Perspective - Imaging in Medicine (2013) Volume 5, Issue 1
FDG PET/CT as a marker for grading sarcomas and for the individualization of disease management
Rajan Rakheja*1 and Stephan Probst1
1Division of Nuclear Medicine, Jewish General Hospital, Montreal, Quebec, Canada
- *Corresponding Author:
- Rajan Rakheja
Division of Nuclear Medicine
Jewish General Hospital, Montreal
Quebec, Canada
Tel: +1 514 295 6104
Fax:+1 514 340 7919
E-mail: rajan.rakheja@gmail.com
Abstract
Keywords
FDG PET/CT; necrosis; sarcoma; tumor grading
part of PERSPECTIVE FDG PET/CT as a marker for grading sarcomas and for the individualization of disease management Sarcomas are a diverse group of relatively rare malignant solid tumors arising from the embryonic mesoderm. They comprise over 50 pathologic subtypes and are broadly grouped into sarcomas of soft tissue and sarcomas of bone. Further classification is based on presumed tissue of origin and histopathologic features. Sarcomas can occur anywhere in the body and at any age; however, the incidence is strongly dependant on the tumor subtype. While soft tissue sarcomas are more frequent in older adults, they are an important cause of death in the 14–29-year-old age group due to the relatively low incidence of other lethal disease in this cohort of the population [1].
The risk factors and etiology of most sarcomas are poorly understood; however, associations with hereditary syndromes, viral infections, occupational exposure to carcinogens, chemotherapy and radiotherapy for a prior malignancy have emerged in the literature [2]. Prior radiotherapy has emerged as one of the strongest risk factors for the development of sarcomas. The symptoms and clinical presentation of different sarcomas are widely variable and depend on the site of origin of the cancer.
Sarcomas account for 0.7% of all adult malignancies and 6.5% of childhood cancers, although some authors believe the true incidence to be underestimated [3]. Despite their low incidence, patients have a high mortality rate and account for 3–4% of annual cancer-related deaths. Regardless of aggressive treatment with surgery and chemoradiotherapy, the prognosis of most histologic subtypes of sarcomas remains poor and the survival has not changed significantly in the past several decades [4]. The 5-year metastasis-free rate, a strong prognostic factor, is most dependant on histologic grade and not histologic type. Overall, 91% of patients with grade 1 tumors are metastasis-free at 5 years, whereas the rate for grade 3 lesions is only 44% [5]. The 5-year overall survival of patients with all grades soft tissue sarcoma is approximately 50%.
Staging & grading sarcomas
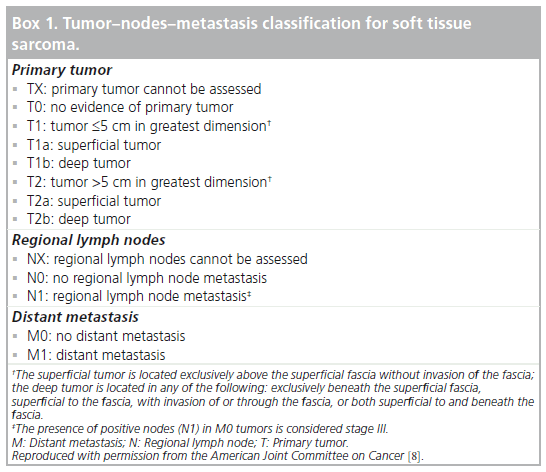
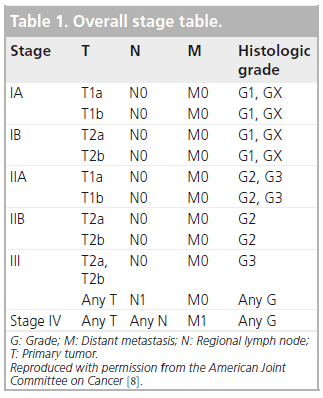
Effective management of sarcomas involves both staging and grading, which allows prognostic assessment, and will dictate appropriate treatment and follow-up of patients. The standard tumor–nodes–metastasis stage, widely used in many malignancies, is relevant, but the histologic grade plays a prominent role in overall staging, as is clear from Box 1 & Table 1. Grading of sarcomas is early standard management and is performed histologically in parallel with anatomic tumor–nodes–metastasis staging.
