Review Article - Interventional Cardiology (2015) Volume 7, Issue 1
Expanding opportunities in interventional cardiology: reducing aortic insufficiencies with transcatheter aortic valve replacement
- Corresponding Author:
- Samir R Kapadia
Department of Cardiovascular Medicine
Heart & Vascular Institute, Cleveland Clinic
Cleveland, OH 44195, USA
Tel: +1 216 444 6735
Fax: +1 216 445 6142
E-mail: kapadis@ccf.org
Abstract
Aortic insufficiency (AI) is common after transcatheter aortic valve replacement (TAVR). It has been linked to mortality that has limited the expansion of this promising technique. Inappropriate valve sizing relative to the native annulus, calcification in the aortic annulus and imprecise implantation (too low relative to the annulus) are the most common causes for post-TAVR AI. A thorough understanding of these factors may therefore allow overcoming this complication. In this review, we describe the prediction and reduction of these factors to minimize post-TAVR AI.
Keywords
aortic annulus, aortic insufficiency, aortic regurgitation, aortic regurgitation index, aortic root angiography, aortic stenosis, balloon valvuloplasty, Edwards SAPIEN valve, Medtronic CoreValve, transcatheter aortic valve replacement, transcatheter heart valve
Transcatheter aortic valve replacement (TAVR) is a viable alternative to surgical aortic valve replacement in high surgical risk patients. However, TAVR has significant limitations that restrict its expansion to the intermediate and low surgical risk population. Aortic insufficiency (AI) is one such limitation that is frequent with both the balloon-expandable (BE) and selfexpanding (SE) valves. It can be either central, supra-skirtal or paravalvular, with the latter being most common. Inadequate seal of the TAVR prosthesis to the aortic annulus has been implicated in its occurrence. Even mild AI after TAVR is of concern because of its potential to negatively impact on survival. Every effort should therefore be made to avoid any AI with comprehensive preprocedural planning and meticulous procedural execution. In addition, newer generation TAVR devices have been designed in an attempt to decrease AI by improving valve positioning and annular seal. This review will discuss the available literature on post-TAVR AI with emphasis on prediction, reduction and upcoming novel valve designs.
Mechanisms for AI after TAVR
During TAVR, balloon aortic valvuloplasty is initially performed to facilitate passage of the prosthetic valve followed by appropriate positioning and anchoring of the valve in the virtual aortic annulus. Unlike surgical aortic valve replacement, sizing of the virtual annulus and positioning are not performed under direct vision but using multimodality imaging. Also, in TAVR, the valve is not sutured but anchored, and thus the annulus is not modified to conform to the implanted valve. Additionally, the native valve leaflets and calcification are not removed but crushed and displaced. Collectively these differences contribute to high rates of AI after TAVR [1-7]. Three types of AI have been identified after TAVR (Figure 1) [8,9].
Central or transvalvular AI is due to structural valve failure causing inadequate coaptation of the leaflets (frozen leaflet, damaged leaflets from crimping or over dilation, asymmetrical balloon deployment) or incorrect sizing of the valve. It is an uncommon type of AI after successful TAVR.
Supra-skirtal insufficiency as the name suggests is from leakage above the skirt of the prosthesis. In the first-generation transcatheter heart valves (THVs), the skirt covers only the lower half of the stent frame. Low implantation of the valve therefore causes AI from the uncovered stent portion.
Paravalvular AI is by far the most common type after TAVR. Any paravalvular AI is estimated to occur in the range of 65–94% [10-15], while moderate-tosevere paravalvular AI is seen in 2–21% of cases [2-7,16]. Incomplete seal of the TAVR prosthesis to the aortic annulus from heavily calcified aortic root, annulus prosthesis mismatch or suboptimal implantation depth can all result in paravalvular insufficiency.
Grading of AI
Aortic root angiography can be performed safely and easily during the TAVR procedure. It provides a quick qualitative assessment to initiate corrective measures if necessary (greater than mild AI). To match the VARC2 grading system [17], three degrees of AI can be identified on angiography [18]: mild – reflow of a small amount of contrast into the left ventricular (LV) outflow tract (LVOT) and mid-LV cavity that washes out completely with each cardiac cycle; moderate – reflow of contrast into the entire LV with incomplete washout and with opacification less than that seen in the ascending aorta; and severe – reflow of contrast into the entire LV, absence of washout and with opacification similar to that in the ascending aorta. It has to be recognized that the dye load and rate of injection have to be proper to assess AR as per sellar’s criteria [10]. In the CHOICE trial [19], investigators used this method with core lab oversight to grade AR. Although simple, angiographic grading of AI can be subjective and should be used in conjunction with other measures (hemodynamic and echocardiography) in the presence of any AI.
Invasive hemodynamic assessment of AI using the ‘AR index’ has been used to evaluate and stratify the degree of AI in the peri-implant period when in doubt [20]. AR index is calculated as a ratio of the gradient between diastolic blood pressure (DBP) and left ventricular end-diastolic pressure (LVEDP) to systolic blood pressure (SBP): [(DBP – LVEDP)/SBP] × 100. The AR index should not be performed immediately after valve deployment to avoid confounding by an increased LVEDP due to myocardial ischemia from rapid pacing. It is recommended that the AR index be performed approximately 10 min after valve deployment. Sinning et al. showed an inverse relationship between the AR index and degree of AI. An AR index less than 25 was associated with higher degrees of AI and mortality at follow-up. However, an AR index less than 25 was not specific for moderate/severe AI, and there was considerable overlap between grades making it less useful on its own. The AR index is also sensitive to rapid heart rates and should ideally be measured over several cardiac cycles averaging 60–80 beats per minute and with extra systolic beats [21].
Echocardiography is the most commonly used modality to assess the type and grade of post-procedural AI. Immediately after valve deployment, transesophageal echocardiography (TEE) imaging should be used to assess stent positioning, leaflet motion and AI. TEE is superior to transthoracic echocardiography (TTE) to identify the type and degree of AI immediately postimplant. For paravalvular AI, the short-axis plane of imaging should be just below the TAVR stent and skirt and just within the LVOT. If the imaging plane is above the stent, regurgitation might not be visualized or color flow just above the annulus but contained within the sinuses of Valsalva might be mistaken for regurgitant jets into the LV [22]. The deep gastric view allows imaging of the LVOT without acoustic shadowing. Multiple echocardiographic views should be performed to assess severity. Imaging the entire annulus is mandatory and requires rotating 180° while centered on the valve. The severity of AR typically lessens from that right after implantation. Thus, small central or paravalvular regurgitants do not require intervention. Echocardiographic assessment of AI post-TAVR is nevertheless challenging due to the eccentric and irregular contour of para-valvular jets and from acoustic shadowing and reverberation from the metal prosthesis of the THV [23-25]. Qualitative assessment of AI is therefore rarely adequate. Quantification of the regurgitant volume, effective regurgitant orifice area and regurgitant fraction to assess severity is required in all cases of AI post-TAVR. The regurgitant volume may be calculated as the difference between the stroke volume across any nonregurgitant valve and the stroke volume across the LVOT. Quantitative measures of regurgitant orifice and volume using 3D TTE are particularly useful in this circumstance [26-28]. Quantitative measures to grade AI have now been standardized in the updated VARC2 criteria [17].
