Review Article - Interventional Cardiology (2012) Volume 4, Issue 5
Factors influencing the outcomes of percutaneous coronary intervention in the stent era
- Corresponding Author:
- Jinnette Dawn Abbott
Division of Cardiology, Rhode Island Hospital
Warren Alpert Medical School, Brown University
814 APC, 593, Eddy Street, Providence, RI 02903, USA
Tel: +1 401 444 5328
Fax: +1 401 444 4652
E-mail: jabbott@lifespan.org
Abstract
Keywords
complications,coronary intervention,outcomes,restenosis,stents,stent thrombosis
Introduction
Since percutaneous coronary intervention (PCI) was first performed over 30 years ago tremendous progress has been made with regards to making the procedure safer and more effective, while at the same time expanding the indications and patient populations treated. Although this article will focus on the factors influencing PCI outcomes in contemporary practice, in the era of stents and aggressive pharmacology, an understanding of the progress in the field thus far is warranted. Several large studies have documented temporal trends in the characteristics of patients treated with PCI and the in-hospital outcomes of the procedure. The National Heart, Lung and Blood Institute-sponsored 1985–1986 percutaneous transluminal coronary angioplasty and 1997–2006 Dynamic Registries, the Northern New England Registry and the Mayo Clinic PCI Registry have examined outcomes for over 68,000 consecutive PCI procedures over time [1–3]. All of these studies demonstrated an increase in the complexity of patients treated over time including older age, increased comorbidity and increasing treatment of acute coronary syndromes (ACSs). However, with the routine use of stents supplanting percutaneous transluminal coronary angioplasty, procedural success has significantly increased from 78–82 to 94–95%. Rates of major complications over the same time period have decreased including: emergent coronary artery bypass grafting (CABG) 3.7–5 down to 0.4%, and mortality from 3 to 0.7–1.8% [1,3].
PCI outcomes
A successful PCI can be defined angiographically, procedurally or clinically. A summary of PCI outcome definitions, complications and late outcomes is presented in Box 1. There has been intense interest in the incidence and risk factors for bleeding complications including relationship to procedural access site and stent thrombosis. These specific complications are highly associated with myocardial infarction (MI) and mortality. The goal of understanding these outcomes is to make PCI safer. In addition, the late clinical outcome of target-vessel revascularization is an indicator of the efficacy of PCI with stents and the patient and lesion subsets that would benefit from further developments in stent technology. A comprehensive discussion of the factors associated with all PCI-related outcomes is beyond the scope of this article, but several important outcomes will be covered.
Periprocedural bleeding
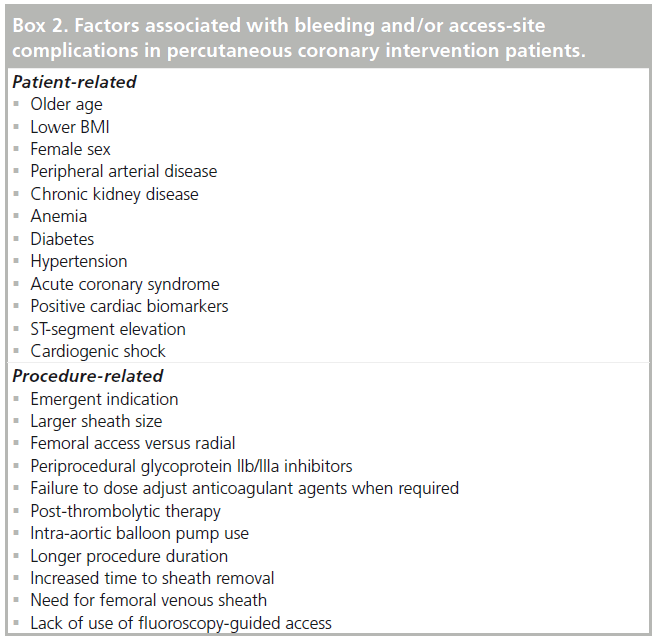
Patients undergoing PCI have a significant risk of hemorrhagic complications that can occur at the access site or remotely. Avoidance of bleeding complications is very important since periprocedural bleeding is now recognized to be significantly associated with subsequent mortality. Bleeding events may directly result in mortality or result in changes to medications that increase ischemic risk. In the REPLACE-2 trial of elective or urgent PCI, major hemorrhage occurred in 3.2% of patients and was independently associated with a 2.7-fold increase in 1-year mortality [4]. In the larger ACUITY study (n = 13,819) of ACS patients, major bleeding resulted in higher rates of 30 day mortality, ischemia and stent thrombosis. Absolute rates of bleeding were nearly double in patients receiving glycoprotein (GP) IIb/IIIa inhibitors with heparin or bivalirudin compared with bivalirudin alone that had a 3.0% major bleeding rate. At 30 days, the odds ratio (OR) for mortality in patients with major bleeding was 7.55 (95% CI: 4.68–12.18; p < 0.0001) [5]. These studies and other have identified numerous independent risk factors for bleeding which are summarized in Box 2 [6]. The most commonly noted factors include advanced age, female gender, low BMI, chronic kidney disease and intensity of antiplatelet therapy. Since the majority of patient variables cannot be modified at the time of PCI, an appreciation for patients at risk of bleeding can assist in procedure-related decisions including the access site and anticoagulation strategy.
In the most recent PCI guidelines, evaluation of bleeding risk before PCI received a Class I recommendation [7]. To assist physicians in assessing the risk of bleeding after PCI, risk scores have been developed. For periprocedural bleeding in elective and urgent PCI, a risk score was derived using seven variables (age >55 years, female gender, estimated glomerular filtration rate <60 ml/min/1.73 m2, pre-existing anemia, low-molecular-weight heparin within 48 h pre-PCI, use of GP IIb/IIIa inhibitors and intra-aortic balloon pump use). The risk of major bleeding in patients without any risk factors was 1% compared with those with several factors in whom the risk was greater than 5% [8]. These types of risk score are helpful due the variability of bleeding risk that has been demonstrated in PCI patients. In ACS patients, major bleeding rates varied from 1 to 40% depending on the number of independent risk factors for bleeding with the only modifiable predictor being the treatment-related variable of heparin plus a GP IIb/IIIa compared with bivalirudin alone [9]. The overall approach to PCI should be based on a balance of ischemic and bleeding risks for the individual patient.
