Review Article - Interventional Cardiology (2013) Volume 5, Issue 4
Examining access routes and bleeding risk in women undergoing percutaneous coronary intervention
- Corresponding Author:
- Angela Hoye
Hull York Medical School, Department of Cardiology, Castle Hill Hospital
Castle Road, Cottingham, East Yorkshire, HU16 5JQ, UK
Tel: +44 148 262 2024
Fax: +44 148 262 2093
E-mail: angela.hoye@hull.ac.uk
Abstract
Recent improvements in antithrombotic strategies during percutaneous coronary intervention (PCI) have led to a reduced incidence of periprocedural ischemic events and stent thrombosis. However, as a consequence of this, bleeding complications have perhaps gained recognition as the most common problem observed. When such an event occurs, the outcomes are inferior and can add significantly to the morbidity and mortality of the individual requiring intervention.
Keywords
antithrombotic agents, bleeding, female sex, percutaneous coronary intervention, vascular access
Recent improvements in antithrombotic strategies during percutaneous coronary intervention (PCI) have led to a reduced incidence of periprocedural ischemic events and stent thrombosis. However, as a consequence of this, bleeding complications have perhaps gained recognition as the most common problem observed. When such an event occurs, the outcomes are inferior and can add significantly to the morbidity and mortality of the individual requiring intervention [1–3].
A number of studies have demonstrated that female sex is associated with an increased risk of periprocedural bleeding; indeed, female sex has been shown to be an independent predictor of bleeding in both stable coronary artery disease and acute coronary syndromes (ACSs). This leads to poorer outcomes in this sex and, therefore, reluctance by the treating interventional cardiologist to administer effective evidence-based therapy is common.
There are a number of strategies that can be employed with an aim to reduce the risk of bleeding in this population of patients. Although the benefits of antithrombotic treatment are well established, consideration should be given to ensure accurate dosage and the most appropriate therapy. In addition, each patient should be assessed on an individual basis with regard to their bleeding risk; hence, the therapy can be tailored for each patient. Moreover, the use of the radial access route may lead to a reduction in major vascular complications and, thus, lower the frequency of significant bleeding, thereby improving procedural outcomes further.
The aim of this review is to discuss bleeding issues in the female sex undergoing PCI and suggest methods by which these can be reduced in everyday clinical practice.
The impact of bleeding complications in PCI
It has been widely demonstrated that bleeding has a detrimental effect on outcomes following PCI. In the GRACE study, major bleeding was shown to be an independent predictor of in-hospital mortality (adjusted odds ratio [OR]: 1.64; 95% CI: 1.18–2.28) [4]. In addition, bleeding has been shown to have an impact on outcomes at 30 days [2] and also up to 1 year [3,5]. A large (n = 17,034) patient-level pooled analysis from the REPLACE-2, ACUITY and HORIZONS-AMI studies demonstrated that major bleeding significantly increased the risk of mortality at 1 year (hazard ratio [HR]: 4.2; 95% CI: 3.1–5.7; p < 0.001) [5]. Furthermore, bleeding related to PCI in the context of ST-elevation myocardial infarction has been demonstrated to lead to a higher mortality (24.6 vs 5.4%; p < 0.0001) and more major adverse cardiovascular events (40.3 vs 20.5%; p < 0.0001) at 3-years follow-up. Indeed, major bleeding was an independent predictor of mortality in this study (HR: 2.80; 95% CI: 1.89–4.16; p < 0.0001) [6].