Underestimation by either modality is not infrequent, and therefore the assessment of severity should include at least two of the above modalities. In situations of discordance, the more severe assessment should be used to decide further management. An approach to severity assessment and subsequent management has been proposed by Sinning et al. [29].
Impact of AI
AI after TAVR has been associated with adverse outcomes. Even mild AI after TAVR was found to increase all-cause mortality in a meta-analysis (hazard ratio [HR]: 1.82; 95% CI: 1.005–3.329; p = 0.048) [1]. However, this finding was not robust on sensitivity analysis, suggesting that distinction of mild and moderate AR may be problematic and responsible for this instability in the model. PARTNER IA results implicated mild AR for increasing mortality, but it is possible that some patients with a diagnosis of mild AR may have moderate mitral regurgitation that was under appreciated [30]. The negative impact of mild AI has however not always been evident. In the FRANCE2 TAVR registry [3] that reported on 3195 consecutive patients, there was no association between mild AI and mortality. This discrepancy in outcomes has been attributed to underestimation of the degree of AI due to the challenges in its identification and quantification of post-TAVR [1,24]. Moderate or severe AI has however uniformly been associated with mortality. In the FRANCE2 TAVR registry, moderate/severe AI was associated with higher mortality (HR: 2.33; 95% CI: 1.82–2.99). Similar results were reported from other large registries [2,5] and the aforementioned meta-analysis [1]. It is thus critical to assess AR carefully after TAVR and try to avoid any AR that is more than minimal.
Predictors of AI post-TAVR
Multiple studies have identified annulus-prosthesis mismatch, suboptimal device implantation and aortic root calcification as the major culprits for post-TAVR AI [1]. The aortic annulus is a key component of the TAVR procedure as it serves as the anchor point for the transcatheter aortic valve and also because its dimension is used as a standard measurement to choose prosthesis size. Any error in obtaining the aortic annulus diameter therefore compromises procedural success. A thorough understanding of the anatomy of the aortic annulus and pitfalls in its measurement are critical to improve outcomes after TAVR.
Annulus sizing
The aortic annulus is not a true anatomical structure but a virtual basal ring formed by the lowest anchor points of the three aortic leaflets [22,31]. Although called the annulus, the virtual basal ring is not circular in shape but oval and somewhat saddle shaped. No single measurement can therefore describe the annulus. More importantly, different imaging modalities provide different measures of annular dimension [32-34]. Multislice computerized tomography (MSCT) with its 3D capability clearly identifies the noncircular shape of the aortic annulus [35]. The maximal diameter of the aortic annulus (Dmax) using MSCT is routinely obtained in the coronal view and the minimal diameter (Dmin) in the sagittal view [35,36]. The base-to-base measurement of the annulus on 2D TTE/TEE approximates the Dmin obtained on MSCT. In general, the TEE-measured annulus is 1 mm bigger than that on TTE and 1–1.5 mm smaller than MSCT-derived measurements [22,32,33]. To overcome limitations of using a single measurement, an average annular diameter (Da) derived by averaging the Dmax and Dmin values had been used in the past. More recently, an Da derived from the planimetered area (AreaDa) or circumference (CircDa) of the virtual basal ring obtained by 3D imaging has been adapted to the size the annulus [37]. This is because Da obtained from the planimetered area or circumference is more reproducible, reliable and clinically useful as it takes into account the geometry of the annulus. 3D TEE-derived annular measurements are comparable to that obtained on MSCT, with a 9.6–12.89% underestimation of cross-sectional area [38,39].
Schultz et al. [40] evaluated the effect of applying current- sizing guidelines to different MSCT-derived annular measurements in 75 patients referred for TAVR. They found that Dmax and Dmin differed substantially with a mean difference of 6.5 mm (95% CI: 5.7–7.2). Selection based on Dmin would have made 26% of patients ineligible for TAVR, predominantly due to a small annulus, while use of Dmax would have made 39% of patients ineligible due to large annuli. Sizing based on Da or AreaDa would only make 11 and 9% of patients ineligible for TAVR. Similarly, Shahgaldi et al. [41] found that applying current-sizing guidelines would make 36, 12, 4 and 2% of patients ineligible for TAVR due to small annuli based on TTE, 2D TEE, TEE biplane and 3D TEE annular measurements. Jilaihawi et al. [38] investigated the clinical utility of annular diameter obtained from CT/3D TEE cross-sectional area as opposed to 2D TEE in 256 patients undergoing TAVR with the BE valve. They found that Da obtained from cross-sectional techniques using either CT or 3D TEE had the greatest discriminatory value for paravalvular AI. There was a 7.3-fold excess of paravalvular AI (greater than mild) for undersizing by CT and 11.7-fold excess for undersizing by 3D TEE. Pontone et al. identified similar discriminatory value for cross-sectional area obtained by MSCT for predicting moderate-to-severe paravalvular insufficiency was [39]. They reported that a mismatch of 61.5 mm2 between prosthesis size and MSCT-derived cross-sectional area to be a good predictor of paravalvular insufficiency. In a multicenter study that compared the impact of integrating MSCT annulus area sizing algorithm to routine 2D TEE measurements of annular size, there was a significant reduction in paravalvular insufficiency with implementation of the MSCT annulus area [42]. More than mild AI was found in 5.3% of patients in the MSCT group and in 12.8% of patients in the standard treatment group (p = 0.032).
Multimodality imaging provides useful information that should be used in a complimentary fashion to balance the risk of oversizing and relative undersizing. This is especially relevant in complex situations with borderline annulus size or massive calcifications. We recommend routine use of mean annulus/area measurements obtained from cross-sectional data of MPR images obtained on MSCT or 3D TEE as opposed to 2D TEE/TEE to choose prosthesis size. The impact of valve selection based on cross-sectional data on post- TAVR AI is shown in Table 1. When making measurements, the variability in annular dimension and area throughout the cardiac cycle should also be recognized [43-45]. Prior to TAVR, standard measurements of the aortic diameter were performed on gated scans of the thoracic aorta using images at end diastole because of higher resolution from the lack of wall motion at this time. Emerging data in the TAVR era however indicate that imaging of the aortic root and annulus in diastole may be undesirable because of the risk of undersizing of the THV valve that in some instances may lead to significant paravalvular leak [34,46]. In 110 patients with severe aortic stenosis, Blanke et al. [46] found that valve sizing based on systolic AreaDa or CircDa resulted in no undersized valves as opposed to 13.5 and 5.5% undersized valves with corresponding diastolic measurements.
| Assessment of annulus | Study | n | Moderate AR post- procedure, n (%) | Ref. |
|---|---|---|---|---|
| 2D echocardiography | PARTNER A PARTNER B UK TAVI German TAVI France TAVI Binder RK Hansson NC |
348 179 870 690 3195 133 80 |
34 (12.3) 21 (11.8) 115 (13.6) 119 (17.2) 527 (16.5) 17 (12.8) 23 (28.8) |
[47] [30] [4] [48] [49] [42] [50] |
| Cross-sectional annulus assessment using 3D TEE/MSCT or both | Smith LA (3D TEE mean diameter) Jilaihawi H (mean diameter, area and perimeter-derived diameter from MSCT) Wilson AB (CT area >THV nominal area) Binder RK (5–10% area oversizing) Hansson NC (mean annular diameter) |
256 40 56 133 58 |
9 (3.4) 3(7.5) 4(7.1) 7(5.3) 5(8.6) |
[51] [34] [52] [42] [50] |
Table 1: Impact of cross-sectional annulus assessment pre-transcatheter aortic valve replacement on post-transcatheter aortic valve replacement aortic regurgitation.
