Device Evaluations - Interventional Cardiology (2012) Volume 4, Issue 6
Evolution in transcatheter aortic valve replacement: the CoreValve self-expanding prosthetic aortic valve
- Corresponding Author:
- Jon C George
Division of Interventional Cardiology and Endovascular Medicine
Deborah Heart and Lung Center, Browns Mills, NJ, USA
E-mail: jcgeorgemd@hotmail.com
Abstract
Keywords
aortic stenosis, Edwards SAPIEN aortic valve, Medtronic CoreValve® prosthesis, surgical aortic valve replacement, transcatheter aortic valve implantation, transcatheter aortic valve replacement, valvular heart disease
Surgical aortic valve replacement has been the sole treatment option available for severe symptomatic aortic stenosis (AS) for decades. Although highly effective, patients undergoing this major operative procedure often possess multiple comorbid risk factors, which increase peri-procedural morbidity and mortality. Many patients are at a prohibitively high surgical risk and are, therefore, managed medically with dismal survival rates. With a prevalence of severe AS between 2 and 4% in patients over the age of 65 years, up to one-third of these patients are considered high-risk operative candidates [1–3].
Current status
The Medtronic CoreValve® (Medtronic Inc., MN, USA) is one of two percutaneous aortic valves currently available for clinical use in patients with symptomatic severe AS, who are deemed either inoperable due to prohibitively high surgical risk or designated as high risk for procedural morbidity and mortality, as determined by a EuroSCORE >20% or Society of Thoracic Surgeons score >10%. The CoreValve was approved for clinical use in Europe in May 2007, and is currently under evaluation in the USA with the large, multicenter, randomized Medtronic CoreValve US Pivotal Trial, which is currently enrolling patients. This study will evaluate transcatheter aortic valve replacement (TAVR) versus surgical aortic valve replacement in patients with symptomatic severe AS, who are at high surgical risk, as well as TAVR versus optimal medical therapy in patients at extreme operative risk [101].
Valve design
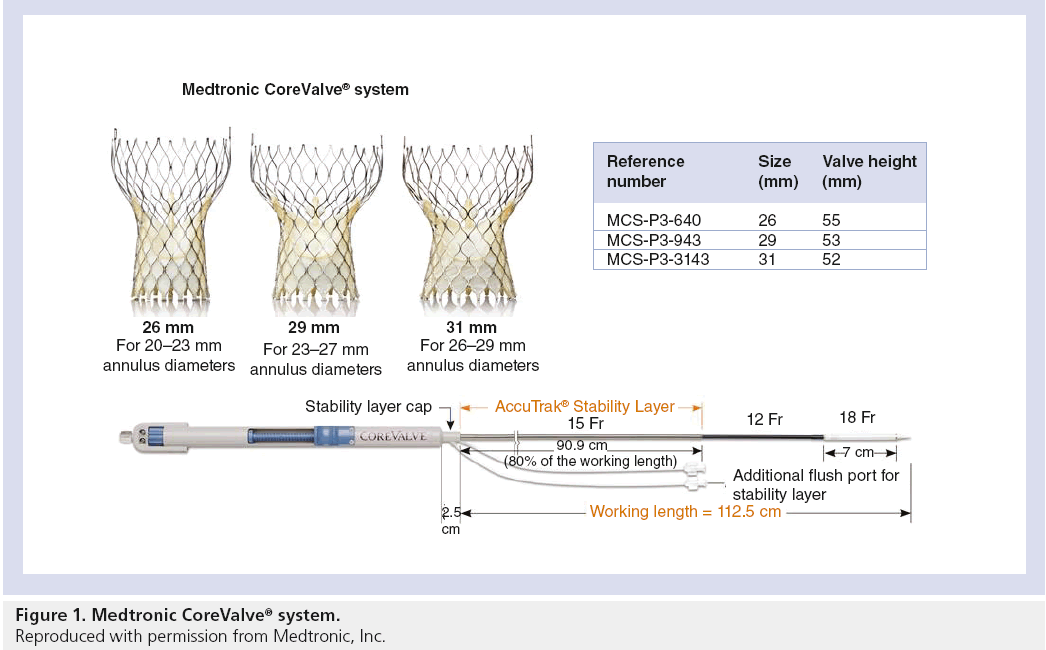
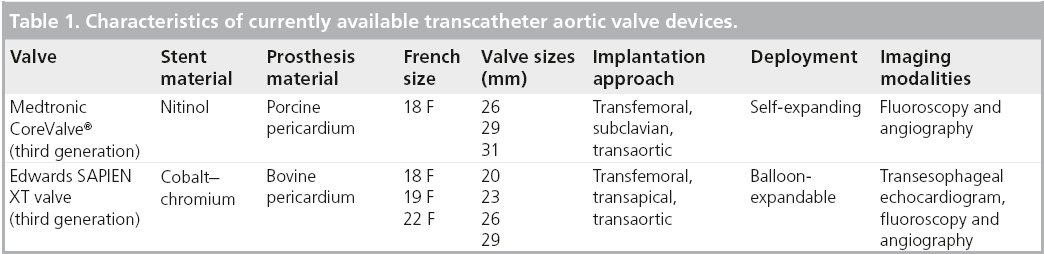
The CoreValve aortic prosthesis consists of a trileaflet bioprosthetic porcine pericardial tissue valve, which is mounted and sutured in a self-expanding nitinol stent (Figure 1). The prosthetic laser-cut frame has an overall length of approximately 50 mm. The prosthesis has three distinct segments: the base portion exerts a high radial force that expands and pushes aside calcified leaflets avoiding recoil; the central portion carries the valve; and the top portion flares to fixate and stabilize the valve in the ascending aorta [4–7]. The first-generation CoreValve was a 24-French system, the second-generation CoreValve was a 21-French system, and the newer third generation is an 18-French system.