There are a number of factors that impact upon the increased risk following a significant bleeding event. First, prompt bleeding leads to loss of circulatory blood volume and therefore the development of hypovolemic shock. This may result in coronary ischemia and hence post-PCI myocardial infarction, left ventricular dysfunction and poorer outcomes. Second, if there is a marked drop in the hemoglobin level of the patient owing to significant blood loss, the treating physician may discontinue the dual antiplatelet therapy, which can lead to subsequent stent thrombosis and myocardial infarction [7,8]. Furthermore, in this situation, the patient may undergo a transfusion of packed red blood cells, which again has been demonstrated to lead to poorer outcomes, especially in ACS patients [9,10]. This is believed to be secondary to an increase in inflammatory mediators, a depletion of nitric oxide and 2,3-diphosphoglyceric acid, which leads to increased platelet aggregation, and impaired oxygen delivery to tissues [11–14]. Finally, following a significant bleeding episode owing to hypovolemia and possible impaired renal function, the individual may be prescribed fewer evidence-based therapies, which have been demonstrated to improve outcomes following PCI. In the HORIZONS-AMI study of 3345 patients with ST-elevation myocardial infarction, major bleeding occurred in 8% of cases and, as predicted, patients with a bleed were significantly less likely to be discharged home on clopidogrel (96.1 vs 98.1%; p = 0.05). However, an additional interesting observation was that these patients were also significantly less likely to be discharged home on a b-blocker (83.4 vs 91.2%; p < 0.0001) and a statin (90 vs 6%; p < 0.0001) [6].
Bleeding risk in women
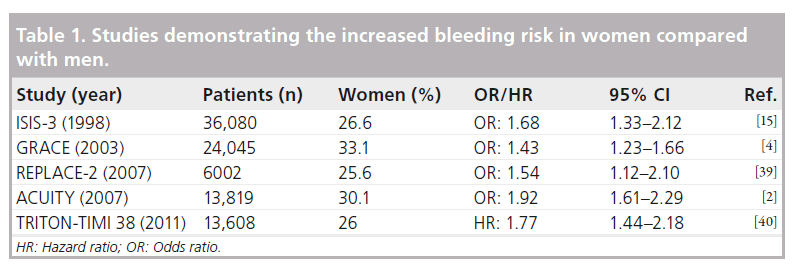
Women have consistently been shown to be at a higher risk of bleeding compared with men following PCI in both randomized controlled trials and large-scale registry studies (Table 1). In the large GRACE registry, female sex was shown to confer an increased risk of major bleeding (OR: 1.43; 95% CI: 1.23–1.66; p < 0.0001) [4]. The ISIS 3 study analyzed 36,080 patients (of whom 9600 were women) with a clear indication for fibrinolytic therapy. This demonstrated a higher occurrence of major bleeding in women compared with men (1.5 vs 0.7%; adjusted OR: 1.68; 95% CI: 1.33–2.12) [15].
A large meta-analysis from the CURE, CREDO, CLARITY-TIMI 28, COMMIT and CHARISMA studies, including a total of 79,613 patients of whom 30% were women, demonstrated that the addition of clopidogrel to aspirin therapy resulted in a higher risk of bleeding in women (1.7 vs 1.2%; OR: 1.43; 95% CI: 1.15–1.79). However, this was also observed among the men (1.3 vs 1.1%; OR: 1.22; 95% CI: 1.05–1.42) [16].
Newer therapies used in the treatment of coronary disease have also assessed the differences according to the sex of the patient. The REPLACE-2 study, which assessed the use of bivalirudin with provisional glycoprotein IIb/ IIIa inhibition (GPI) versus heparin with planned GPI in 6002 patients, demonstrated increased major bleeding in women compared with men (4.8 vs 2.7%; p < 0.001). In addition, there was more access site bleeding (2.9 vs 1.2%; p < 0.001) and a greater need for blood transfusion (3.8 vs 1.5%; p < 0.001) in this cohort [17]. Another study assessing the use of GPI, the CRUSADE prospective registry of 32,601 patients, demonstrated that women treated with GPI had a higher rate of major bleeding than men (15.7 vs 7.3%; p < 0.0001), although this was also true in the cohort not receiving this therapy (8.5 vs 5.4%; p < 0.0001) [18].
A further large registry study, including 13,653 female and 32,334 male patients demonstrated (after adjustment for baseline differences) that female sex remained a significant predictor of increased risk for bleeding (OR: 2.6; 95% CI: 1.74–3.91). Notably, in this study, women with major bleeding or a significant vascular complication were more likely to die in hospital (6.2 vs 1.7%; p < 0.001) [19]. Reassuringly, however, this study demonstrated an improvement in the incidence of bleeding complications over time.