Despite the tremendous advances in noninvasive imaging, sizing by balloon valvuloplasty (BaV) may still be needed in extreme situations (conflicting noninvasive measurements). This was originally described by Cribier [53]. He determined the stop flow diameter of the aortic valve by performing simultaneous balloon inflation and aortic root contrast injection while looking for AR of injected contrast material. Babaliaros et al. modified the above technique and calculated an ‘additional intraballon pressure’ that they used to size the THV [54]. On an average, the annulus measured by BaV was greater than 1 mm larger than that found on 2D TEE with discrepancies up to 3.5 mm. More importantly selecting prosthesis size based on BaV changed prosthesis selection in 26% of patients with no significant post-procedural AI. The safety and clinical utility of BaV for prosthesis selection was further elucidated by Patsalis et al. [55]. They showed an improvement in valve size selection, degree of paravalvular AI and survival when compared with conventional valve sizing.
A three-step approach called the ‘turnaround rule’ to easily and quickly size the annulus by TEE and MSCT has been proposed by Kasel et al. [56]. It is based on imaging in three planes locked at a 90° angle.
Valve selection
Controlled oversizing of the THV relative to the annulus is performed during TAVR to achieve stable anchoring of the THV and to minimize paravalvular AI (Tables 2 & 3). The THV valves have traditionally been oversized by 4–30% relative to the diameter measurement of the aortic annulus [57,58]. Self- expandable valves are oversized more than BE valves for the same annular size.
| Valve type | Valve size | 2D TTE/TEE annular diameter (mm) | Absolute oversizing (mm) | Relative oversizing (%) |
|---|---|---|---|---|
| Medtronic CoreValve | 23 26 29 31 |
18–20 20–23 23–27 26–29 |
3–5 3–6 2–6 2–5 |
15–28 13–30 7–26 7–19 |
| Edwards SAPIEN | 23 26 29 |
18–22 21–25 24–28 |
1–5 1–5 1–5 |
4–28 4–24 4–21 |
| SAPIEN XT | 23 26 29 |
18–22 21–25 24–27 |
1–5 1–5 2–5 |
4–28 4–24 7–21 |
Table 2: Selection of valve size based on annular diameter.
| Valve type | Valve size (mm) | Annulus size (mm) | Absolute oversizing (mm) | Relative oversizing (%) | Ref. |
|---|---|---|---|---|---|
| Direct Flow Medical Valve | 25 27 23 25 27 |
19–24 24–26 21–22.9 23–24.9 25–27 |
1–6 1–3 0.1–2 0.1–2 0–2 |
4–32 4–12 0.5–10 0.5–9 0–8 |
[59] |
| Portico Valve | 23 | 19–21 | 2–4 | 10–21 | [60] |
| Sadra Lotus Valve | 23 27 |
19–22 23–27 |
1–4 0–4 |
5–21 0–17 |
[61] |
| ACURATE TF Valve | S M L |
21–23 23–25 25–27 |
0–2 0–2 0–2 |
0–10 0–9 0–8 |
[62] |
| ACURATE TA Valve | 23 25 27 | 20–23 24–25 26–27 | 0–3 0–1 0–1 | 0–15 0–4 0–4 | [63] |
| ENGAGER Valve | 23 26 |
19–23 23–26 |
0–4 0–3 |
0–21 0–13 |
[64] |
| CENTERA Valve | 26 | 20–23 | 3–6 | 13–30 | [65] |
| CoreValveEvolut | 23 | 18–20 | 3–5 | 15–28 | [66] |
| SAPIEN 3 Valve | 23 26 29 |
18–22 21–25 24–28 |
1–5 1–5 1–5 |
5–28 4–24 4–21 |
[67] |
Table 3: Selection of valve size for second-generation valves based on annular diameter.
Detaint et al. [68] studied the effect of undersizing using the cover index 100 × (prosthesis diameter – TEE annulus diameter)/prosthesis diameter. A low cover index was found to be an independent predictor of significant AR (odds ratio [OR]: 1.22; 95% CI: 1.03–1.51). Patients with a cover index greater than 8% did not have significant AR after implantation of the Edwards valve. These results have since been replicated in other studies [20,69]. Oversizing the BE valve to achieve a cover index of 12.4 ± 4.3 decreased the occurrence of moderate and severe AI after TAVR in a study by Samim et al. [69]. With use of the SE THV, a cover index of 16.0 ± 4.7 or more resulted in none to mild para-valvular leak [20]. This difference in cover index between the BE and SE THV is consistent with the work of Buzzatti et al. [70], who showed that a 2% oversizing may be sufficient for the BE valve, whereas 11.5% oversizing is required for the SE valve to avoid significant paravalvular leak. An indirect evidence to support the concept of oversizing to prevent paravalvular AI has also been provided by Kalavrouziotis et al. [71]. Significant AR was never observed in patients with an aortic annulus less than 22 mm, after implantation of a 23-mm Edwards valve. Willson et al. [52] dichotomized patients implanted with the CoreValve into those with oversized valves (prosthesis diameter ≥1 mm of CT-measured annulus) and those with undersized valves (prosthesis diameter <1 mm than the CT-measured annulus). The incidence of post-TAVI AR was 2.2% in patients with an oversized prosthesis as opposed to 21.4% in patients with an undersized prosthesis (OR: 9.4; 95% CI: 2.15–88.8; p < 0.01).
Lately oversizing based on MSCT annular area or annular circumference has become the standard. In a retrospective analysis of 109 patients, Willson and colleagues [52] showed that a THV area greater than 14.2 ± 18.3 of the annular area was associated with none/trivial AI, while a THV area less than the annular area (−7.0 ± 9.5) was associated with moderate to severe AR with use of the BE valve. The same authors showed that use of MSCT annular area for prosthesis sizing would have resulted in a larger valve size being implanted in 33.3% of patients when compared with 2D TEE [72]. Mild or higher grades of paravalvular AI were seen in 85% of these patients suggesting undersizing of prosthesis using 2D TEE. From our experience, CircDa may be preferred over AreaDa for SE valves to prevent undersizing, and AreaDa over CircDa to avoid oversizing. This is likely due to alteration of the annular geometry to a circular configuration over time with the BE valve [46,73].