Grading is often initially performed on core biopsy specimens and finalized on complete resection of the sarcoma. The WHO recognizes the French Federation of Cancer Centers Sarcoma Group grading system (FNCLCC system) and the National Cancer Institute (NCI) grading systems. The NCI system utilizes tumor histologic type and subtype, and the amount of tumor necrosis, but cellularity, nuclear pleomorphism and mitotic index are also taken into account for some tumor types.
This article will focus on the FNCLCC system, as it is standard for pathologists at our institution for grading and it has demonstrated slightly better ability to predict distant metastasis and overall survival [6]. The prognostic values of the FNCLCC and NCI grading systems were compared by Guillou et al. in a series of 410 patients [6]. Both systems had good prognostic value in predicting overall and metastasis-free survival; however, in the NCI system, there were more patients in the intermediate group of grade 2 tumors. Grade 2 comprises a heterogeneous group, which most clinicians find unhelpful as their prognosis is widely variable. Moreover, a multivariate analysis demonstrated that the FNCLCC system ranked first in predicting both overall and metastasis-free survival [7].
The FNCLCC grade is based on three parameters: differentiation, mitotic activity and necrosis. Each of these parameters receives a score: differentiation (1–3), mitotic activity (1–3) and necrosis (0–2). Of the three, tumor differentiation is the most problematic aspect of the FNCLCC system. Its use is subjective and is not defined for every sarcoma subtype. Nonetheless, it is an integral part of the system, and every attempt should be made to assign a differentiation score.
Tumor differentiation is the most challenging aspect of the FNCLCC system. It is highly subjective and does not include every subtype of sarcoma. Nonetheless, it is an integral part of the system, and every attempt should be made to assign a differentiation score.
Tumor differentiation is scored as follows: a score of 1 is assigned to sarcomas closely resembling normal adult mesenchymal tissue; a score of 2 for sarcomas of certain histologic type; and a score of 3 for synovial sarcomas, embryonal sarcomas, undifferentiated sarcomas, and sarcomas of unknown tumor type.
Mitotic activity is determined using the mitotic count, obtained from the most mitotically active area in ten successive high-power fields (HPFs); a high-power field at 40× is 0.1734 mm2.
A score of 1 is 0–9 mitoses per ten HPFs; a score of 2 is 10–19 mitoses per ten HPFs; and score of 3 is 20 or more mitoses per ten HPFs.
Tumor necrosis: the presence and the amount of tumor necrosis is determined on histologic sections. A score of 0 indicates that there is no tumor necrosis; a score of 1indicates that there is tumor necrosis of 50% or less; and score of 2 indicates that there is tumor necrosis of more than 50%.
The 7th Edition of the American Joint Committee on Cancer (AJCC) staging system for soft tissue tumors recommends the FNCLCC three-grade system but further simplifies the classification into high grade and low grade; thus using FNCLCC, grade 2 tumors are considered as ‘high grade’ for the purposes of staging, even if they, on average, have a more favorable prognosis than grade 3 lesions [8].
Pathological assessment
While the standard grading systems are part of the essential work-up for every patient diagnosed with sarcoma, there are some limitations with these grading schemes. Sarcomas by nature show extreme internal heterogeneity, and large samples often show areas of low- and high-grade tumor. Similarly, there are frequently areas of necrosis that can further complicate pathological evaluation of the primary lesion. These factors lead to poor interobserver agreement on histopathological assessment; Presant et al. found total agreement between the primary pathologist and the reviewer in only 66% of cases in a histopathologic peer review of specimens from 216 consecutive patients with soft-tissue or bone sarcomas. Furthermore, this review demonstrated that the subtype of sarcoma was incorrectly identified in 27% of cases [9]. When the reproducibility of the FNCLCC system was tested by 15 pathologists on 25 cases, the crude proportion in agreement was 75% for tumor grade, 73% for mitotic index and 74% for differentiation. Reproducibility of diagnosis of histologic type was significantly lower with an agreement rate of 61% [7]. Several other histopathological ‘second reader’ peer reviews have established similar results [10,11].
Tumor necrosis
Tumor necrosis identified on surgical specimen has frequently been shown to be a significant prognostic factor in soft tissue sarcomas with respect to overall survival; patients with marked necrosis have poorer survival than those without necrosis [12]. Unfortunately, necrosis is challenging to recognize grossly and pathologists are apt to underestimate necrosis microscopically, as these areas are avoided during surgical sampling [13]. Similar interobserver variation in the histopathologic assessment of necrosis has been documented in other studies [10]; Coindre tested the reproducibility of the French grading system with 15 pathologists and specifically found that the crude proportion in agreement was 81% for tumor necrosis [7].
