available for sale) received CE approval with a specific acute myocardial infarction indication. Both MGuard and MGuard Prime are not available in the USA and Japan.
Executive summary
Advantages of the MGuard™ stent
▪ No special training is required.
▪ No specific distal or proximal landing zone is required.
▪ Deliverability and crossing profile remain virtually unchanged and deployment pressures are not affected.
▪ The sleeve may reduce the impact on the arterial wall and may reduce injury, thereby possibly lowering the restenosis rate.
▪ Mesh may facilitate re-endothelialization by serving as a scaffold.
▪ Optimal stent upsizing or postdilatation with less concern of embolization.
Disadvantages of the MGuard stent
▪ Not recommended in vessels with extreme tortuosity or heavy calcifications.
▪ Not recommended for lesions located distally to previously implanted coronary stents.
▪ Data concerning the use of mesh-covered stent within bifurcation lesions, including accessibility of side branches originating at the site of stent implantation and possibility of postdilatation with kissing balloons technique, are rather limited.
Clinical efficacy in ST-segment elevation myocardial infarction
▪ More than 600 ST-segment elevation myocardial infarction patients treated with MGuard stent have been reported.
▪ High procedural success rate.
▪ Final Thrombolysis In Myocardial Infarction grade 3 flow in 90–100% of patients.
▪ Final myocardial blush grade 3 in 73–90% of patients.
▪ Complete ST-segment elevation resolution >70% after percutaneous coronary intervention in 61–90% of patients.
▪ Distal embolization in 1–5% of patients.
▪ Low rates of major adverse cardiac events at 30 days and 6 months.
▪ Large ‘real-world’ registry is ongoing.
▪ Randomized clinical trials planned.
Availability
▪ CE marked.
▪ Not available in the USA and Japan.
Recommendations
▪ May be considered for percutaneous coronary intervention of highly thrombotic or coronary vein graft lesions (IIb C, according to European Society of Cardiology/European Association for Cardio-Thoracic Surgery 2010 Guidelines on myocardial revascularization).
Conclusion
The use of MGuard stent during primary PCI for STEMI is an attractive option as the completed studies have shown that use of the stent is safe and effective. The MGuard stent and aspiration catheters could be used as complementary, rather than competitive devices during primary PCI in STEMI.
Future perspective
The mesh can also potentially be used as a platform for drug delivery, especially for drug molecules with reduced diffusion capabilities. The mesh may provide more uniform coverage of arterial wall than a regular drug-eluting stent and thus may give better control of vessel healing and re-endothelialization. Case report data support the use of the MGuard stent in the case of coronary vessel perforation, however, the efficacy of such an approach was not tested in clinical studies [29]. The MGuard concept may also be used during stenting of carotid or peripheral arteries by applying the mesh onto a dedicated self-expanding stent.
Information resources
▪ www.inspire-md.com/; InspireMD website with MGuard brochures, presentations and study results.
▪ http://cardio.pl/; educational website with lectures, scientific reports and case presentations.
Financial & competing interests disclosure
Dariusz Dudek, received a research grant as Principal Investigator for the MAGICAL study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- Van De Werf F, Bax J, Betriu A et al.:Management of acute myocardial infarction inpatients presenting with persistent ST-segmentelevation: the Task Force on the Management ofST-Segment Elevation Acute MyocardialInfarction of the European Society of Cardiology.Eur. Heart J. 29(23), 2909–2945 (2008).
- Stone GW, Peterson MA, Lansky AJ et al.:Impact of normalized myocardial perfusionafter successful angioplasty in acute myocardialinfarction. J. Am. Coll. Cardiol. 39(4), 591–597(2002).
- Henriques JP, Zijlstra F, van ‘t Hof AW et al.:Angiographic assessment of reperfusion in acutemyocardial infarction by myocardial blushgrade. Circulation 107(16), 2115–2119 (2003).
- Henriques JP, Zijlstra F, Ottervanger JP et al.:Incidence and clinical significance of distalembolization during primary angioplasty foracute myocardial infarction. Eur. Heart J.23(14), 1112–1117 (2002).
- Napodano M, Ramondo A, Tarantini G et al.:Predictors and time-related impact of distalembolization during primary angioplasty. Eur.Heart J. 30(3), 305–313 (2009).
- De Luca G, Dudek D, Sardella G et al.:Adjunctive manual thrombectomy improvesmyocardial perfusion and mortality in patientsundergoing primary percutaneous coronaryintervention for ST-elevation myocardialinfarction: a meta-analysis of randomizedtrials. Eur. Heart J. 29(24), 3002–3010(2008).
- De Luca G, Suryapranata H, Stone GW et al.:Adjunctive mechanical devices to prevent distalembolization in patients undergoingmechanical revascularization for acutemyocardial infarction: a meta-analysis ofrandomized trials. Am. Heart J. 153(3),343–353 (2007).
- Stone GW, Webb J, Cox DA et al.: Distalmicrocirculatory protection duringpercutaneous coronary intervention in acuteST-segment elevation myocardial infarction: arandomized controlled trial. JAMA 293(9),1063–1072 (2005).
- Muramatsu T, Kozuma K, Tsukahara R et al.:Comparison of myocardial perfusion by distalprotection before and after primary stentingfor acute myocardial infarction: angiographicand clinical results of a randomized controlledtrial. Catheter Cardiovasc. Interv. 70(5),677–682 (2007).