Why do women have a higher risk of bleeding?
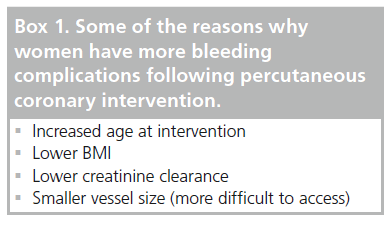
There is clear evidence to demonstrate that bleeding complications are an unfavorable occurrence following PCI, and woman are more likely to develop such an event owing to various inherent factors (Box 1). This group of patients has different pharmacokinetics due to smaller body mass with relatively more fat and lower creatinine clearance. This leads to a higher circulating level of common antithrombotic agents administered throughout the PCI procedure and, hence, such medication should be accurately weight-adjusted to ensure that excess dosage does not occur.
The CRUSADE registry reported that women treated with GPI were more likely to receive excess drug than their male counterparts (46.4 vs 17.2%; p < 0.001; adjusted OR: 3.81; 95% CI: .39–4.27). Excess dosing led to an increased risk of bleeding in women (OR: 1.72; 95% CI: 1.30–2.28) and men (OR: 1.27; 95% CI: 0.97–1.66); however, the bleeding risk attributable to dosing was much higher in women (25.0 vs 4.4%) [18].
A further prospective observational analysis from the CRUSADE registry of 30,136 non- ST-elevation ACS patients reported an excess of unfractionated heparin in 32.8% of patients, excess low molecular weight heparin in 13.8% of patients and excess GPI in 26.8% of patients. Predictors of excessive anticoagulation were older age, lower weight, chronic kidney disease, diabetes mellitus, congestive heart failure and, notably, female sex. Indeed, in this study, there was a relationship between in-hospital mortality and the degree of anticoagulation with both unfractionated heparin (recommended 3.9 vs excess 5.8%; p = 0.04) and GPI (recommended 4.4 vs excess 12.4%; p < 0.001) [20].
Such excess dosing may occur as the weight of the patient and information, such as creatinine clearance, are not immediately available to the operator; however, this may mean that those patients at an intrinsically higher risk, for example women, are also more likely to receive an excessive dose. Such details are important and physicians need to ensure accurate dosing to reduce the risk of bleeding in all patients.
Further factors that may contribute to the bleeding risk of women are older age at presentation and more comorbidities, such as renal impairment and diabetes mellitus. A lower BMI, again more common in the female population, has also been shown to increase risk [19,21–23]. In addition, the vascular access site may play a role, which is discussed in the next section, as the vessels are often smaller in women and may be more difficult to puncture, for example in obese patients.
What can be done to reduce bleeding complications in females?
▪Vascular access approach
It is absolutely essential in each individual patient requiring an invasive approach for the treatment of coronary artery disease that the site of access (femoral vs radial) is considered carefully. There is a growing body of evidence that reports improved outcomes with the utilization of the radial artery; however, in some cases it may still be necessary to perform the procedure via the transfemoral route, for example if a large-caliber sheath is necessary.
In the case that a transfemoral approach is required, it is imperative that the puncture is performed as accurately and cleanly as possible. Too often, the puncture itself may be delegated to a junior trainee with the possibility of increased access site complications. In general, the ‘inguinal crease’ should not be used as a landmark, and a puncture between the lower border of the inferior epigastric artery and above the common femoral artery bifurcation has fewer complications [24]. However, in women this may pose more of a challenge as the common femoral artery is typically smaller in diameter and shorter, owing to the smaller size of the patient [25]. A large registry demonstrated female sex and lack of safe-zone arteriotomy as independent predictors of retroperitoneal bleeding [26].
Furthermore, adequate training should be provided to those healthcare professionals undertaking removal of the arterial sheath as this may lead to vascular complications if not performed appropriately. Vascular closure devices can be considered to allow for more effective hemostasis.