Similar to trends in major in-hospital clinical outcomes, the rate of major bleeding in unselected PCI patients has decreased over time. From 2005 to 2009 bleeding rates were examined for elective and ACS patients including ST-elevation MI (STEMI) in over 1.7 million patients in the CathPCI Registry. In all clinical scenarios, elective PCI, unstable angina/non- STEMI and STEMI, an approximate 20% reduction in post-PCI bleeding was observed. Half of the reduction in bleeding was attributed to changes in anticoagulation strategies, with an increase in bivalirudin use and decrease in GP IIb/IIa inhibitors. During the period of this study, radial approach was quite low at <3% [10].
▪ Access-site complications
The catheterization access site is responsible for 50–60% of bleeding events in PCI patients. The femoral artery is the most common access site for PCI in the USA. Femoral vascular complications include access-site bleeding, hematoma, retroperitoneal hematoma, pseudoaneurysm, arteriovenous fistula and arterial dissection and/ or thrombosis. The incidence of complications ranges from 2 to 6% and has decreased over time in both men and women, but female sex is still associated with a twofold risk [11,12]. Other independent risk factors for vascular complications include sheath size, intensity and duration of anticoagulation with heparin and procedure time [13]. In femoral access cases, when femoral angiography shows suitable anatomy, closure devices are often used to shorten the time-to-ambulation and have a Class IIa recommendation for this purpose. It should be recognized, however, that vascular closure devices do not reduce vascular complications (Class III indication for routinue use of a vascular closure device for the purpose of reducing vascular complications) [7,14].
▪ Radial access
Compared with femoral access, radial access reduces complications. A meta-analysis of randomized trials showed that radial access reduced major bleeding by 73% compared with femoral access (0.05 vs 2.3%, OR: 0.27 [95% CI: 0.16–0.45]; p < 0.001). Additionally, there was a trend for a reduction in the composite of death, MI or stroke (2.5 vs 3.8%, OR: 0.71 [95% CI: 0.49–1.01]; p = 0.058) as well as death (1.2 vs 1.8%, OR: 0.74 [95% CI: 0.42–1.30]; p = 0.29) [15]. The more contemporary RIVAL trial randomized 7021 patients undergoing coronary angiography or intervention to a radial or femoral approach. There was a lower rate of local vascular complications with the use of radial access. In patients treated at high volume radial centers and in those with STEMI, there was a lower risk of the primary composite end point of death, MI, stroke or non-CABG-related major bleeding at 30 days [16]. The benefit of radial compared with femoral access was confirmed in ST-segment elevation ACS patients in the multicenter, randomized, RIFLE-STEACS study. The primary end point, a 30-day composite of cardiac death, stroke, MI, target-lesion revascularization and bleeding, occurred in 13.6% of patients assigned to radial compared with 21.0% of femoral access patients (p = 0.003). Individual end points of bleeding and mortality were also significantly lower with radial access [17]. An analysis of nearly 600,000 PCI procedures in the National Cardiovascular Data Registry showed that the radial approach had a 58% lower risk of bleeding than femoral. The reduction in bleeding complications with the radial approach was more pronounced among patients <75 years old, women and patients undergoing PCI for an ACS [18]. Use of radial access to decrease access-site complications carries a Class IIa recommendation in the PCI guidelines [7].
Stent thrombosis
Stent thrombosis was recognized as a serious complication in the bare-metal stent (BMS) era; however, the use of high-pressure stent deployment and dual antiplatelet therapy with aspirin and a thienopyridine decreased the incidence to an acceptable level of less than 0.5% at 30 days [19]. With the rapid adoption of drug-eluting stents (DES) after approval in the USA, reports of stent thrombosis at later time points, even after 1 year, raised concerns about this new technology that has led to intense investigation into the factors associated with its occurrence in both BMS and DES. Although the incidence is low, approximately 1% at 30 days, and 0.2–0.6% per year thereafter, this complication results in a high rate of MI, repeat revascularization and death. Therefore, an understanding of the risk factors for stent thrombosis is of paramount importance [20–24]. As with other complications, identification of patients at risk for stent thrombosis can assist in the developed preventative strategies.
Standard definitions have been developed in order to compare stent thrombosis rates across trials and observational studies. Early stent thrombosis occurs within 30 days, late is from 30 days to 1 year and very late stent thrombosis is beyond 1 year. Definite stent thrombosis is confirmed by pathology or angiography in the appropriate clinical setting. Probable stent thrombosis is unexplained death within 30 days of stent placement or an MI in the territory of the stent at any time. Most studies report definite or the composite of definite and probable. Possible stent thrombosis is unexplained death beyond 30 days of the stent.
Risk factors for stent thrombosis can be categorized into patient related, including clinical and anatomical, or procedure related, which encompasses stent characteristics (Box 3). There are several potential mechanisms for stent thrombosis including reduced coronary flow and inadequate suppression of thrombin and platelet aggregation during critical time periods, such as during stent deployment and the period of stent re-endothelialization. At later time points, delays in complete neointimal coverage or a functional endothelium can contribute to risk [25]. Large clinical studies, autopsy reports and intracoronary imaging findings have contributed to our knowledge in this area.