- Gick M, Jander N, Bestehorn HP et al.:Randomized evaluation of the effects offilter-based distal protection on myocardialperfusion and infarct size after primarypercutaneous catheter intervention inmyocardial infarction with and withoutST-segment elevation. Circulation 112(10),1462–1469 (2005).
- Wijns W, Kolh P, Danchin N et al.:Guidelines on myocardial revascularization:The Task Force on MyocardialRevascularization of the European Society ofCardiology (ESC) and the European Association for Cardio-Thoracic Surgery(EACTS). Eur. Heart J. 31(20), 2501–2555(2010).
- Mehta SK, Frutkin AD, Milford-Beland Set al.: Utilization of distal embolic protectionin saphenous vein graft interventions (ananalysis of 19,546 patients in the AmericanCollege of Cardiology-NationalCardiovascular Data Registry). Am. J Cardiol.100(7), 1114–1118 (2007).
- Dudek D, Mielecki W, Burzotta F et al.:Thrombus aspiration followed by directstenting: a novel strategy of primarypercutaneous coronary intervention inST-segment elevation myocardial infarction.Results of the Polish-Italian-HungarianRAndomized ThrombEctomy Trial(PIHRATE Trial). Am. Heart J. 160(5),966–972 (2010).
- Svilaas T, Vlaar PJ, van der Horst I et al.:Thrombus aspiration during primarypercutaneous coronary intervention. N. Engl.J. Med. 358(6), 557–567 (2008).
- Vlaar PJ, Svilaas T, van der Horst I et al.:Cardiac death and reinfarction after 1 year inthe Thrombus Aspiration duringPercutaneous coronary intervention in Acutemyocardial infarction Study (TAPAS): a1-year follow-up study. Lancet 371(9628),1915–1920 (2008).
- Burzotta F, De Vita M, Gu YL et al.:Clinical impact of thrombectomy in acuteST-elevation myocardial infarction: anindividual patient-data pooled analysis of 11trials. Eur. Heart J. 30(18), 2193–2203(2009).
- Fokkema ML, Vlaar PJ, Svilaas T et al.:Incidence and clinical consequences of distalembolization on the coronary angiogram afterpercutaneous coronary intervention forST-elevation myocardial infarction. Eur.Heart J. 30(8), 908–915 (2009).
- Kaluski E, Groothuis A, Klapholz M, SeifartP, Edelman E: Coronary stenting withM-Guard: feasibility and safety porcine trial.J. Invasive Cardiol. 19(8), 326–330 (2007).
- Kaluski E, Tsai S, Klapholz M: Coronarystenting with MGuard: from conception tohuman trials. Cardiovasc. Revasc. Med. 9(2),88–94 (2008).
- Jain A, Weerackody R, Kennon S, RothmanM: Prevention of thrombus embolizationduring primary percutaneous interventionusing a novel mesh covered stent. CatheterCardiovasc. Interv. 74(1), 88–93 (2009).
- Piscione F, Danzi GB, Cassese S et al.:Multicentre experience with MGuard netprotective stent in ST-elevation myocardialinfarction: safety, feasibility, and impact onmyocardial reperfusion. Catheter. Cardiovasc.Interv. 75(5), 715–721 (2010).
- Kaluski E, Hauptmann KE, Muller R et al.:Coronary stenting with MGuard: first-in-mantrial. J. Invasive. Cardiol. 20(10), 511–515(2008).
- Maia F, Costa JR Jr, Abizaid A et al.:Preliminary results of the INSPIRE trial withthe novel MGuard stent system containing aprotection net to prevent distal embolization.Catheter Cardiovasc. Interv. 76(1), 86–92(2010).
- Costa JR Jr, Abizaid A, Costa R et al.: Finalresults of the INSPIRE Trial with the novelmguard stent for thrombus-containg lesions.J. Am. Coll. Cardiol. 56, B70 (2010).
- Dudek D, Dziewierz A, Rzeszutko L et al.:Mesh covered stent in ST-segment elevationmyocardial infarction. EuroIntervention 6(5),582–589 (2010).
- Apro D: Six-months clinical and angiographicresults of mguard net protective stent in primaryPCI. J. Am. Coll. Cardiol. 56, B107 (2010).
- Weerackody R, Jain M, Archbold A et al.: Amesh covered stent effectively reduces risk ofdistal embolisation during primarypercutaneous intervention for ST-segmentelevation myocardial infarction.EuroIntervention 6, H106 (2010).
- Danzi GB: iMOS Interim Analysis – onbehalf of international MGuardObservational Study (iMOS) Registry StudyInvestigators. Presented at: EuroPCR Congress.Paris, France, 25–28 May 2010.
- Jain A: A novel mesh covered stent for thetreatment of coronary artery perforation.Presented at: EuroPCR Congress. Barcelona,Spain, 19–22 May 2009.
▪ Important meta-analysis of studies with adjunctive manual thrombectomy devices showing mortality benefit.
▪ Largest study with MGuard™ stent during primary angioplasty in acute myocardial infarction.
▪ The second largest study with MGuard stent during primary angioplasty in acute myocardial infarction.
▪ Website
101 MGuard Stent in ST-elevation Myocardial Infarction (GUARDIAN) http://clinicaltrials.gov/ct2/show/ NCT01124942