Radial access has now become many operators’ access route of choice, with a reduction in complications when this route is chosen. Indeed, one meta-analysis of 12 randomized trials has shown a 78% reduction in access site complications when using this approach [27]. However, it must be noted that this can be more challenging in women, since women are still at an increased risk of access site bleeding, even with the transradial approach. A group of 1348 ACS patients underwent transradial PCI in the EASY trial, with female sex shown to be an independent predictor for a hematoma (OR: 4.40; 95% CI: 2.49–7.81; p < 0.0001) [28]. One reason may relate to the dimensions of the radial artery, which have been shown to be significantly smaller in women compared with men (2.43 ± 0.38 mm vs 2.69 ± 0.40 mm; p = 0.001) [29]. This can lead to increasing problems with radial artery spasm, hematoma, false aneurysm, causalgia, radial artery evulsion following sheath removal and radial artery occlusion in this group of patients. Indeed, female sex is a predictor of radial artery spasm [30] and occlusion following PCI [31].
Hence, it is important when proceeding via the radial route to ensure a careful puncture after adequate training, with liberal use of intra-arterial nitrates as tolerated by the patient. Heparin should be given in order to ensure arterial patency following the procedure and careful attention should be paid to sheath removal. A recent consensus document has been published on the radial approach for PCI, which highlights the need for adequate training to reduce problems that can occur with radial access, such as prolonged procedural time, possibly increasing the risk of cerebral embolization and also increased radiation dose [32]. Moreover, the ongoing multicenter study SAFE PCI for Women aims to provide further insight into the safest vascular access site in women undergoing PCI. A total of 3000 women undergoing coronary angiography will be randomized to transfemoral versus transradial procedures, with a primary efficacy end point of Bleeding Academic Research Consortium types 2, 3 and 5 bleeding [33] or vascular complications at 72 h.
As discussed, there are a number of strategies that can be used to try and reduce the occurrence of bleeding in all patients undergoing PCI, whether it is considering access or pharmacology. A recent study by Daugherty et al. analyzed the results of 570,777 patients (32.5% women) undergoing PCI from the National Cardiovascular Data Registry’s CathPCI Registry® (American College of Cardiology Foundation and Society for Cardiovascular Angiography and Interventions, DC, USA). [34]. The authors specifically looked at the use of any bleeding-avoidance strategy, which included the use of vascular closure devices, bivalirudin and the radial approach (or any combinations of these). The results showed that women had significantly higher rates of bleeding than men when bleeding-avoidance strategies were not used (12.5 vs 6.2%; p < 0.01), with a greater reduction in the absolute risk with the use of bleeding-avoidance strategies (women 6.3 vs men 3.2%; p < 0.01) [34].
▪Antithrombotic therapies
Vascular access bleeds account for only a proportion of bleeding complications. Indeed, women still have a significantly higher rate of nonaccess site bleeding complications. Therefore, it is important to consider optimizing the antithrombotic strategy utilized. In addition to ensuring that the accurate dose of antithrombotic therapy is given to the patient, it is also important to consider which agent is used as part of the PCI procedure. Ideally, such agents need to protect against ischemic complications without leading to bleeding. Traditional standard therapy utilizes unfractionated heparin; however, its efficacy is limited owing to a rather unpredictable effect. More recent data have questioned the optimal anticoagulation regimen used in elective PCI. From a cohort of 698 patients (28% female), 35% were simply given a bolus of 3000 units of unfractionated heparin as compared with the remaining 65%, who were treated with a standard 70-units/kg dose of unfractionated heparin. The study found no significant difference in the level of troponin release between the two groups, bringing the traditional regimen into question and emphasizing the importance of future research [35].
The individual response to low molecular weight heparin (i.e., enoxaparin) is more predictable and a number of newer agents have become available in recent years with the aim for a more constant effect and can be considered when planning the PCI procedure.
Fondaparinux, a synthetic factor Xa inhibitor, was assessed in the OASIS trial. This study randomized 20,078 ACS patients to a fixed dose of fondaparinux versus weight-adjusted enoxaparin. At 9 days, the rate of major bleeding was significantly reduced with the use of fondaparinux compared with enoxaparin (2.2 vs 4.1%; HR: 0.52; 95% CI: 0.44–0.61; p < 0.001) [30,36]. It has to be stated, however, that there are currently no benefits of fondaparinux in individuals with a planned invasive strategy, as this may lead to more guide catheter thromboses.