Clinical data
The first human implantation of the CoreValve took place in 2005, which was followed by a feasibility and safety study in 25 high-risk surgical patients with symptomatic severe AS. The CoreValve was successfully implanted in 22 of 25 patients (88%), with a major adverse cardiovascular and cerebral event rate of 32%. The average peak pressure gradient decreased from 69.3 mmHg to 22.1 mmHg, and the New York Heart Association functional class improved 1–2 grades in all treated patients [5].
Further advancement led to the development of the newer second- (21-French) and third- (18-French) generation CoreValve devices. The safety and efficacy of these valves were evaluated in a multicenter trial, which enrolled 86 high-risk patients (50 patients received a second- generation device, 36 patients received a third-generation device). Acute device success was achieved in 76 (88%) patients, with a major adverse cardiovascular and cerebral event rate of 26%. There was significant reduction in the mean transvalvular gradient from 43.7 mmHg to 9.0 mmHg (p < 0.001), with an improvement in mean New York Heart Association functional class from 2.85 to 1.85 (p < 0.001), and overall 30-day mortality of 12% [6].
A review of all three generation CoreValve devices showed that the procedural success rate increased from generations one and two to three; from 70% and 70.8%, to 91.2%, respectively (p = 0.003) [8], likely a reflection of improving operator expertise and better device delivery from reduced profile size. Mid-term follow-up of the third-generation CoreValve demonstrated continued clinical benefit at 3 years, with a sustained post-TAVR mean transvalvular gradient of 10.3 mmHg (pre-TAVR mean pressure gradient 52.2 mmHg), and aortic valve area of 1.8 cm2 (pre-TAVR aortic valve area 0.6 cm2). Additionally, survival free of death, major stroke, myocardial infarction or life-threatening bleeding was 69.6% at 1 year, 63.5% at 2 years, and 59.7% at 3 years [9].
Procedure details
The TAVR procedure is performed in the cardiac catheterization lab or in a hybrid operating room. Aortic balloon valvuloplasty is initially performed to prepare the calcified native valve for device implantation, followed by delivery, positioning and deployment of the CoreValve. However, a recent pilot study of TAVR without balloon predilation showed equivalent feasibility, safety and efficacy as that with predilation [10]. The CoreValve, as opposed to the Edwards valve (Edwards Lifesciences, CA, USA), is a self-expanding device, which can be deployed in a ‘step-wise’ fashion, theoretically permitting accurate device placement prior to final deployment. The third-generation, 18-French CoreValve allows a complete percutaneous approach, without femoral cut-down, feasible with lower vascular complications. In addition, general anesthesia and transesophageal echocardiography are often no longer mandatory for procedural success, as fluoroscopic and angiographic guidance might suffice. The CoreValve can be safely postdilated without altering structural integrity if significant paravalvular regurgitation is present. Post-deployment balloon dilation was reported in up to 24% of treated patients in one study [6]. Although postdilation after valve deployment may reduce paravalvular regurgitation, there are risks associated with excessive dilation, including aortic rupture and increased central aortic regurgitation.