▪ Patient-related factors for stent thrombosis
Cessation of antiplatelet therapy before the recommended duration, which has varied from 3 to 12 months, is the strongest risk factor for stent thrombosis. One of the original studies that brought this to attention showed a 90-fold increase in stent thrombosis in patients that prematurely discontinued antiplatelet therapy [20]. Several other studies have confirmed the importance of compliance to dual antiplatelet therapy. In the Dutch Stent Thrombosis Registry, premature discontinuation of clopidogrel was the most predictive factors for stent thrombosis therapy (OR: 36.5) [23]. In a study of 10,778 patients treated with DES, patients that discontinued antiplatelet therapy had a late stent thrombosis rate of 1.76% and very late stent thrombosis rate of 2.1% compared with 0.1 and 0.14% of compliant patients [26]. These studies highlight the importance of ensuring that patients can comply with prolonged dual antiplatelet therapy prior to placement of a DES.
Presence of an ACS at the time of stenting is another well-described risk factor for stent thrombosis. The plaque characteristics and thrombotic milieu in ACS are contributing mechanisms. Compared with patients with stable angina, the rates of stent thrombosis for ACS are three- to nine-fold higher, both in BMS and DES, with the highest risk group being STEMI patients [27,28]. In ACS patients the use of more potent oral antiplatelet therapies than clopidogrel, including prasugrel or ticagrelor, reduces the risk of stent thrombosis about 50%. This is due to several factors including a greater level of platelet inhibition and less interindividual variability in response and the lack of influence of genetic polymorphisms known to influence clopidogrel metabolism [29–32].
Numerous other clinical factors have been associated with an increased risk of stent thrombosis, including but not limited to diabetes, chronic kidney disease and hypersensitivity to DES [20,33]. These factors result in inflammation, delayed healing and endothelial dysfunction. In addition, several anatomic- or lesion-related variables have been identified, such as low ejection fraction and bifurcation lesion. A more comprehensive list patient-related variables shown to be associated with stent thrombosis in one or more studies is provided in Box 3 [34–36].
▪ Procedure-related factors for stent thrombosis
The majority of procedure-related factors increase the risk of stent thrombosis through an influence on coronary flow or delay in healing. Intravascular ultrasound (IVUS) identified inadequate postprocedure lumen dimensions, dissection, thrombus or tissue prolapse to be associated [37]. Incomplete stent apposition, either at the time of deployment or acquired, and overlapping stents have also been observed in patients with stent thrombosis [34,38]. Intravascular optical coherence tomography, which has approximately tenfold greater resolution than IVUS, can also be instructional in assessing risk for stent thrombosis as it has a higher sensitivity to detect stent strut malapposition, plaque protrusion and stent-edge dissection [39]. Autopsy studies have identified stenting across branch ostia, disruption of adjacent vulnerable plaques and extensive plaque prolapse as possible precipitants of stent thrombosis [40]. Use of IVUS, therefore, should be considered in patients with complex anatomy to optimize stent deployment and assess for contributors to the risk for stent thrombosis [41]. The PCI guidelines give a Class IIb recommendation for the use of IVUS to determine the mechanism of stent thrombosis [7].
The influence of stent type on stent thrombosis risk has been controversial. After extensive analysis of randomized trials and observational studies of BMS compared with DES, it is accepted that the rates of stent thrombosis are similar except for very late stent thrombosis, which is higher with DES [42–44]. In 2012, a network meta-analysis including 49 randomized trials compared either one DES to a BMS or to another DES. This meta-analysis suggests that, compared with BMS, paclitaxel-eluting stents (PES) and zotalrolimus-eluting stents (ZES) have comparable rates of definite early stent thrombosis, but the rates with everolimuseluting stents (EES) and sirolimus-eluting stents (SES) may be lower. Cumulative rates of stent thrombosis at 2 years showed no difference in DES versus BMS with the exception of EES, which had a significantly lower rate. However, since few studies directly compared newer generation DES, such as EES and ZES with BMS, not all types of DES have been directly compared, and other changes such as BMS design and practice changes over time cannot be assessed, these findings should be considered exploratory [45]. Much less data is available for the newer DES, particularly the Resolute® (Medtronic, MN, USA) ZES. In a registry study that included 12,339 patients who received SES, PES or EES, the cumulative incidence rate of definite stent thrombosis over 4 years was lower with EES compared with PES or SES (adjusted hazard ratios: 0.33, 95% CI: 0.23–0.48 and 0.41, 95% CI: 0.27–0.62, respectively). The difference was primarily driven by a lower incidence of very late stent thrombosis with EES [46]. The above studies suggest that the newer DES may have a more favorable late safety profile.
Periprocedural MI
The definition of periprocedural MI has evolved over time. According to the universal definition of MI, PCI-related MI is considered a type 4 MI and is defined as an increase of biomarker greater than threefold the 99th percentile upper reference level (in patients with normal baseline levels) or a new rise of greater than 20% in serum biomarkers over and beyond the last nadir [47]. The incidence is 15–30% depending on the definition used and patient population studied [48,49]. The clinical significance of periprocedural MI has also been argued and many studies have found no independent association between periprocedural MI and outcomes. A meta-analysis of 20 studies including over 15,000 patients, however, suggested an association between troponin elevation and mortality during follow-up (OR: 1.35; 95% CI: 1.13–1.60) [50]. Increased mortality in patients with troponin elevation after PCI was also observed in the Evaluation of Drug Eluting Stents and Ischemic Events Evaluation Registry and was related to the degree of elevation with the hazard of mortality increased from 1.02 at a threefold to 1.67 at a 20-fold elevation [51].