Fluorodeoxyglucose PET/CT
Where available, fluorodeoxyglucose (FDG) PET/CT imaging is routinely performed on sarcoma patients at the time of diagnosis, and to evaluate response to therapy if recurrence is suspected, in order to guide additional therapy. FDG PET/CT imaging provides a rapid and early overview of the metabolic activity of sarcomas and has been shown to be extremely sensitive; a retrospective study by Charest et al. included 212 consecutive patients with known soft tissue or osseous sarcoma, demonstrated that FDG PET/CT detected 93.9% of all sarcomas, with a sensitivity of 93.7% for soft tissue sarcomas and 94.6% for osseous sarcomas [14]. Kole et al. showed that FDG PET/CT is useful for detection of local recurrence [15]. Many studies have revealed that FDG PET/CT is a valuable tool for detecting metastatic disease in sarcoma patients [16], and for evaluating response to chemotherapy [17].
FDG PET/CT can be viewed as a surrogate method for grading sarcomas, given the strong correlation between the standardized uptake value (SUV) and tumor grade. Several studies have also shown significant differences between tumor SUVmax in benign versus high-grade malignant soft tissue tumors [18,19]. FDG uptake generally correlates well with cellularity, mitotic activity, histopathologic Ki-67 labelling and p53 overexpression. Eary et al. demonstrated in a study including a total of 70 sarcoma patients that FDG uptake correlates with the histologic grade of soft tissue tumors, allowing PET/CT to accurately distinguish between three histologic grades of the NCI grading system [5]. In a separate study, Eary et al. demonstrated that tumor SUVmax and metabolic rate of FDG had a consistent relationship in 42 sarcoma patients, with an overall correlation coefficient of 0.94 [20]. A meta-analysis, which included 15 studies with 441 soft tissue lesions (227 malignant and 214 benign), by Ioannidis et al. noted that practically all intermediate/high-grade soft tissue sarcomas are accurately diagnosed on the basis of qualitative interpretation and almost all of them have SUVmax ≥2.0 [21]. Another large meta-analysis of 29 studies including 1163 patients with soft tissue and bone sarcomas demonstrated that PET/CT can discriminate between low- and high-grade sarcomas based on the average lesion SUV (SUVmean) [22]. Although PET/CT is limited for differentiating grade 2 from grade 3 sarcomas, PET cannot be used to reliably distinguish between grade 2 and 3 soft tissue sarcomas [23,24]. This is a similar problem for pathologists and has resulted in effectively collapsing the FNCLCC three-grade system into high- and low-grade sarcomas [8]. Other studies have demonstrated an association between SUVmax and pathologic makers of soft tissue and bone sarcomas, including cellularity, p53 and Ki-67 [25]. Our previous work demonstrated that there was a strong statistical correlation between SUVmax and tumor mitotic count and necrosis [26]. It is the authors’ opinion that SUVmean should generally not be used in the evaluation of sarcomas, as significant intratumoral heterogeneity may underestimate the true glycolytic rate.
Given the strong statistical correlation between SUV and the criteria that compose the FNCLCC grading scheme (tumor differentiation, mitotic count and necrosis), the authors feel there is a strong case for metabolic data from PET/CTs to act as a surrogate marker for tumor grade. This could be very helpful clinically as PET/CT data are often available early on in management, often prior to complete resection and histopathologic assessment. Thus, metabolic data may provide an early estimation of tumor grade, information that may be helpful to the oncology team for deciding on management. For example, at our institution, a core needle biopsy is often performed on large tumors, and clinical and imaging staging is performed once the core biopsy conf irms a sarcoma. When these results are obtained, the case is discussed by the surgical, oncology, radiation oncology and imaging teams. While the core biopsy provides an initial tumor grade, sarcomas can be very large and often only a segment of the tumor is biopsied. Thus it is possible – and even likely – that the highest grade region is not sampled, and that the metabolic information from FDG PET could yield a more accurate estimation of grade. Theoretically, if the low-grade area of a sarcoma was biopsied, then a discrepant high SUV result in another region of the tumor could suggest that this is indeed a higher tumor grade, perhaps warranting the administration of neoadjuvant radio-chemotherapy. Alternatively, PET/CT could pinpoint high-grade moieties of the tumor, highlighted by the most intensely hypermetabolic segments, and guide further biopsy. Moreover, metabolic data from PET/CT could help direct pathologists to the most aggressive non-necrotic part of the tumor, thus facilitating pathological sarcoma grading.