Vascular access
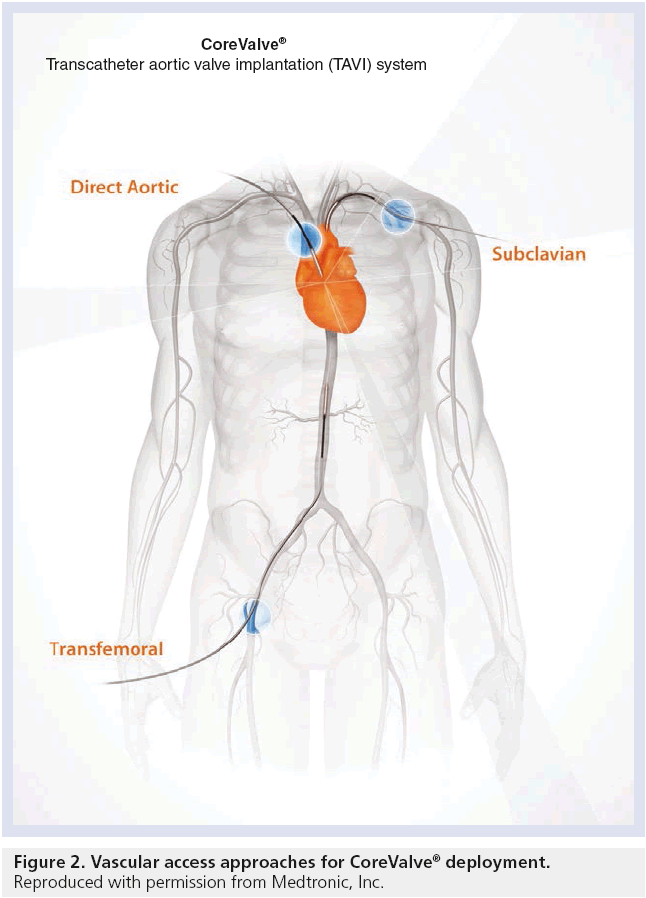
In determining the suitability of TAVR via transfemoral approach, the workup begins with sizing the iliofemoral arteries, by either computed tomography angiography or iliofemoral angiography. The dimensions of the aortic annulus, for appropriate valve sizing, may be acquired by transthoracic echocardiogram, transesophageal echocardiogram, or computed tomography angiography. Despite the smaller third-generation CoreValve devices, the primary limiting factor for TAVR is the availability of transfemoral vascular access [11]. In these patients with limited options, various alternative access sites have been explored, including transapical [12], subclavian or axillary [13], transaortic [14], and even carotid [15] arteries with reported success (Figure 2). Whilst the transapical approach is not feasible with the self-expanding CoreValve system, the transsubclavian approach has been described with tremendous success. Future studies with larger patient populations within each subgroup are necessary to delineate the best options for alternative TAVR access when traditional routes are unavailable.
Complications
Although the early and mid-term results of TAVR appear promising, the overall mortality and major adverse cardiovascular and cerebral event rates need improvement. Several complications hinder the widespread use of TAVR, including major stroke (1.2–6.7%), vascular complications (5–10%), myocardial infarction (0–16.3%), coronary obstruction (<1%), atrioventricular block requiring permanent pacemaker implantation (9–40%), renal injury (11.7–28%) and peri-procedural death (10%) [16]. The incidence of stroke following implantation is highly variable between published reports and represents a preventable cause of morbidity. Clinically silent stroke has been detected in up to 84% of patients undergoing TAVR as new foci on diffusion-weighted magnetic resonance imaging [17]. Although multiple, these foci were not associated with any apparent neurological events or neurocognitive decline during follow-up. Paravalvular aortic regurgitation has been observed in the majority of patients following TAVR [18], and has been attributed to prothesis–annulus discongruence [19] and the angle of left ventricular outflow tract to ascending aorta [20]. Nevertheless, these findings have remained stable in follow-up studies following CoreValve implantation without progression to moderate or severe regurgitation [9]. However, utmost care during implantation, along with postdilation, may be necessary to avoid significant paravalvular regurgitation. Careful imaging using transesophageal echocardiography in addition to fluoroscopy is warranted to address these factors associated with postprocedural paravalvular regurgitation [21]. Rarely, CoreValve dislocation has been described during TAVR [22], significantly increasing perioperative risk for severe complications or death. If recapture of the device is unsuccessful, implantation in the descending aorta may be required. Snaring devices attached to one of the two frame loops of the CoreValve bioprosthesis have been successfully used via a transfemoral or transbrachial approach to correct a position too low in the left ventricular outflow tract. Alternatively, a dislocated valve may be managed percutaneously with a second valve implantation or open surgical extraction and replacement.