Another therapy that is becoming increasingly utilized during coronary intervention is bivalirudin, a direct thrombin inhibitor. A specific sex analysis of the ACUITY study, which randomized 13,819 patients with non-ST-elevation ACS to heparin plus a GPI, bivalirudin plus a GPI or bivalirudin alone, demonstrated that bivalirudin monotherapy resulted in significantly decreased major bleeding risk compared with a GPI strategy (bivalirudin 6.0% vs unfractionated heparin plus GPI 12%; p < 0.001); however, importantly, similar rates of composite ischemia and mortality at 1 year were observed [37]. A recent sex-based analysis of the ISAR REACT-4 study demonstrated similar results, with major bleeding occurring in 4.5% of women treated with bivalirudin alone versus 7.5% in those treated with GPI plus heparin (HR: 0.60; 95% CI: 0.26–1.39) [38]. It must be taken into account that each of the studies assessing antithrombotic therapies were performed for different indications and, as such, cannot be extrapolated to the overall PCI population.
Conclusion
Women are subject to an increased risk of bleeding compared with their male counterparts. Such bleeding and access site complications increase procedural morbidity and both short- and longterm mortality; hence, aggressive bleeding-avoidance strategies should be utilized in all patients undergoing PCI. The incidence of such events can be reduced by using newer pharmacological agents, for example bivalirudin, with careful weight-adjusted dosing and a limited duration of therapy. With regard to access, the transradial approach can minimize vascular access complications; however, close attention should be paid to optimizing the puncture technique and sheath removal in whichever route is chosen.
Women have historically been underrepresented in coronary trials and, as such, further research is needed focussing specifically on the female population and we await the results of SAFE PCI for Women. This and other ongoing studies will provide further insight into the optimal management to avoid such events in women in the future.
Future perspective
Contemporary interventional cardiology has made rapid progress, but despite the numerous advances in recent years leading to improved outcomes, certain complications of PCI are still present. Bleeding is one such complication and can result in significant morbidity and, indeed, mortality for the patient affected.
Recent developments have demonstrated newer antithrombotic agents to be more effective in the reduction of ischemic complications and the event of stent thrombosis; however, this has to be balanced against the risk of bleeding associated with more potent agents. Refinements and further new pharmacology may be produced and investigated in the near future to enable a balance with the risk of bleeding. Further studies may help better understand the differing pharmacokinetics seen in the female population, thereby enabling refinement of current pharmacological protocols. Ideally, this may enable us to tailor antithrombotic therapy on a more individualized basis for each patient undergoing PCI.
Vascular access may be performed primarily via the radial access route, with operators becoming increasingly experienced to overcome the problems associated with the learning curve. Newer methods may be devised to allow for adequate hemostasis and reduce the risk of postprocedural bleeding.
Importantly, women have been historically under-represented in major coronary clinical trials. In the next 5 years, there may be more awareness of the sex differences that exist within the field of interventional cardiology, enabling us as interventional cardiologists to provide the safest and most effective therapy based on strong clinical evidence.
Executive summary
▪ Bleeding complications are a common problem during percutaneous coronary intervention (PCI) and can lead to significant morbidity and mortality.
▪ Female sex is associated with an increased risk of periprocedural bleeding and has been shown to be an independent predictor of bleeding.
▪ Female patients have different pharmacokinetics to males, leading to higher circulating levels of antithrombotic agents and are older at presentation, with more comorbidities, such as renal impairment and diabetes mellitus. The site of access is often smaller and more difficult in the female sex.
▪ The incidence of bleeding events can be reduced using newer pharmacological agents, for example bivalirudin, with careful weight-adjusted dosing and a limited duration of therapy.
▪ The transradial approach can minimize vascular access complications; however, close attention should be paid to optimizing the puncture technique and sheath removal in whichever route is chosen.