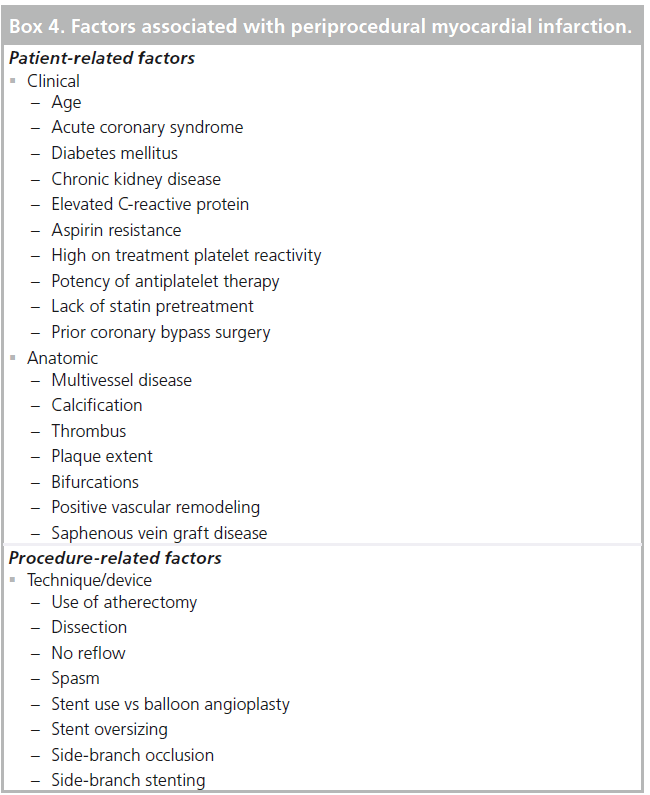
Procedural myonecrosis can be caused by a number of mechanisms, including macrovascular and microvascular obstruction and the thrombotic and neurohormonal milieu. Angiographically evident causes of epicardial obstruction include side-branch occlusion, plaque or thrombus embolization, dissection or spasm. Additional factors for procedural biomarker elevation identified by IVUS include de novo lesions, atheroablative technique, plaque burden at the lesion and reference segments and stent overexpansion [52,53]. Angiographic noreflow is a sign of microvascular damage. In one study of elective and emergent PCI, the incidence was 2%, but rates were as high as 11.5% for STEMI patients. Additional risk factors for no reflow are PCI of saphenous vein grafts and use of stent or atherectomy devices compared with balloon angioplasty [54]. The above and additional patient and procedure-related factors that have been associated with periprocedural MI are presented in Box 4.
Adequate antiplatelet therapy at the time of PCI is important for preventing thrombotic complications. Patients undergoing elective PCI demonstrated to have aspirin resistance had a significantly higher rate of periprocedural MI despite pretreatment with clopidogrel. After adjustment, aspirin resistance (OR: 2.9) and bifurcation lesions (OR: 2.8) remained independently associated with biomarker elevation after PCI [55]. High platelet reactivity after clopidogrel as defined by a point of care assay is also an independent predictor of periprocedural MI [56].
Factors associated with a decreased risk of preand periprocedural MI are pretreatment with clopidogrel and statins, respectively [57]. A metaanalysis of six randomized trials demonstrated a 50% lower risk of periprocedural MI in the statin pretreatment group compared with controls [58]. In the PCI guidelines, administration of a high-dose statin before PCI to reduce the risk of periprocedural MI received a Class IIa recommendation for patients naive and on chronic therapy [7].
Mortality risk in the stent era
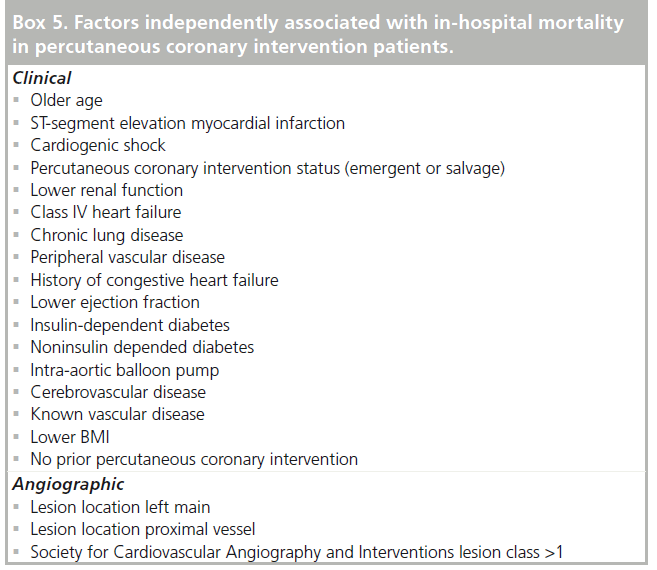
Mortality for patients undergoing PCI has decreased over time [1,3]. The ability to predict the risk of in-hospital mortality following PCI in contemporary practice is important for comparing outcomes and appropriately informing patients. Risk models delineate the factors associated with the outcome of interest. Using data from 588,398 PCI procedures in the National Cardiovascular Data Registry, patient-related variables associated with in-hospital mortality were examined. Mortality was highly related to the clinical circumstances of PCI, 0.65% in elective cases compared with 4.81% in STEMI. Unadjusted in-hospital mortality was higher in patients over 70 years of age, women and those with diabetes mellitus. Female sex, however, was not independently associated with mortality after adjustment for differences in comorbidities. The strongest clinical predictors of mortality were cardiogenic shock, renal function and age. Clinical factors were more prognostic than angiographic variables; however, lesion location in the left main or proximal left anterior descending artery were predictive. Additional factors independently associated with in-hospital mortality are presented in Box 5 and include diabetes, peripheral vascular disease and PCI for stent thrombosis [59].
In-stent restenosis & target-vessel revascularization
Stents were developed to improve upon the results of balloon angioplasty, to decrease acute complications and increase durability of PCI. BMS reduced the incidence of restenosis to 20–30% and of target-lesion revascularization to 15%, about half that of balloon angioplasty [60,61]. Restenosis is due to vessel injury and subsequent neointimal hyperplasia and is defined angiographically as a greater than 50% luminal narrowing. The clinical significance of restenosis is better measured by rates of target-lesion or -vessel revascularization. To inhibit neointimal hyperplasia and reduce restenosis risk, DES were developed. DES elute an antirestenotic drug from a polymer applied to a BMS platform. In the USA, four types of DES have been approved: SES, PES, ZES and EES. DES are very effective and, compared with BMS, reduce restenosis by 40–60% depending on patient and lesion complexity [43,62].