Alternative devices
There are currently only two types of percutaneous aortic valves approved for use in the clinical setting: the balloon-expandable Edwards prosthesis [23] and the self-expandable Medtronic CoreValve prosthesis [8]. The PARTNER trial investigated the efficacy and safety of TAVR using the first-generation Edwards valve in otherwise inoperable patients with severe symptomatic AS and demonstrated significantly reduced rates of death from any cause, the composite end point of major adverse cardiovascular events [23]. Both devices have been extensively studied and utilized in several clinical trials with comparable results. The first two generations of the Edwards valve, the Cribier-Edwards valve (first), and the Edwards SAPIEN valve (second) were composed of a stainless steel frame with three bovine pericardial leaflets. The Edwards SAPIEN XT is the third-generation Edwards valve with a frame composed of cobalt–chromium and a trileaflet bovine pericardial valve mounted within. Modifications to the frame’s design have allowed for a smaller valve size and better profile for delivery. The SAPIEN XT valve is available in several sizes, as detailed in Table 1.
Future perspective
The realm of TAVR continues to evolve as both valve technology and operator experience advance. When the first-in-man implant was performed in 2002, components such as general anesthesia, surgical cut-down, transesophageal echocardiography and cardiothoracic surgical back-up were necessary for TAVR. Today, after considerable technological progress, only percutaneous access, conscious sedation and f luoroscopy are needed for procedural success, with procedural times less than 45 min in experienced hands [8]. However, there remain many challenges in TAVR that need improvement, including even smaller device profiles, which would allow for smaller vascular access sites and potentially less vascular complications. Furthermore, future generations of TAVR devices with improved subannular fixation design or external space-filling materials may also reduce incomplete apposition [24]. Major stroke may be preventable as the development of embolic protection devices, such as the Embrella Embolic Deflector™ (Embrella Cardiovascular Inc., PA, USA) [25], and the Claret CE Pro™ device (Claret Medical Inc., CA, USA) [26] for the great vessels during device implantation shows promise. In addition, the development of a TAVR risk score may help identify those patients at highest risk for periprocedural complications. Finally, the projected efficacy of TAVR in the intermediate-risk patient needs to be addressed in the future. Meanwhile, other patient subsets including valve implantation within previous bioprosthetic aortic valves [27] and degenerated aortic homografts with aortic insufficiency [28] are currently being explored.
Executive summary
Clinical rationale
▪ Transcatheter aortic valve replacement (TAVR) was derived out of the need for better treatment options for patients with severe symptomatic aortic stenosis who are at prohibitively high or high risk for surgical valve replacement.
▪ Percutaneous balloon aortic valvuloplasty alone has poor long-term durability.
Device description
▪ The Medtronic CoreValve® is a self-expanding transcatheter aortic valve system.
▪ Transfemoral implantation is the usual approach, with transaortic or subclavian arteries as alternatives.
▪ The periprocedural limitations of TAVR include major vascular complications, stroke, myocardial infarction, procedural death, kidney injury, conduction abnormalities and aortic insufficiency.
Alternative devices
▪ The Edwards valve (Cribier-Edwards, SAPIEN, SAPIEN XT) is another percutaneous aortic valve currently available for clinical use, and several other competing products are currently under investigation.
Potential use
▪ TAVR has proven to be an effective treatment in patients who would otherwise not undergo surgical aortic valve replacement.
▪ The decision for TAVR should be made by an interdisciplinary heart team based on current guideline recommendations.
▪ The future of TAVR lies in determining its efficacy in intermediate surgical risk patients, as well as lowering procedural limitations such as vascular complications and stroke.
Conclusion
The Medtronic CoreValve prosthesis represents the continued advancement and progress in the field of TAVR. With the third-generation, 18-French CoreValve, TAVR has become a completely percutaneous procedure, often performed without general anesthesia or transesophageal echocardiogram. In experienced hands, procedural success can be obtained in excess of 90% of cases. Recent clinical trial data have solidified its role in effectively treating inoperable and high-surgical-risk patients, offering alternative therapies for what was once primarily a surgical disease. The results of the Medtronic CoreValve US Pivotal Trial, involving more than 1300 patients at up to 40 hospitals in the USA, are expected to be available around 2014, hopefully proving TAVR benefit with CoreValve in the high-risk population. Meanwhile, the CoreValve SURTAVI trial will evaluate the safety and efficacy of the device in symptomatic AS patients who are at intermediate risk for surgery.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 29, 630–634 (1997).
- Iung B, Cachier A, Baron G et al. Decisionmaking in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur. Heart J. 26, 2714–2720 (2005).