▪ The multicenter study SAFE PCI for Women is ongoing. This and further research aim to provide further insight into bleeding prevention in females undergoing PCI.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 114(8), 774–782 (2006).
- Manoukian SV, Feit F, Mehran R et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J. Am. Coll. Cardiol. 49(12), 1362–1368 (2007).
- Ndrepepa G, Berger PB, Mehilli J, Seyfarth M, Neumann FJ, Schomig A, Kastrati A. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J. Am. Coll. Cardiol. 51(7), 690–697 (2008).
- Moscucci M, Fox KA, Cannon CP et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur. Heart J. 24(20), 1815–1823 (2003).
- Mehran R, Pocock S, Nikolsky E et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc. Interv. 4(6), 654–664 (2011).
- Suh JW, Mehran R, Claessen BE et al. Impact of in-hospital major bleeding on late clinical outcomes after primary percutaneous coronary intervention in acute myocardial infarction the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J. Am. Coll. Cardiol. 58(17), 1750–1756 (2011).
- Chan MY, Sun JL, Wang TY et al. Patterns of discharge antiplatelet therapy and late outcomes among 8,582 patients with bleeding during acute coronary syndrome: a pooled analysis from PURSUIT, PARAGON-A, PARAGON-B, and SYNERGY. Am. Heart J. 160(6), 1056–1064 (2010).
- Wang TY, Robinson LA, Ou FS et al. Discharge antithrombotic strategies among patients with acute coronary syndrome previously on warfarin anticoagulation: physician practice in the CRUSADE registry. Am. Heart J. 155(2), 361–368 (2008).
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N. Engl. J. Med. 345(17), 1230–1236 (2001).
- Willis P, Voeltz MD. Anemia, hemorrhage, and transfusion in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am. J. Cardiol. 104(5 Suppl.), 34C–38C (2009).
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur. Heart J. 28(10), 1193–1204 (2007).
- McMahon TJ, Moon RE, Luschinger BP et al. Nitric oxide in the human respiratory cycle. Nat. Med. 8(7), 711–717 (2002).
- Welch HG, Meehan KR, Goodnough LT. Prudent strategies for elective red blood cell transfusion. Ann. Intern. Med. 116(5), 393–402 (1992).
- Fransen E, Maessen J, Dentener M, Senden N, Buurman W. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest 116(5), 1233–1239 (1999).
- Malacrida R, Genoni M, Maggioni AP et al. A comparison of the early outcome of acute myocardial infarction in women and men. The Third International Study of Infarct Survival Collaborative Group. N. Engl. J. Med. 338(1), 8–14 (1998).
- Berger JS, Bhatt DL, Cannon CP et al. The relative efficacy and safety of clopidogrel in women and men a sex-specific collaborative meta-analysis. J. Am. Coll. Cardiol. 54(21), 1935–1945 (2009).
- Chacko M, Lincoff AM, Wolski KE et al. Ischemic and bleeding outcomes in women treated with bivalirudin during percutaneous coronary intervention: a subgroup analysis of the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE)-2 trial. Am. Heart J. 151(5), 1032 e1031–e1037 (2006).
- Alexander KP, Chen AY, Newby LK et al. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines) initiative. Circulation 114(13), 1380–1387 (2006).
- Ahmed B, Piper WD, Malenka D et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ. Cardiovasc. Interv. 2(5), 423–429 (2009).
- Alexander KP, Chen AY, Roe MT et al. Excess dosing of antiplatelet and antithrombin agents in the treatment of non- ST-segment elevation acute coronary syndromes. JAMA 294(24), 3108–3116 (2005).
- Cox N, Resnic FS, Popma JJ, Simon DI, Eisenhauer AC, Rogers C. Comparison of the risk of vascular complications associated with femoral and radial access coronary catheterization procedures in obese versus nonobese patients. Am. J. Cardiol. 94(9), 1174–1177 (2004).
- Ellis SG, Elliott J, Horrigan M, Raymond RE, Howell G. Low-normal or excessive body mass index: newly identified and powerful risk factors for death and other complications with percutaneous coronary intervention. Am. J. Cardiol. 78(6), 642–646 (1996).