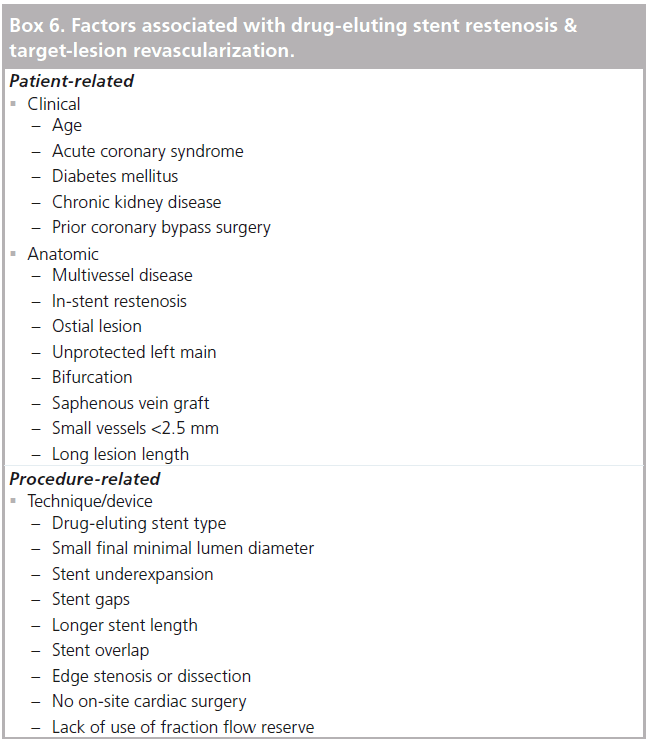
DES restenosis occurs in 4–10% of patients by 1 year and is lowest in randomized trials of do novo lesions and higher in unselected patients in routine practice or in subsets considered to be off-label from the initial indications studied [63]. DES reduce restenosis compared with BMS in all patient and lesion subsets. In BMS, several factors predicted the need for target-lesion revascularization, including smaller pretreatment minimum lumen diameter, smaller final minimum lumen diameter, longer stent length, diabetes mellitus, unstable angina and hypertension [64]. Risk factors for restenosis and target-lesion revascularization in the DES are similar. In patients with DES, angiographic follow-up multivariate predictors of restenosis included: in-stent restenosis, ostial lesion location, diabetes mellitus, stented length >36 mm, reference vessel diameter <2.17 mm, and vessel other than left anterior descending coronary artery [65]. Predictors of target-lesion revascularization in other studies include: age <60, prior PCI, unprotected left main PCI, saphenous vein graft PCI, minimum stent diameter ≤2.5 mm, total stent length ≥40 mm, complex lesions (B2/C) [66,67]. Multivariable analysis showed that vessel size, final diameter stenosis and DES type (SES adjusted OR of 0.60 compared with PES) were the strongest predictors of restenosis [68]. As with the PCI outcomes discussed above, factors associated with restenosis and target-lesion or -vessel revascularization can be grouped according to whether they are patient or procedure related (Box 6). Additional factors determined by angiography or IVUS include stent under expansion (minimum stent area <5.0 mm2), stent gaps, stent overlap, edge stenosis or dissection [69,70]. A recent randomized study demonstrated that patients that have PCI at sites without surgical back-up have higher rates of target-vessel revascularization. The exact mechanism for this is unclear but may relate to operators being less aggressive with stent deployment in the absence of surgical back-up [71]. Procedural variables that reduce the risk of restenosis or repeat revascularization include an IVUS cross-sectional stent area of greater than 9 mm2 and the use of fractional flow reserve to guide multivessel PCI [72].
▪ Complex anatomy
Improvements in technology and operator skill have made the percutaneous treatment of complex anatomy such as left main, multivessel disease and chronic total occlusions more common. While acute outcomes for multivessel PCI with DES are favorable, restenosis remains an issue. In the SYNERGY™ (Boston Scientific, MA, USA) between PCI with the SYNTAX trial, the rate of repeat revascularization was higher in PCI compared with CABG (13.5 vs 5.9%; p < 0.001) [73]. To assist in the decision of whether a patient is better suited for PCI or CABG the anatomic SYNTAX score can be calculated [101]. Higher SYNTAX scores indicate more complex coronary disease. Patients with low (≤22) or intermediate (23–32) scores have similar outcomes with PCI or CABG and treatment decision can be individualized, whereas those with a high score (≥33) should, in general, have surgical revascularization. Determining the lesions causing ischemic in multivessel disease is also important. The use of fractional flow reserve, an index of the physiological significance of a coronary stenosis, is superior to angiography in guiding multivessel PCI. In the FAME trial, patients randomized to fractional flow reserve compared with angiography guided PCI received fewer stents and had a lower 1-year rate of death, nonfatal MI and repeat revascularization with similar rates of angina [72].
Several other lesion subsets remain procedurally challenging in the current era. In the treatment of chronic total occlusions, several novel devices and techniques have been developed to improve upon acute procedural success. In experienced hands, dedicated chronic total occlusion wires and microcatheters can be used in antegrade, retrograde and subintimal approaches to achieve success [74]. Improving guiding support and device delivery with the mother-in-child catheter technique has also allowed more complex PCI, particularly via radial access, where small guiding catheters are required [75].
▪ Stent type
There have been several studies comparing one DES to a second DES. Based on available data, SES and EES have lower rates of target-lesion revascularization than PES. The Resolute ZES has similar efficacy as EES [76]. The data are limited, however, with respect to differences in specific patient and lesion subsets. For example, in patients with diabetes mellitus no differences in target-vessel revascularization have been observed according to DES type.