Valve implantation
Post-procedural paravalvular AI is also influenced by the implantation depth [1,74-75]. Valve positioning is widely performed under fluoroscopy and angiography with or without echocardiographic guidance. Choosing the correct fluoroscopic plane is critical for the success of TAVR. Ideally, valve positioning is performed in a perpendicular valve view with all three cusps separately detected in one plane. On occasion, it is time-consuming to perform multiple aortic root injections to find the perpendicular view, or finding it may be impossible on fluoroscopy and angiography alone. TAVR may then be performed in incorrect projects with the risk of sub optimal valve positioning and its implications. When misplaced either high or low, the skirt of the prosthetic valve does not provide an adequate seal around the annulus resulting in AR. The unequal geometry of the CoreValve with a narrow and tapered midsection further contributes to paravalvular leak from an inadequate seal when misplaced. Takagi et al. [76] showed that low CoreValve implantation increased the odds of significant AR (3.67; 95% CI: 1.01–13.35). Sheriff et al. [77] and Jilaihawi et al. [78] showed that a 9.5- and 5–10-mm-device depth, respectively, from the noncoronary cusp minimized the risk of significant AR for the CoreValve. More recently, MSCT and 3D angiographic reconstruction with or without aortic valve guide (AVG) have been used to predict the optimal implant angles. In 106 consecutive patients, Poon et al. [79] compared the use of AVG with 3D angiographic reconstruction to MSCT alone, or fluoroscopic alone, in optimal implant angle prediction and impact on paravalvular AI. They found that excellent implant angles were obtained using AVG on top of 3D angiographic reconstruction, when compared with MSCT alone and fluoroscopy alone (83.7 vs 52.3 vs 42.1%; p = 0.001). An excellent implant angle was more likely to be associated with no paravalvular AI (41.3 vs 21.6%; p = 0.045). Newer generation delivery catheters have also impacted device positioning. Chorianopoulos et al. [80] demonstrated that use of the new AccuTrak delivery system for the CoreValve decreased post-procedural significant AR by superior device positioning. The use of the AccuTrak delivery catheter resulted in higher and more accurate positioning of the CoreValve in the LVOT (distance from annulus to lower edge of prosthesis 7.0 mm for the AccuTrak group vs 8.8 mm for the original system).
Landing zone calcification
Aortic root calcium has also been implicated in post- TAVR AI [1,81-83]. Calcification in the device-landing zone causes post-TAVR AI by hindering uniform expansion and tight sealing of the valve to the native annulus. We previously reported a positive correlation between aortic valve calcium score and development of significant AR post-TAVR (r = 0.47; 95% CI: 0.30–0.61; p = 0.001) in a pooled analysis of four studies [1]. The role of calcium in causing paravalvular leak is however not entirely clear due to mixed results. Staubach et al. [84] in the analysis of the German TAVI registry on 1365 patients found that the extent of aortic valve calcification did not influence the severity of post-TAVR AR. A similar finding was reported by Wood et al. [85] in a small study of 26 patients. Other investigators however found that an Agatston score greater than 3000 predicted significant AR after initial release of the CoreValve and the need for postdilation [82,83,86,87]. Similarly, other quantitative calcium scores have also been shown to correlate positively with the occurrence of post-TAVR AR. Ewe et al. [88] further showed that calcium at the aortic wall of each valve cusp was more predictive of post-TAVR AR than calcium at the valvular edge or body. Colli et al. [89] observed that calcification at the commissure was significantly associated with the occurrence of significant AR post-TAVR. Despite the conflicting evidence, precise quantitation of the extent and location of calcification in the aortic root may provide further insight into the selection of appropriate candidates and devices for TAVR.
CoreValve versus Edwards valve
The wealth of worldwide experience in TAVR is shared by the SE Medtronic CoreValve and the BE Edwards SAPIEN valve. Both valves though trileaflet and supported by a metallic frame have significant differences. The Edwards valve consists of three bovine pericardial leaflets mounted on a BE stainless steel or cobalt chromium stent that measures 14.3 or 16.1 mm in height. A fabric skirt at the inflow provides an effective seal for 7–8 mm of the stent to prevent paravalvular leak. The CoreValve on the other hand consists of three porcine pericardial leaflets mounted on a SE nitinol stent frame that measures 53–55 mm in height. The stent frame has three levels described as inlet (high radial force to expand against the calcified leaflets), valvular (the narrowest portion to avoid coronary obstruction) and outlet (flared to fix and align in the ascending aorta). Similar to the Edwards design, the CoreValve has a skirt that measures 8 mm in height to prevent paravalvular leak. While the leaflets of the Edwards THV function intra-annular those of the CoreValve function supra annular. Furthermore, both valves also differ in valve mounting, delivery and implantation. Whether these differences influence the occurrence of paravalvular leak is a topic of large-scale interest with expansion of TAVR to the intermediate risk population.
In the French TAVR registry, the influence of valve design on post-procedural AI was explored in 2769 patients (BE: 67.6%; SE: 32.4%) [3]. Post-procedural AI more than mild was more frequent in the SE group (21.5 vs 13%; p = 0.0001). On multivariate analysis, the use of the SE valve was an independent predictor of greater than mild AI (OR: 2.03; 95% CI: 1.46–2.83). Interestingly annulus dimension, prosthesis diameter and cover index were predictors of AI with use of the BE valve but not with the SE valve. We previously reported the higher risk of paravalvular AI with the SE device in a pooled analysis of 45 reports (16.1 vs 9.1%) [1]. In the only head-to-head randomized comparison of the two devices (CHOICE trial [19]), more than mild AI was again significantly higher in the SE valve group (18.3 vs 4.1%; relative risk: 4.34; 95% CI: 1.72–11.11; p < 0.001). There was also no reduction in AI over time (30 days). Longer follow up results are however needed to confirm the evolution of AI because earlier reports have suggested a decrease in AI over time with the SE valve. In the CoreValve Extreme Risk Registry [90], AI at 1 year was much less frequent than at early follow-up. The higher incidence of post-procedural AI with the SE valve is likely due to impaired apposition of the prosthetic valve to the native annulus and wall of the LVOT from incomplete device expansion. Differences in annular measurement techniques can also influence post-procedural AI. Overtime, unlike the SE valve, the BE valve alters the annular anatomy to an almost circular shape [46,73]. Area-derived annular diameter is therefore suitable for BE valve size selection and perimeter-derived annular area for SE valve size selection. This may be particularly relevant in highly calcific lesions. An increasing angle between the LVOT and the ascending aorta also has a similar effect on the CoreValve by reducing its ability to form a tight seal to close the paravalvular space [77]. Balloon postdilation (BPD) and a greater oversizing as opposed to the Edwards valve may overcome the under expansion of the CoreValve in these situations. Another factor that is important for reducing AR with the CoreValve, which is not frequently mentioned, is the height of implantation [76-78]. Because of the noncylindrical shape of the valve, the depth of implantation determines the effective diameter of the valve in the annulus. Particularly in larger annuli, the sealing of the CoreValve at the level of the virtual ring is dependent on a high implantation in order to take advantage of the diameter of the lower part of the valve.