Similarly, metabolic data representing tumor grade could be used as a valuable tool for directing biopsies of sarcomas. A study of 20 patients by Hain et al. suggested that FDG PET/CT can be used to correctly direct biopsy in soft tissue sarcomas. Hain attained suitable results by obtaining biopsies from the site with the highest SUVmax [13]. Given the aggressive nature of sarcomas and that they often become large soft tissue masses at the time of surgical resection, the most biologically or metabolically active portion of the tumor may not always be chosen for further pathological evaluation, resulting in an underestimation of the grade. By convention, SUVmax has been used for metabolic quantification, although SUVmean has also been shown in some reports to correlate with tumor grade [22]. SUVmax is used in nearly all clinical studies since it is virtually independent of tumor size and shape, while SUVmean is influenced by the threshold for tumor boundary and, thus, is operator dependent. The authors envisage future PET/CT reports, describe the sarcoma and offer advice on guiding biopsy, if appropriate or requested by the referring team. As a recent example at our hospital, a 30-year-old male presented with a 19-cm lower extremity shin and ankle round cell liposarcoma. Round cell liposarcoma is a high-grade tumor and a poorly differentiated form of myxoid liposarcoma, which contains a high-grade round cell component arising from a lower grade myxoid liposarcoma [27]. The PET demonstrated that the tumor was highly heterogeneous and had a SUVmax of 4 in the inferior component at the ankle, while most of the remainder of the tumor in the shin showed low grade uptake, around SUVmean 2. The nuclear physician’s impression read “heterogeneous high-grade sarcoma, highest-grade area based on metabolic data suggested to be in lateral soft tissues of the ankle, 3 cm cranial from tibio-talar joint.” The surgeon and pathologist used this report to assess the most metabolically active area of the tumor near the ankle joint. We speculate that this approach reduces the underestimation of aggressive tumors via core biopsies.
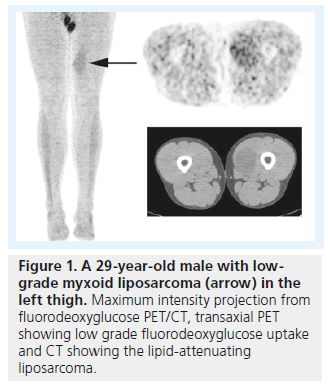
While FDG PET/CT has been shown to be highly sensitive for diagnosis of osseous and soft tissue sarcomas, it should be noted that PET/CT is less sensitive for lower grade sarcomas. The classic low-grade sarcoma is liposarcoma (Figure 1), and false-negative liposarcomas have been described on PET/CT scans [28,29]. However, a meta-analysis of 15 studies with 441 soft tissue tumors demonstrated that FDG PET/CT was positive in all intermediate- and high-grade liposarcomas [21]. PET/CT can be less sensitive for low-grade gastrointestinal stromal tumors, but have been shown to have a similar sensitivity compared with CT, and was superior to CT in predicting early response to therapy in patients with recurrent or metastatic gastrointestinal stromal tumors [30]. While malignant peripheral nerve sheath tumors can demonstrate low-grade metabolic activity, it has been shown that PET/CT is highly sensitive/specific and SUVmax can help in differentiating benign neurofibromas from malignant peripheral nerve sheath tumors [31].