Based on the above findings, several potential mechanisms for restenosis have been described. Failure to inhibit neointimal hyperplasia may result from resistance to the antirestenotic drug or a hypersensitivity reaction to the polymer. Issues with stent deployment and integrity include underexpansion, nonuniform expansion, fracture, polymer disruption, barotrauma outside the stented segment and incomplete lesion coverage. The PCI guidelines give a Class IIa recommendation for the use of IVUS to determine the mechanism of stent restenosis [7].
Conclusion & future perspective
Currently available DES are composed of a metallic stent platform, a durable polymer and an antirestenotic drug. While DES have improved outcomes for patients, they have several limitations. The drug–polymer combination delays vessel healing, including endothelialization and function and can cause a hypersensitivity reaction, therefore, increasing risk of stent thrombosis. The metallic component prevents positive vascular remodeling, can limit side-branch access or targets for surgical revascularization and interferes with noninvasive coronary imaging such as computed tomography angiography. Novel technologies under development that should be available over the next few years including DES with bioabsorbable polymers and completely bioresorbable vascular scaffolds. The SYNERGY stent, an EES with a bioabsorbable polymer is in human trials and initial data are promising, with low rates of restenosis and no stent thrombosis events reported [77]. The ABSORB™ (Abbott Vascular, IL, USA) stent consists of a bioresorbable backbone of poly-l-lactide coated with a biodegradable polymer that controls the release of everolimus. The device has been termed a scaffold rather than a stent since it is not permanent. The vascular scaffold degrades over several years and is replaced by a proteoglycan matrix. The most recent iteration studied has stable scaffold area over time and sufficient inhibition of neointimal hyperplasia. A randomized trial is planned comparing the ABSORB scaffold and the Xience® (Abbott Vascular) EES [78]. These devices may reduce the risk of stent thrombosis and the need for prolonged dual-antiplatelet therapy, which in turn would lower risk of bleeding. Benefits on vascular function are also expected.
In addition to advances in technology, heightened attention to the appropriateness of PCI and the use of outcomes as reimbursement and quality measures will further generate interest in understanding the factors that influence procedural safety and efficacy. Similar to risk models developed for mortality, prediction models for complications such as bleeding and periprocedural MI and for outcomes such as restenosis will be an important way to individualize care and assure similar outcomes across institutions.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Percutaneous coronary intervention outcomes
▪▪ Over the past three decades, our understanding of the patient and procedure-related factors that influence percutaneous coronary intervention (PCI) outcomes have advanced the field of interventional cardiology.
▪▪ Advances in techniques, equipment, pharmacotherapy and patient management have reduced the risk of bleeding, vascular complications, and myocardial infarction (MI) and improved the durability of PCI by reducing restenosis.
▪▪ Although the risk factors for various outcomes differ, several clinical variables consistently predict increased risk including advanced age, acute coronary syndromes, diabetes mellitus and chronic kidney disease.
▪▪ Operators should be aware of high-risk variables and guideline recommendations that allow the safe performance of PCI in patients with cardiovascular disease.
Periprocedural bleeding
▪▪ PCI related bleeding, which may be due to an access or nonaccess site source, is significantly associated with mortality.
▪▪ The use of bivalirudin and radial access reduce periprocedural bleeding.
Stent thrombosis
▪▪ Stent thrombosis is uncommon but results in devastating consequences including MI and death.
▪▪ Dual antiplatelet therapy and optimal stent deployment are critical for reducing the risk of stent thrombosis.
Periprocedural MI
▪▪ Procedural myonecrosis can be caused by a number of mechanisms.
▪▪ Pretreatment with antiplatelet therapy and statins can reduce the risk of periprocedural MI.
Mortality risk in the stent era
▪▪ Models can be used to predict the risk of in-hospital mortality following PCI in contemporary practice.
In-stent restenosis & target-vessel revascularization
▪▪ Drug-eluting stents reduce restenosis compared with bare-metal stents in all patient and lesion subsets.
▪▪ Several anatomic and clinical factors increase the risk of restenosis in drug-eluting stent-treated patients.
Future perspective
▪▪ Drug-eluting stents with bioabsorbable polymers and completely bioresorbable vascular scaffolds are under development and may improve upon the safety of PCI.
▪▪ Individualizing care with risk models may reduce complications and improve outcomes.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Venkitachalam L, Kip KE, Selzer F et al. Twenty-year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the National Heart, Lung, and Blood Institute-sponsored, multicenter 1985–1986 PTCA and 1997–2006 Dynamic Registries. Circ. Cardiovasc. Interv. 2, 6–13 (2009).
- McGrath PD, Malenka DJ, Wennberg DE et al. Changing outcomes in percutaneouscoronary interventions: a study of 34,752 procedures in northern New England, 1990 to 1997. Northern New England Cardiovascular Disease Study Group. J. Am. Coll. Cardiol. 34, 674–680 (1999).
- Singh M, Rihal CS, Gersh BJ et al. Twenty-five-year trends in in-hospital and long-term outcome after percutaneous coronary intervention: a single-institution experience. Circulation 115, 2835–2841 (2007).
- Feit F, Voeltz MD, Attubato MJ et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 trial. Am. J. Cardiol. 100, 1364–1369 (2007).
- anoukian SV, Feit F, Mehran R et al. Impact of major bleeding on 30‑day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY trial. J. Am. Coll. Cardiol. 49, 1362–1368 (2007).
- Nikolsky E, Mehran R, Aymong ED et al. Impact of anemia on outcomes of patients undergoing percutaneous coronary interventions. Am. J. Cardiol. 94, 1023–1027 (2004).
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 58, e44–e122 (2011).
- Nikolsky E, Mehran R, Dangas G et al. Development and validation of a prognostic risk score for major bleeding in patients undergoing percutaneous coronary intervention via the femoral approach. Eur. Heart J. 28, 1936–1945 (2007).
- Mehran R, Pocock SJ, Nikolsky E et al. A risk score to predict bleeding in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 55, 2556–2566 (2010).