Intraprocedural management of AI
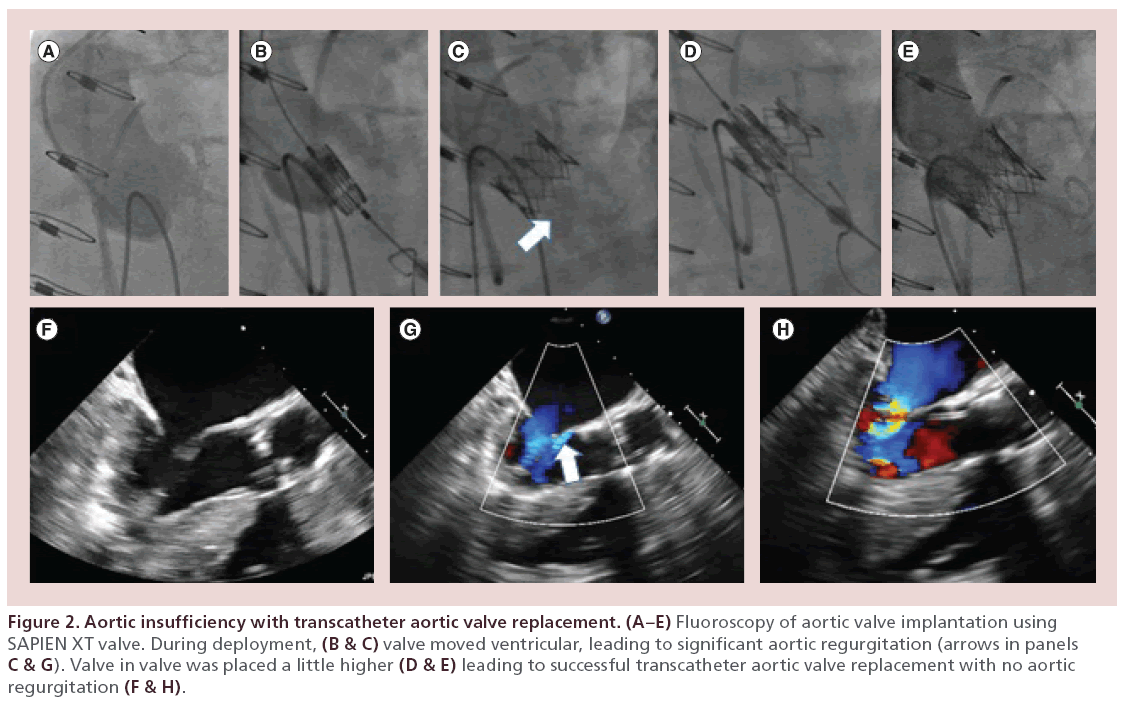
Any AI of greater than mild severity should be treated. The strategy depends on the etiology of post-TAVR AI. BPD is especially useful when there is heavy calcification or possible under expansion of the THV. Care has to be taken to make sure that the size of the balloon used should be less than the diameter of the original aortic valve dimension as otherwise complications can include aortic rupture or central AI. Prior experience suggests that a balloon with a diameter of 22, 25, 28 and 29 mm is recommended for the 23, 26, 29 and 31 mm CoreValve, respectively, and in case of the Edwards SAPIEN valve, expansion should be done with the same balloon as used for delivery of the THV initially but adding 1 ml saline to the total volume to increase its diameter [29]. The Valve-in-Valve implantation strategy can be used in cases where malposition or prosthetic leaflet dysfunction is the cause of the AI. When the initial THV implantation is felt to be either too aortic or too ventricular causing malposition and subsequent AI, a Valve-in-Valve implantation seems to be viable treatment strategy to reduce the AI [91]. Severe transvalvular AI from mechanical dysfunction of the THV can be treated similarly by implanting a second valve (Figure 2). Potential challenges to the Valve-in-Valve approach are under expansion of the second valve or coronary obstruction. Snaring the misplaced CoreValve by hooking and pulling with a snare to facilitate repositioning, when the original implant is too low or ventricular, has also been described in literature. Aortic dissection or embolization of the valve is a possible complication of this technique. In some cases of failed or risky BPD, the localized paravalvular AI may be treated using an Amplatzer Vascular Plug (Amplatzar Vascular Plug, St Jude Medical Inc., St Paul, MN, USA) to seal the leak [92,93].
Figure 2: Aortic insufficiency with transcatheter aortic valve replacement. (A–E) Fluoroscopy of aortic valve implantation using SAPIEN XT valve. During deployment, (B & C) valve moved ventricular, leading to significant aortic regurgitation (arrows in panels C & G). Valve in valve was placed a little higher (D & E) leading to successful transcatheter aortic valve replacement with no aortic regurgitation (F & H).
Emerging valve designs to reduce AI
Newer second-generation transcatheter aortic valves have hence been developed to overcome limitations of the SE and BE valve to improve deliverability, sealing to prevent paravalvular leak and the ability to reposition or retrieve when necessary. A number of secondgeneration devices with these desirable features are in investigation and have undergone successful clinical evaluation with promising results. Eight different second-generation valves have so far received the CE mark approval and are available for commercial use in Europe. The Direct Flow Medical [59] THV (Direct Flow Medical, Inc, Santa Rosa, CA, USA) is a trileaflet bovine pericardial valve mounted on a stentless-covered frame that has an upper and lower circular ring connected by tubular supports. Design of the valve offers precise positioning, ability to reposition and retrieve and hemodynamic assessment prior to final deployment. In the DISCOVER trial [94], mild or no AI was seen in 99% of the patients. The Portico Valve [60] (St Jude Medical Inc., minneapolis, MN, USA) is a SE valve that allows for full resheathing and repositioning prior completing valve deployment. In the Portico Transfemoral CE Mark trial [95], 95% of patients had mild to no AI at 30 days. The Boston Lotus Valve [96] (Boston Scientific, Natick, MA, USA) trileaflet bovine pericardial tissue valve is mounted on a nitinol stent. The valve has an adaptive seal to that is designed to conform to the irregular annulus to minimize paravalvular leak. In the REPRISE II trial [97] at 30 days, there was no case of severe AI and only one case of moderate AI. Ninety-eight percent of patients had no, trivial or mild AI. The Edwards SAPIEN 3 [67] (S3) has an internal and external skirt to reduce paravalvular leak. The early experience with the S3 device reported no postmoderate or severe AI at 30 days [98]. The Symetis ACURATE TA and TF valves [63] (Symetis Inc., Switzerland) have a unique self-seating and self-sealing architecture. In the ACURATE TF [99] study, 80 patients underwent transfemoral implantation of the ACURATE TF valve. No or mild paravalvular leak was seen in 94.6% of patients. The JenaValve [100] (JenaValve, Munich, Germany) has a unique anchoring system that holds the valve in place and is designed to reduce paravalvular leak. In the JUPITER registry [101], 97.6% of patients had no or mild AI at 30 days. The Medtronic Engager valve [64] (Medtronic Inc., minneapolis, MN, USA) has a support frame the arms of which are designed to be placed in the aortic sinuses to achieve accurate implantation. In the European pivotal trial [102], there were no cases of moderate-to-severe AI. In total, 96% of patients had no or trivial AI. The Edwards CENTERA valve [65] (Edwards Lifescience, Irvine, CA, USA) has a unique motorized delivery system. In the feasibility study [103] that enrolled 15 patients, no or trace AI was seen in 3 (23%), mild AI in 9 (69%) and moderate AI in 1 patient (8%). In a pooled analysis [Athappan G et al., Unpublished Data] of all trials reporting experiences with newer generation valves, we found an incidence rate of 3.3% (95% CI: 1.8–6.6) for moderate to severe AI after TAVR.
Conclusion
Post-TAVR AI is common with use of first-generation THV. Mismatching of the prosthesis to the annulus and suboptimal device implantation are the major determinants of AI. Systolic 3D measurements of annular area and annular perimeter are the more reproducible, accurate and clinically relevant compared with 2D annular measurements. Incorporation of data from multimodality imaging with emphasis on 3D valve sizing will reduce post-TAVR AR. Use of MSCT or 3D angiographic reconstruction data to predict the optimal plane for implantation will improve implantation and reduce AI. Newer generation valves with specialized anchors, redesigned skirts, adaptive seals and ability to reposition and retrieve provide hope for further reduction in post-TAVR AI.