- Bonow RO, Carabello BA, Kanu C et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114, e84–e231 (2006).
- Grube E, Laborde JC, Gerckens U et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 114, 1616–1624 (2006).
- Grube E, Laborde JC, Zickmann B et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc. Interv. 66, 465–469 (2005).
- Grube E, Schuler G, Buellesfeld L et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current thirdgeneration self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J. Am. Coll. Cardiol. 50, 69–76 (2007).
- Singh I, Tuzcu E, Shishehbor M, Kapadia S, Christofferson R. Percutaneous treatment of aortic valve stenosis. Cleve. Clin. J. Med. 75(11), 805–812 (2008).
- Grube E, Buellesfeld L, Mueller R et al. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the corevalve revalving system. Circ. Cardiovasc. Interv. 1, 167–175 (2008).
- Ussia G, Barbanti M, Petronio A et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur. Heart J. 33(8), 969–976 (2012).
- Grube E, Naber C, Abizaid A et al. Feasibility of transcatheter aortic valve implantation without balloon pre-dilation: a pilot study. JACC Cardiovasc. Interv. 4(7), 751–757 (2011).
- Mouillet G, Desgranges P, Teiger E. Transcatheter aortic valve implantation when classical access routes are unavailable. Catheter Cardiovasc. Interv. 78(7), 1004–1007 (2011).
- Lange R, Schreiber C, Gotz W et al. First successful transapical aortic valve implantation with the CoreValve revalving system: a case report. Heart Surg. Forum 10(6), E478–E479 (2007).
- Modine T, Sudre A, Collet F et al. Transcutaneous aortic valve implantation using the axillary/subclavian access with patent left internal thoracic artery to left anterior descending artery: feasibility and early clinical outcomes. J. Thorac. Cardiovasc. Surg. doi:10.1016/j.jtcvs.2012.01.031 (2012) (Epub ahead of print).
- Bruschi G, De Marco F, Botta L et al. Direct transaortic CoreValve implantation through right minithoracotomy in patients with patent coronary grafts. Ann. Thorac. Surg. 93(4), 1297–1299 (2012).
- Modine T, Lemesle G, Azzaoui R, Sudre A. Aortic valve implantation with the CoreValve ReValving system via left carotid artery access: first case report. J. Thorac. Cardiovasc. Surg. 140(4), 928–929 (2010).
- Rodes-Cabau J. Transcatheter aortic valve implantation: current and future approaches. Nat. Rev. Cardiol. 9, 15–29 (2011).
- Kahlert P, Knipp SC, Schlamann M et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 121, 870–878 (2010).
- Abdel-Wahab M, Zahn R, Horack M et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 97(11), 899–906 (2011).
- Detaint D, Lepage L, Himbert D et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. J. Am. Coll. Cardiol. Interv. 2, 821–827 (2009).
- Sherif MA, Abdel-Wahab M, Stocker B et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the medtronic CoreValve Bioprosthesis. J. Am. Coll. Cardiol. 56(20), 1623–1629 (2010).
- Chin D. Echocardiography for transcatheter aortic valve implantation. Eur. J. Echocardiogr. 10, i21–i29 (2009).
- Geisbusch S, Bleiziffer S, Mazzitelli D et al. Incidence and management of CoreValve dislocation during transcatheter aortic valve implantation. Circ. Cardiovasc. Interv. 3(6), 531–536 (2010).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Kodali SK, Williams MR, Smith CR et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 366(18), 1686–1695 (2012).
- Nietlispach F, Wijesinghe N, Gurvitch R et al. An embolic deflection device for aortic valve interventions. J. Am. Col. Cardiol. 3, 1133–1138 (2010).
- Naber CK, Ghanem A, Abizaid AA et al. First-in-man use of a novel embolic protection device for patients undergoing transcatheter aortic valve implantation. EuroIntervention 8(1), 43–50 (2012).
- Cockburn J, Trivedi U, Hildick-Smith D. Transaortic transcatheter aortic valve implantation within a previous bioprosthetic aortic valve replacement. Catheter Cardiovasc. Interv. 78(3), 479–484 (2011).
- Olsen LK, Engstrom T, Sondergaard L. Transcatheter valve-in-valve implantation due to severe aortic regurgitation in a degenerated aortic homograft. J. Invasive Cardiol. 21(10), E197–E200 (2009).
▪ Website
- Adams D, Popma J. Safety and efficacy of the medtronic CoreValve system in the treatment of symptomatic severe aortic stenosis in high risk and very high risk subjects who need aortic valve replacement. ClinicalTrials.Gov. NCT01531374.