- Subherwal S, Peterson ED, Dai D et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: a report from the National Cardiovascular Data CathPCI Registry. J. Am. Coll. Cardiol. 59, 1861–1869 (2012).
- Applegate RJ, Sacrinty MT, Kutcher MA et al. Trends in vascular complications afterdiagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc. Interv. 1(3), 317–326 (2008).
- Ahmed B, Piper WD, Malenka D et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ. Cardiovasc. Interv. 2, 423–429 (2009).
- Doyle BJ, Ting HH, Bell MR et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc. Interv. 1(2), 202–209 (2008).
- Biancari F, D’Andrea V, Di Marco C, Savino G, Tiozzo V, Catania A. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am. Heart J. 159, 518–531 (2010).
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am. Heart J. 157, 132–140 (2009).
- Jolly SS, Yusuf S, Cairns J et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 377, 1409–1420 (2011).
- Romagnoli E, Biondi-Zoccai G, Sciahbasi A et al. Radial versus femoral randomizedinvestigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (radial versus femoral randomized investigation in ST-elevation acute coronary syndrome) study. J. Am. Coll. Cardiol. doi:10.1016/j.jacc.2012.06.017 (2012) (Epub ahead of print).
- Rao SV, Ou F-S, Wang TY et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc. Interv. 1(4), 379–386 (2008).
- Leon MB, Baim DS, Popma JJ et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N. Engl. J. Med. 339, 1665–1671 (1998).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- Mauri L, Hsieh W, Massaro JM, Ho K, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356, 1020–1029 (2007).
- Kimura T, Morimoto T, Nakagawa Y et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation 125, 584–591 (2012).
- van Werkum JW, Heestermans AA, Zomer AC et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 53, 1399–1409 (2009).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369, 667–678 (2007).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115, 2435–2441 (2007).
- Kimura T, Morimoto T, Nakagawa Y et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation 119, 987–995 (2009).
- Aoki J, Lansky AJ, Mehran R et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation 119, 687–698 (2009).
- Wiviott SD, Braunwald E, McCabe CH et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 371, 1353–1363 (2008).
- Gurbel PA, Bliden KP, Samara W et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J. Am. Coll. Cardiol. 46, 1827–1832 (2005).
- Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
- Steg PG, James S, Harrington RA et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation 122, 2131–2141 (2010).
- Cayla G, Hulot J-S, O’Connor SA et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA 306, 1765–1774 (2011).
- Fujii K, Carlier SG, Mintz GS et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J. Am. Coll. Cardiol. 45, 995–998 (2005).
- Ellis SG, Colombo A, Grube E et al. Incidence, timing, and correlates of stent thrombosis with the polymeric paclitaxel drug-eluting stent: a TAXUS II, IV, V, and VI meta-analysis of 3,445 patients followed for up to 3 years. J. Am. Coll. Cardiol. 49, 1043–1051 (2007).
- Kuchulakanti PK, Chu WW, Torguson R et al. Correlates and long-term outcomes ofangiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation 113, 1108–1113 (2006).
- Pilgrim T, Vetterli F, Kalesan B et al.The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ. Cardiovasc. Interv.
5, 202–210 (2012). - Cheneau E, Leborgne L, Mintz GS et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation 108, 43–47 (2003).
- Cook S, Wenaweser P, Togni M et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 115, 2426–2434 (2007).
- Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review: clinical and research applications. JACC Cardiovasc. Interv. 2(11), 1035–1046(2009).
- Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 108, 1701–1706 (2003).
- Moses JW, Dangas G, Mehran R, Mintz GS. Drug-eluting stents in the real world: how intravascular ultrasound can improve clinical outcome. Am. J. Cardiol. 102, 24J–28J (2008).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356, 998–1008 (2007).
- Kirtane AJ, Gupta A, Iyengar S et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119, 3198–3206 (2009).
- Roukoz H, Bavry AA, Sarkees ML et al. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am. J. Med. 122, 581 e1–e10 (2009).
- Palmerini T, Biondi-Zoccai G, Della Riva D et al. Stent thrombosis with drug-eluting andbare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 379, 1393–1402 (2012).
- Raber L, Magro M, Stefanini GG et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 125, 1110–1121 (2012).
- Thygesen K, Alpert JS, White HD et al. Universal definition of myocardial infarction. Circulation 116, 2634–2653 (2007).
- Alcock RF, Roy P, Adorini K et al. Incidence and determinants of myocardial infarction following percutaneous coronary interventions according to the revised Joint Task Force definition of troponin T elevation. Int. J. Cardiol. 140, 66–72 (2010).
- Testa L, Van Gaal WJ, Biondi Zoccai GGL et al. Myocardial infarction afterpercutaneous coronary intervention: a meta-analysis of troponin elevation applying the new universal definition. Q JM 102, 369–378 (2009).
- Nienhuis MB, Ottervanger JP, Bilo HJG, Dikkeschei BD, Zijlstra F. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Cath. Cardiovasc. Interv. 71, 318–324 (2008).
- Novack V, Pencina M, Cohen DJ et al. Troponin criteria for myocardial infarction after percutaneous coronary intervention. Arch. Int. Med. 172, 502–508 (2012).
- Mehran R, Dangas G, Mintz GS et al. Atherosclerotic plaque burden and CK-MB enzyme elevation after coronary interventions: intravascular ultrasound study of 2256 patients. Circulation 101, 604–610 (2000).
- Iakovou I, Mintz GS, Dangas G et al. Increased CK-MB release is a ‘trade-off’ for optimal stent implantation: an intravascular ultrasound study. J. Am. Coll. Cardiol. 42, 1900–1905 (2003).
- Piana RN, Paik GY, Moscucci M et al. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation 89, 2514–2518 (1994).
- Chen W-H, Lee P-Y, Ng W, Tse H-F, Lau C-P. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J. Am. Coll. Cardiol. 43, 1122–1126 (2004).