Future perspective regarding TAVR
TAVR has emerged as a safe and effective alternative to surgical aortic valve replacement. It continues to evolve rapidly with many new devices/valves in different stages of clinical testing. With the new devices, there is an emphasis on flexibility, deliverability, easier positioning and retrievability. Following success in high surgical risk patients, intermediate surgical risk patients (Society of Thoracic Surgeons, STS score of 3–8) seem the next logical group of patients who may benefit from it. Other groups of patients who may benefit include patients with failed surgical prosthesis and AR. While there is an obvious temptation to rapidly expand the potential indications of TAVR, it would be prudent to tread cautiously in this direction until there is robust evidence supporting the same.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Mechanisms for aortic insufficiency after transcatheter aortic valve replacement
• The native stenosed valve is crushed and displaced but not removed.
• The transcatheter heart valve is anchored under imaging guidance and not sutured.
• The annulus is not modified to conform to the implanted valve.
Grading of aortic insufficiency
• Aortic root angiography, aortic regurgitation (AR) index and echocardiography should all be used to assess severity of aortic insufficiency (AI) in the peri-implant period.
• Underestimation by either modality is not infrequent, and therefore, the assessment of severity should include at least a combination of two of the above modalities.
Impact of AI
• Even mild AI after transcatheter aortic valve replacement (TAVR) can increase mortality (hazard ratio: 1.82; 95% CI: 1.005–3.329; p = 0.048).
Predictors of AI post-TAVR
• Annulus-prosthesis mismatch, suboptimal device implantation and aortic root calcification are known predictors of AI.
Intraprocedural management of AI
• Balloon postdilation, valve-in-valve implantation, snaring and amplatzar plugging are available options to manage post-TAVR AI.
Emerging valve designs to reduce AI
• Second-generation valves have improved deliverability, sealing and ability to reposition or retrieve when necessary.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- Athappan G, Patvardhan E, Tuzcu EM et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J. Am. Coll. Cardiol. 61(15), 1585–1595 (2013).
- Abdel-Wahab M, Zahn R, Horack M et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 97(11), 899–906 (2011).
- Van Belle E, Juthier F, Susen S et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FR ANCE2 registry. Circulation 129(13), 1415–1427 (2014).
- Moat NE, Ludman P, De Belder MA et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) registry. J. Am. Coll. Cardiol. 58(20), 2130–2138 (2011).
- Tamburino C, Capodanno D, Ramondo A et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 123(3), 299–308 (2011).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364(23), 2187–2198 (2011).
- Stahli BE, Gebhard C, Falk V, Corti R, Jenni R, Tanner FC. Regurgitation after Edwards SAPIEN valve implantation: truly paravalvular or ‘supra-skirtal’? Eur. Heart J. 34(16), 1214 (2013).
- Stahli BE, Maier W, Corti R, Luscher TF, Jenni R, Tanner FC. Aortic regurgitation after transcatheter aortic valve implantation: mechanisms and implications. Cardiovasc. Diag. Ther. 3(1), 15–22 (2013).
- Dvir D, Barbash IM, Ben-Dor I et al. Paravalvular regurgitation after transcatheter aortic valve replacement: diagnosis, clinical outcome, preventive and therapeutic strategies. Cardiovasc. Revasc. Med. 14(3), 174–181 (2013).
- Walther T, Simon P, Dewey T et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 116(Suppl. 11), I240–I245 (2007).
- Rodes-Cabau J, Dumont E, De Larochelliere R et al. Feasibility and initial results of percutaneous aortic valve implantation including selection of the transfemoral or transapical approach in patients with severe aortic stenosis. Am. J. Cardiol. 102(9), 1240–1246 (2008).
- Cribier A, Eltchaninoff H, Tron C et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J. Am. Coll. Cardiol. 47(6), 1214–1223 (2006).
- Rajani R, Kakad M, Khawaja MZ et al. Paravalvular regurgitation one year after transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 75(6), 868–872 (2010).
- Webb JG, Pasupati S, Humphries K et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116(7), 755–763 (2007).
- Thomas M, Schymik G, Walther T et al. Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 122(1), 62–69 (2010).
- Kappetein AP, Head SJ, Genereux P et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 60(15), 1438–1454 (2012).
- Werner N, Sinning JM. Aortic regurgitation after transcatheter aortic valve replacement. Circulation J. 78(4), 811–818 (2014).
- Abdel-Wahab M, Mehilli J, Frerker C et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 311(15), 1503–1514 (2014).
- Sinning JM, Hammerstingl C, Vasa-Nicotera M et al. Aortic regurgitation index defines severity of peri- prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 59(13), 1134–1141 (2012).
- Sinning JM, Werner N, Nickenig G, Grube E. Challenges in transcatheter valve treatment: aortic regurgitation after transcatheter aortic valve implantation. EuroIntervention 9, S72–S76 (2013).
- Bloomfield GS, Gillam LD, Hahn RT et al. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc. Imaging 5(4), 441–455 (2012).
- Raffa GM, Malvindi PG, Settepani F et al. Aortic valve replacement for paraprosthetic leak after transcatheter implantation. J. Card. Surg. 27(1), 47–51 (2012).
- Tuzcu EM, Kapadia SR, Svensson LG. Valve design and paravalvular aortic regurgitation: new insights from the French registry. Circulation 129(13), 1378–1380 (2014).
- Noble S BA, Ibrahim R. Transcatheter prosthetic paravalvular leak closure. Cardiovasc. Med. 15, 245–252 (2012).
- Genereux P, Head SJ, Hahn R et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles’ heel? A comprehensive review of the literature. J. Am. Coll. Cardiol. 61(11), 1125–1136 (2013).
- Zamorano JL, Goncalves A. Three-dimensional echocardiography for quantification of valvular heart disease. Heart 99(11), 811–818 (2013).
- Goncalves A, Almeria C, Marcos-Alberca P et al. Three- dimensional echocardiography in paravalvular aortic regurgitation assessment after transcatheter aortic valve implantation. J. Am. Soc. Echocardiogr. 25(1), 47–55 (2012).
- Sinning JM, Vasa-Nicotera M, Chin D et al. Evaluation and management of paravalvular aortic regurgitation after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 62(1), 11–20 (2013).
- Kodali SK, Williams MR, Smith CR et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 366(18), 1686–1695 (2012).
- Piazza N, De Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ. Cardiovasc. Interv. 1(1), 74–81 (2008).
- Messika-Zeitoun D, Serfaty JM, Brochet E et al. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 55(3), 186–194 (2010).
- Leipsic J, Gurvitch R, Labounty TM et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc. Imaging 4(4), 416–429 (2011).
- Jilaihawi H, Kashif M, Fontana G et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J. Am. Coll. Cardiol. 59(14), 1275–1286 (2012).
- Tops LF, Wood DA, Delgado V et al. Noninvasive evaluation of the aortic root with multislice computed tomographic implications for transcatheter aortic valve replacement. JACC Cardiovasc. Imaging 1(3), 321–330 (2008).
- Altiok E, Koos R, Schroder J et al. Comparison of two- dimensional and three-dimensional imaging techniques for measurement of aortic annulus diameters before transcatheter aortic valve implantation. Heart 97(19), 1578–1584 (2011).
- Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 6(6), 366–380 (2012).