- Gurm HS, Brennan DM, Booth J, Tcheng JE, Lincoff AM, Topol EJ. Impact of body mass index on outcome after percutaneous coronary intervention (the obesity paradox). Am. J. Cardiol. 90(1), 42–45 (2002).
- Fitts J, Ver Lee P, Hofmaster P, Malenka D. Fluoroscopy-guided femoral artery puncture reduces the risk of PCI-related vascular complications. J. Interv. Cardiol. 21(3), 273–278 (2008).
- Sandgren T, Sonesson B, Ahlgren R, Lanne T. The diameter of the common femoral artery in healthy human: influence of sex, age, and body size. J. Vasc. Surg. 29(3), 503–510 (1999).
- Ellis SG, Bhatt D, Kapadia S, Lee D, Yen M, Whitlow PL. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc. Interv. 67(4), 541–545 (2006).
- Agostoni P, Biondi-Zoccai GG, de Benedictis ML et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials. J. Am. Coll. Cardiol. 44(2), 349–356 (2004).
- Tizon-Marcos H, Bertrand OF, Rodes-Cabau J et al. Impact of female gender and transradial coronary stenting with maximal antiplatelet therapy on bleeding and ischemic outcomes. Am. Heart J. 157(4), 740–745 (2009).
- Yoo BS, Yoon J, Ko JY et al. Anatomical consideration of the radial artery for transradial coronary procedures: arterial diameter, branching anomaly and vessel tortuosity. Int. J. Cardiol. 101(3), 421–427 (2005).
- Rathore S, Stables RH, Pauriah M et al. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: a randomized study. JACC Cardiovasc. Interv. 3(5), 475–483 (2010).
- Uhlemann M, Mobius-Winkler S, Mende M et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. JACC Cardiovasc. Interv. 5(1), 36–43 (2012).
- Hamon M, Pristipino C, Di Mario C et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care and Thrombosis of the European Society of Cardiology. EuroIntervention 8(11), 1242–1251 (2013).
- Mehran R, Rao SV, Bhatt DL et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123(23), 2736–2747 (2011).
- Daugherty SL, Thompson LE, Kim S et al. Patterns of use and comparative effectiveness of bleeding avoidance strategies in men and women following percutaneous coronary interventions: an observational study from the National Cardiovascular Data Registry(R). J. Am. Coll. Cardiol. 61(20), 2070–2078 (2013).
- Kidambi A, Mayurathan G, Viswanathan G, Schechter C, Zaman AG. Unfractionated heparin during elective PCI: fixed dose or weight adjusted? Cardiovasc. Ther. 30(1), 1–4 (2012).
- Yusuf S, Mehta SR, Chrolavicius S et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N. Engl. J. Med. 354(14), 1464–1476 (2006).
- Lansky AJ, Mehran R, Cristea E et al. Impact of gender and antithrombin strategy on early and late clinical outcomes in patients with non-ST-elevation acute coronary syndromes (from the ACUITY trial). Am. J. Cardiol. 103(9), 1196–1203 (2009).
- Mehilli J, Neumann FJ, Ndrepepa G et al. Sex-related effectiveness of bivalirudin versus abciximab and heparin in non-ST-segment elevation myocardial infarction. Am. Heart J. 165(4), 537–543 (2013).
- Feit F, Voeltz MD, Attubato MJ et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 trial. Am. J. Cardiol. 100(9), 1364–1369 (2007).
- Hochholzer W, Wiviott SD, Antman EM et al. Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel--Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38). Circulation 123(23), 2681–2689 (2011).
• This large pooled analysis attempts to establish a risk score to calculate the bleeding risk in patients undergoing percutaneous coronary intervention in order to minimize the risk of bleeding.
•• Overview of the role of the radial approach in modern interventional practice and advice on technique, training needs and optimal clinical indications.
• Provides a standardized reporting system for bleeding complications following percutaneous coronary intervention to allow for comparison of major trials.
• Questions the optimal dose of heparin needed for percutaneous coronary intervention and suggests a need for further clinical trials to assess this.