- Mangiacapra F, Barbato E, Patti G et al. Point-of-care assessment of platelet reactivity after clopidogrel to predict myonecrosis in patients undergoing percutaneous coronary intervention. JACC Cardiovasc. Interv. 3, 318–323 (2010).
- Sabatine MS, Hamdalla HN, Mehta SR et al. Efficacy and safety of clopidogrel pretreatment before percutaneous coronary intervention with and without glycoprotein IIb/IIIa inhibitor use. Am. Heart J. 155, 910–917 (2008).
- Zhang F, Dong L, Ge J. Effect of statins pretreatment on periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention: a meta-analysis. Annal. Med. 42, 171–177 (2010).
- Peterson ED, Dai D, DeLong ER et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 55, 1923–1932 (2010).
- Fischman DL, Leon MB, Baim DS et al.randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl.Med. 331, 496–501 (1994).
- Serruys PW, de Jaegere P, Kiemeneij F et al.comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331, 489–495 (1994).
- Abbott JD, Voss MR, Nakamura M et al. Unrestricted use of drug-eluting stents compared with bare-metal stents in routine clinical practice: findings from the National Heart, Lung, and Blood Institute Dynamic Registry. J. Am. Coll. Cardiol. 50, 2029–2036 (2007).
- Marroquin OC, Selzer F, Mulukutla SR et al.comparison of bare-metal and drug-eluting stents for off-label indications. N. Engl.Med. 358, 342–352 (2008).
- Cutlip DE, Chauhan MS, Baim DS et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J. Am. Coll. Cardiol. 40, 2082–2089 (2002).
- Lemos PA, Hoye A, Goedhart D et al. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the rapamycin-eluting stent evaluated at rotterdam cardiology hospital (RESEARCH) study. Circulation 109, 1366–1370 (2004).
- Stolker JM, Kennedy KF, Lindsey JB et al. Predicting restenosis of drug-eluting stents placed in real-world clinical practice: derivation and validation of a risk model from the EVENT registry. Circ. Cardiovasc. Interv.3, 327–334 (2010).
- Zahn R, Hamm CW, Schneider S et al. Incidence and predictors of target vessel revascularization and clinical event rates of the sirolimus-eluting coronary stent (results from the prospective multicenter German Cypher Stent Registry). Am. J. Cardiol. 95, 1302–1308 (2005).
- Kastrati A, Dibra A, Mehilli J et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation 113, 2293–2300 (2006).
- Takebayashi H, Kobayashi Y, Mintz GS et al. Intravascular ultrasound assessment of lesions with target vessel failure after sirolimus-eluting stent implantation. Am. J. Cardiol. 95, 498–502 (2005).
- Lemos PA, Saia F, Ligthart JMR et al. Coronary restenosis after sirolimus-eluting stent implantation: morphological description and mechanistic analysis from a consecutive series of cases. Circulation 108, 257–260 (2003).
- Aversano T, Lemmon CC, Liu L, Atlantic CI. Outcomes of PCI at hospitals with or without on-site cardiac surgery. N. Engl. J. Med. 366, 1792–1802 (2012).
- Tonino PAL, De Bruyne B, Pijls NHJ et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. Engl. J. Med. 360, 213–224 (2009).
- Serruys PW, Morice M-C, Kappetein AP et al. Percutaneous coronary interventionversus coronary-artery bypass grafting for severe coronary artery disease. N. Engl.Med. 360, 961–972 (2009).
- Sumitsuji S, Inoue K, Ochiai M, Tsuchikane E, Ikeno F. Fundamental wire technique and current standard strategy of percutaneous intervention for chronic total occlusion with histopathological insights. JACC Cardiovasc. Interv. 4, 941–951 (2011).
- Takeshita S, Shishido K, Sugitatsu K et al. In vitro and human studies of a 4F double-coaxial technique (‘mother–child’ configuration) to facilitate stent implantation in resistant coronary vessels. Circ. Cardiovasc. Interv. 4, 155–161 (2011).
- Bangalore S, Kumar S, Fusaro M et al. Short and long-term outcomes with drug eluting and bare metal coronary stents: a mixed treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 125(23), 2873–2891 (2012).
- Meredith IT, Verheye S, Dubois CL et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J. Am. Coll. Cardiol. 59, 1362–1370 (2012).
- Serruys PW, Onuma Y, Dudek D et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12‑month clinical and imaging outcomes. J. Am. Coll. Cardiol. 58, 1578–1588 (2011).
▪ Identified independent predictors of periprocedural major hemorrhage and showed that major hemorrhage was an independent predictor of 1‑year mortality.
▪ This risk score for bleeding highlights seven variables that should be considered when assessing bleeding risk for percutaneous coronary intervention (PCI) with femoral access.
▪ Study of consecutive PCI patients demonstrated that although bleeding rates have decreased over time, female gender is still independently associated with a twofold risk of bleeding and vascular complications compared with men.
▪ Meta-analysis that demonstrates radial access reduced major bleeding by 73% compared with femoral access.
▪▪This study showed that ST-elevation myocardial infarction patients treated with a radial approach had lower mortality compared with femoral approach.
▪▪ One of the first studies to show the strength of the association between premature antiplatelet therapy discontinuation and stent thrombosis.
▪ Elucidated mechanisms of subacute stent thrombosis such as procedurally related dissection, thrombus or tissue prolapse.
▪▪ One of the largest studies to date comparing drug-eluting stents to bare-metal stents and other drug-eluting stents.
▪ Demonstrates the magnitude of biomarker elevation in periprocedural myocardial infarction is related to the risk of mortality.
▪▪ Meta-analysis of randomized controlled trials of statin preloading in PCI patients showed a 50% reduction in periprocedural myocardial infarction.
▪▪ Comprehensive risk model for in-hospital mortality in elective and emergent PCI.