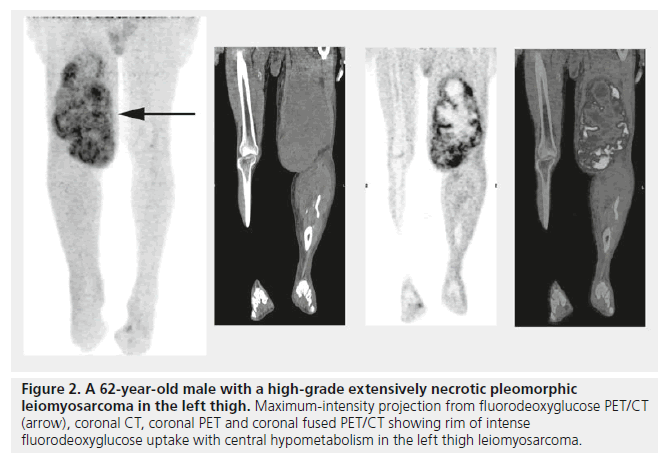
Similarly, it has been shown that PET/CT can accurately identify necrotic areas of tumors (Figure 2); Schwarzbach et al. evaluated PET/CT in soft tissue sarcomas and observed that areas of central absent FDG uptake in bulky masses correlates strongly with spontaneous tumor necrosis [29], thus concluding that FDG PET/CT could identify tumor necrosis. An example of the authors’ future vision is a large aggressive sarcoma that has a large necrotic component. The PET/CT report could read “large intensely FDG-avid tumor with SUVmax 10, with a large 5-cm necrotic zone located centrally.” Thus, the surgeon would target the most metabolically active region for sampling, avoiding the necrotic zone, and thus ensuring the pathologist has the most poorly differentiated area with the highest mitotic count, while using the PET result for necrosis grading. This would allow for the most accurate overall grading with nonexcisional biopsies. Similarly, the pathologist would have an idea of pretreatment necrosis as opposed to those treated with neoadjuvant chemoradiation – as these have expected significant treatment-related necrosis. Lastly, any sarcoma with uniformly low- or high-grade metabolic signal would be reported as “metabolic data suggests uniform tumor grade” and biopsy could be performed at the most convenient location, while minimizing patient morbidity.
Figure 2. A 62-year-old male with a high-grade extensively necrotic pleomorphic leiomyosarcoma in the left thigh. Maximum-intensity projection from fluorodeoxyglucose PET/CT (arrow), coronal CT, coronal PET and coronal fused PET/CT showing rim of intense fluorodeoxyglucose uptake with central hypometabolism in the left thigh leiomyosarcoma.
Conclusion & future perspective
The clinical course of sarcomas is difficult to predict on the basis of current clinical and pathologic prognostic criteria, and further advancements for diagnosis, tumor–nodes– metastasis staging, grading and prognosis are being investigated. The authors have also observed the importance of prediction models such as the sarcoma normogram: postsurgery prediction model by Memorial Sloan Kettering Cancer Center [101]. They are constantly used during tumor boards at our institution and play an important role in dictating treatment regimes. Despite aggressive therapy, 40% of patients with high-grade sarcomas die from metastatic disease [4]. There is a need for better prognostic models to identify higher risk patients as early as possible. The authors’ picture incorporating metabolic information from PET/CT into prediction models. There is ample evidence to support the SUVmax correlation with prognosis. Hawkins et al. showed SUV from FDG PET imaging of Ewing’s sarcomas correlates with progression-free survival independent of initial disease stage [32]. Furthermore, Eary et al. and others have demonstrated that SUV and PET imaging predicts outcomes in both bone and soft tissue sarcoma [5,20,33]. Given this knowledge, SUVmax from PET/CT should be incorporated into these prognostic models. It is also possible that the presence of necrosis from the PET/CT could be incorporated into these models in the future, although more research is needed to clarify its diagnostic and predictive accuracy. New PET tracers such as 3´-deoxy-3´- 18F-fluorothymidine, which has shown a lot of early promise in TNM staging and noninvasive grading; and 18F-fluoromisonidazole, which can be used to asses tumor hypoxia and grade, are actively being investigated [23,34,35].

Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
* of considerable interest
- Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010, 506182 (2010). & Summarizes the guidelines from a consensus meeting of UK sarcoma specialists convened under the auspices of the British Sarcoma Group. They intend to provide a framework for the multidisciplinary care of patients with soft tissue sarcomas.
- Henderson TO, Rajaraman P, Stovall M et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int. J. Radiat. Oncol. Biol. Phys. 84(1), 224–230 (2012).
- Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J. Nucl. Med. 44(6), 930–942 (2003).
- Iagaru A, Chawla S, Menendez L, Conti PS. 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl. Med. Comm. 27, 795–802 (2006).
- Eary JF, Conrad EU, Bruckner JD et al. Quantitative [F-18]fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin. Cancer Res. 4, 1215–1220 (1998).