- Jilaihawi H, Doctor N, Kashif M et al. Aortic annular sizing for transcatheter aortic valve replacement using cross- sectional 3-dimensional transesophageal echocardiography. J. Am. Coll. Cardiol. 61(9), 908–916 (2013).
- Pontone G, Andreini D, Bartorelli AL et al. Aortic annulus area assessment by multidetector computed tomography for predicting paravalvular regurgitation in patients undergoing balloon-expandable transcatheter aortic valve implantation: a comparison with transthoracic and transesophageal echocardiography. Am. Heart J. 164(4), 576–584 (2012).
- Schultz CJ, Moelker A, Piazza N et al. Three dimensional evaluation of the aortic annulus using multislice computer tomography: are manufacturer’s guidelines for sizing for percutaneous aortic valve replacement helpful? Eur. Heart J. 31(7), 849–856 (2010).
- Shahgaldi K, Da Silva C, Back M, Ruck A, Manouras A, Sahlen A. Transesophageal echocardiography measurements of aortic annulus diameter using biplane mode in patients undergoing transcatheter aortic valve implantation. Cardiovasc. Ultrasound 11, 5 (2013).
- Binder RK, Webb JG, Willson AB et al. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J. Am. Coll. Cardiol. 62(5), 431–438 (2013).
- De Heer LM, Budde RP, Mali WP, De Vos AM, Van Herwerden LA, Kluin J. Aortic root dimension changes during systole and diastole: evaluation with ECG-gated multidetector row computed tomography. Int. J. Cardiovasc. Imaging 27(8), 1195–1204 (2011).
- Bertaso AG, Wong DT, Liew GY et al. Aortic annulus dimension assessment by computed tomography for transcatheter aortic valve implantation: differences between systole and diastole. Int. J. Cardiovasc. Imaging 28(8), 2091–2098 (2012).
- Lehmkuhl L, Foldyna B, Von Aspern K et al. Inter-individual variance and cardiac cycle dependency of aortic root dimensions and shape as assessed by ECG-gated multi-slice computed tomography in patients with severe aortic stenosis prior to transcatheter aortic valve implantation: is it crucial for correct sizing? Int. J. Cardiovasc. Imaging 29(3), 693–703 (2013).
- Blanke P, Russe M, Leipsic J et al. Conformational pulsatile changes of the aortic annulus: impact on prosthesis sizing by Comput. Tomogr. for transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 5(9), 984–994 (2012).
- MakkarRR, Fontana GP, Jilaihawi H et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N.Engl. J. Med. 366(18), 1696–1704 (2012).
- ZahnR, Gerckens U, Grube E et al. Transcatheteraortic valve implantation: First results from a multi-centre real- worldregistry. Eur. Heart J. 32(2), 198–204 (2011).
- EltchaninoffH, Prat A, Gilard M et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRenchaortic national CoreValve and Edwards) registry. Eur. Heart J. 32(2), 191–197 (2011).
- HanssonNC, Thuesen L, Hjortdal VE et al. Three- dimensional multidetector computed tomography versus conventional2-dimensional transesophageal echocardiography for annular sizing intranscatheter aortic valve replacement: influence on postproceduralparavalvular aortic regurgitation. Catheter Cardiovasc. Interv. 82(6), 977–986 (2013).
- SmithLA, Dworakowski R, Bhan A et al. Real-time three-dimensional transesophageal echocardiography adds value totranscatheter aortic valve implantation. J. Am. Soc. Echocardiogr. 26(4), 359–369 (2013).
- Willson AB, Webb JG, Labounty TM et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J. Am. Coll. Cardiol. 59(14), 1287–1294 (2012).
- Cribier AEH. Preimplantation percutaneous aortic balloon valvotomy - Aortogram during balloon inflation: technique interest. In: Transcatheter Aortic Valve Implantation. Tips And Tricks To Avoid Failure.Serruys PW, Piazza N, Cribier A, Webb JG, Laborde J-C, de Jaegere P (Eds.). Informa Healthcare Inc., New York, NY (2010).
- Babaliaros VC, Junagadhwalla Z, Lerakis S et al. Use of balloon aortic valvuloplasty to size the aortic annulus before implantation of a balloon-expandable transcatheter heart valve. JACC Cardiovasc. Interv. 3(1), 114–118 (2010).
- Patsalis P, Al-Rashid F, Neumann T. Preparatory balloon aortic valvuloplasty during transcatheter aortic valve implantation for improved valve sizing. JACC Cardiovasc. Interv. 6(9), 965–971 (2013); erratum in JACC Cardiovasc. Interv. 6(10), 1110 (2013).
- Kasel AM, Cassese S, Bleiziffer S et al. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc. Imaging 6(2), 249–262 (2013).
- Mylotte D, Martucci G, Piazza N. Patient selection for transcatheter aortic valve implantation: an interventional cardiology perspective. Ann. Cardiothorac. Surg. 1(2), 206–215 (2012).
- Blanke P, Willson AB, Webb JG et al. Oversizing in transcatheter aortic valve replacement, a commonly used term but a poorly understood one: dependency on definition and geometrical measurements. J. Cardiovasc. Comput. Tomogr. 8(1), 67–76 (2014).
- Bijuklic K, Tubler T, Low RI, Grube E, Schofer J. Direct flow medical valve. EuroIntervention 8(Suppl. Q), Q75–Q78 (2012).
- Manoharan G, Spence MS, Rodes-Cabau J, Webb JG. St Jude Medical Portico valve. EuroIntervention 8(Suppl. Q), Q97–Q101 (2012).
- Jilaihawi H, Bonan R, Asgar A et al. Anatomic suitability for present and next generation transcatheter aortic valve prostheses: evidence for a complementary multidevice approach to treatment. JACC Cardiovasc. Interv. 3, 859–866 (2010).
- Mollmann H, Diemert P, Grube E et al. Symetis acurate TF aortic bioprosthesis. EuroIntervention 9(Suppl.), S107–S110 (2013).
- Kempfert J, Mollmann H, Walther T. Symetis ACUR ATE TA valve. EuroIntervention 8(Suppl. Q ), Q102–Q109 (2012).
- Sundermann SH, Holzhey D, Bleiziffer S, Treede H, Falk V. Medtronic Engager bioprosthesis for transapical transcatheter aortic valve implantation. EuroIntervention 9(Suppl.), S97–S100 (2013).
- Ribeiro HB, Urena M, Kuck KH, Webb JG, Rodes-Cabau J. Edwards CENTER A valve. EuroIntervention 8(Suppl. Q ), Q79–Q82 (2012).
- Sinning JM, Werner N, Nickenig G, Grube E. Medtronic CoreValve Evolut valve. EuroIntervention 8(Suppl.), Q94–Q96 (2012).
- Binder RK, Rodes-Cabau J, Wood DA, Webb JG. Edwards SAPIEN 3 valve. EuroIntervention 8(Suppl. Q ), Q83–Q87 (2012).
- Detaint D, Lepage L, Himbert D et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc. Interv. 2(9), 821–827 (2009).
- Samim M, Stella PR, Agostoni P et al. A prospective “oversizing” strategy of the Edwards SAPIEN bioprosthesis: results and impact on aortic regurgitation. J. Thorac. Cardiovasc. Surg. 145(2), 398–405 (2013).
- Buzzatti N, Maisano F, Latib A et al. Computed tomography-based evaluation of aortic annulus, prosthesis size and impact on early residual aortic regurgitation after transcatheter aortic valve implantation. Eur. J. cardiothorac. Surg. 43(1), 43–50; discussion 50–41 (2013).
- Kalavrouziotis D, Rodes-Cabau J, Bagur R et al. Transcatheter aortic valve implantation in patients with severe aortic stenosis and small aortic annulus. J. Am. Coll. Cardiol. 58(10), 1016–1024 (2011).
- Willson AB, Webb JG, Freeman M et al. Computed tomography-based sizing recommendations for transcatheter aortic valve replacement with balloon- expandable valves: comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J. Cardiovasc. Comput. Tomogr. 6(6), 406–414 (2012).
- Blanke P, Siepe M, Reinohl J et al. Assessment of aortic annulus dimensions for Edwards SAPIEN transapical heart valve implantation by computed tomography: calculating average diameter using a virtual ring method. Eur. J. cardio- thorac. Surg. 38(6), 750–758 (2010).
- Petronio AS, Giannini C, De Carlo M. Mechanisms and prediction of aortic regurgitation after TAVI. EuroIntervention 8(Suppl. Q ), Q18–Q20 (2012).
- Abdel-Wahab M, Comberg T, Buttner HJ et al. Aortic regurgitation after transcatheter aortic valve implantation with balloon- and self-expandable prostheses: a pooled analysis from a 2-center experience. JACC Cardiovasc. Interv. 7(3), 284–292 (2014).
- Takagi K, Latib A, Al-Lamee R et al. Predictors of moderate-to-severe paravalvular aortic regurgitation immediately after CoreValve implantation and the impact of postdilatation. Catheter. Cardiovasc. Interv. 78(3), 432–443 (2011).
- Sherif MA, Abdel-Wahab M, Stocker B et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J. Am. Coll. Cardiol. 56(20), 1623–1629 (2010).
- Jilaihawi H, Chin D, Spyt T et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the Medtronic CoreValve bioprosthesis. Eur. Heart J. 31(7), 857–864 (2010).
- Poon KK, Crowhurst J, James C et al. Impact of optimising fluoroscopic implant angles on paravalvular regurgitation in transcatheter aortic valve replacements - utility of three- dimensional rotational angiography. EuroIntervention 8(5), 538–545 (2012).
- Chorianopoulos E, Krumsdorf U, Pleger ST, Katus HA, Bekeredjian R. Improved procedural results after CoreValve implantation with the New AccuTrak delivery system. J. Interv. Cardiol. 25(2), 174–179 (2012).
- Haensig M, Rastan AJ. Aortic valve calcium load before TAVI. Is it important? Ann. Cardiothorac. Surg. 1(2), 160–164 (2012).
- Haensig M, Lehmkuhl L, Rastan AJ et al. Aortic valve calcium scoring is a predictor of significant paravalvular aortic insufficiency in transapical-aortic valve implantation. Eur. J. Cardiothorac. Surg. 41(6), 1234–1240; discussion 1240–1231 (2012).
- Unbehaun A, Pasic M, Dreysse S et al. Transapical aortic valve implantation: incidence and predictors of paravalvular leakage and transvalvular regurgitation in a series of 358 patients. J. Am. Coll. Cardiol. 59(3), 211–221 (2012).
- Staubach S, Franke J, Gerckens U et al. Impact of aortic valve calcification on the outcome of transcatheter aortic valve implantation: results from the prospective multicenter German TAVI registry. Catheter. Cardiovasc. Interv. 81(2), 348–355 (2013).
- Wood DA, Tops LF, Mayo JR et al. Role of multislice computed tomography in transcatheter aortic valve replacement. Am. J. Cardiol. 103(9), 1295–1301 (2009).
- John D, Buellesfeld L, Yuecel S et al. Correlation of device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc. Interv. 3(2), 233–243 (2010).
- Schultz C, Rossi A, Van Mieghem N et al. Aortic annulus dimensions and leaflet calcification from contrast MSCT predict the need for balloon post-dilatation after TAVI with the Medtronic CoreValve prosthesis. EuroIntervention 7(5), 564–572 (2011).
- Ewe SH, Ng AC, Schuijf JD et al. Location and severity of aortic valve calcium and implications for aortic regurgitation after transcatheter aortic valve implantation. Am. J. Cardiol. 108(10), 1470–1477 (2011).
- Colli A, D’amico R, Kempfert J, Borger MA, Mohr FW, Walther T. Transesophageal echocardiographic scoring for transcatheter aortic valve implantation: impact of aortic cusp calcification on postoperative aortic regurgitation. J. Thorac. Cardiovasc. Surg. 142(5), 1229–1235 (2011).
- Popma JJ. CoreValve US Pivotal Trial. Extreme Risk Iliofemoral Study Results. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 19- 23 October 2013.
- Toggweiler S, Wood DA, Rodes-Cabau J et al. Transcatheter valve-in-valve implantation for failed balloon-expandable transcatheter aortic valves. JACC Cardiovasc. Interv. 5(5), 571–577 (2012).
- Rihal CS, Sorajja P, Booker JD, Hagler DJ, Cabalka AK. Principles of percutaneous paravalvular leak closure. JACC Cardiovasc. Interv. 5(2), 121–130 (2012).
- Feldman T, Salinger MH, Levisay JP, Smart S. Low profile vascular plugs for paravalvular leaks after TAVR. Catheter Cardiovasc. Interv. 83(2), 280–288 (2014).
- Schofer J. Prospective, multicenter evaluation the direct flow medical® transcatheter aortic valve: the discover trial 6-month follow up. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 19- 23 October 2013.
- Manoharan G. Portico ce trial: assessment the St. Jude medical portico™ transcatheter aortic valve implant the transfemoral delivery system. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 19- 23 October 2013.
- Meredith IT, Hood KL, Haratani N, Allocco DJ, Dawkins KD. Boston Scientific Lotus valve. EuroIntervention 8(Suppl. Q ), Q70–Q74 (2012).
- Meredith I T. Reprise II. A prospective registry study transcatheter aortic valve replacement with a repositionable transcatheter heart valve in patients with severe aortic stenosis. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 19-23 October 2013.
- Binder RK, Rodes-Cabau J, Wood DA et al. Transcatheter aortic valve replacement with the SAPIEN 3: a new balloon- expandable transcatheter heart valve. JACC Cardiovasc. Interv. 6(3), 293–300 (2013).
- Mollmann H. Accurate TF technology, clinical results, and case examples. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 19-23 October 2013.
- Treede H, Rastan A, Ferrari M, Ensminger S, Figulla HR, Mohr FW. JenaValve. EuroIntervention 8(Suppl. Q ), Q88–Q93 (2012).
- Ensminger S. JenaValve clinical results JUPITER Registry AR results. Presented at: Transcatheter Valve Therapies. Vancouver, Canada, 12-15 June 2013.
- Treede H. Medtronic’s TAVR Systems: Engager TA. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 19-23 October 2013.
- Binder RK, Schafer U, Kuck KH et al. Transcatheter aortic valve replacement with a new self-expanding transcatheter heart valve and motorized delivery system. JACC Cardiovasc. Interv. 6(3), 301–307 (2013).
• Meta analysis on aortic insufficiency.
• CoreValve versus Edwards valve for aortic regurgitation.
• Assesment of AI.
• Comprehensive review.
• AI severity assesment.
• Paravalvular leak.