- Guillou L, Coindre JM, Bonichon F et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J. Clin. Oncol. 15, 350–362 (1997). & The French Federation of Cancer Centers Sarcoma Group grading system demonstrates a slightly increased ability to predict distant metastasis development and tumor mortality. The use of this system to evaluate soft tissue sarcoma aggressiveness may be favored.
- Coindre JM. Grading of soft tissue sarcomas: review and update. Arch. Pathol. Lab. Med. 130, 1448–1453 (2006). & Grading is the best predictor of metastasis outcome in adult soft tissue sarcomas and should be part of the pathologic report.
- Edge SB, Byrd DR, Compton CC et al. AJCC Cancer Staging Manual (7th Edition). Springer, NY, USA (2009).
- Presant CA, Russell WO, Alexander RW et al. Soft-tissue and bone sarcoma histopathology peer review: the frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J. Clin. Oncol. 4, 1658–1661 (1986).
- Harris M, Hartley AL, Blair V et al. Sarcomas in north west England. I. Histopathological review. Br. J. Cancer 64, 315–320 (1991).
- Meis-Kindblom JM, Bjerkehage B, Bohling T et al. Morphologic review of 1000 soft tissue sarcomas from the Scandinavian Sarcoma Group (SSG) Register. The peer-review committee experience. Acta Orthop. Scand. 285, 18–26 (1999).
- Pisters PW, Leung DH, Woodruff J et al. Analysis of prognostic factors in 1041 patients with localized soft tissue sarcomas of the extremities. J. Clin. Oncol. 14, 1679–1689 (1996).
- Hain SF, O’Doherty MJ, Bingham J et al. Can FDG PET be used to successfully direct preoperative biopsy of soft tissue tumours? Nucl. Med. Commun. 24, 1139–1143 (2004).
- Charest M, Hickeson M, Lisbona R et al. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur. J. Nucl. Med. Mol. Imaging 36(12), 1944–1951 (2009).
- Kole AC, Nieweg OE, van Ginkel RJ et al. Detection of local recurrence of soft-tissue sarcoma with positron emission tomography using [18F]fluorodeoxyglucose. Ann. Surg. Oncol. 4, 57–63 (1997).
- Miraldi F, Adler LP, Faulhaber P. PET imaging in soft tissue sarcomas. Cancer Treat. Res. 91, 51–64 (1997).
- Jones D, McCowage G, Sostman H et al. Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J. Nucl. Med. 37, 1438–1444 (1996).
- Adler LP, Blair HF, Williams RP et al. Grading liposarcomas with PET using [18F] FDG. J. Comput. Assisted Tomogr. 14, 960–962 (1990).
- Kern KA, Brunetti A, Norton JA et al. Metabolic imaging of human extremity musculoskeletal tumours by PET. J. Nucl. Med. 29, 181–186 (1988).
- Eary JF, Mankoff DA. Tumour metabolic rates in sarcoma using FDG PET. J. Nucl. Med. 39, 250–254 (1998).
- Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J. Nucl. Med. 44, 717–724 (2003).
- Bastiaannet E, Groen H, Jager PL et al. The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat. Rev. 30, 83–101 (2004).
- Benz MR, Czernin J, Allen-Auerbach MS et al. 3´-deoxy-3´-[18F]fluorothymidine positron emission tomography for response assessment in soft tissue sarcoma: a pilot study to correlate imaging findings with tissue thymidine kinase 1 and Ki-67 activity and histopathologic response. Cancer 118(12), 3135–3144 (2012).
- Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M et al. Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J. Nucl. Med. 42(5), 713–720 (2001).
- Folpe AL, Lyles RH, Sprouse JT et al. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin. Cancer Res. 6, 1279–1287 (2000).
- Rakheja R, Makis W, Skamene S et al. Correlating metabolic activity on 18F-FDG PET/CT with histopathologic characteristics of osseous and soft-tissue sarcomas: a retrospective review of 136 patients. Am. J. Roentgenol. 198(6), 1409–1416 (2012).
- Smith TA, Easley KA, Goldblum JR. Myxoid/round cell liposarcoma of the extremities. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am. J. Surg. Pathol. 20(2), 171–180 (1996).
- Schwarzbach MH, Dimitrakopoulou-Strauss A, Mechtersheimer G et al. Assessment of soft tissue lesions suspicious for liposarcoma by F18-deoxyglucose (FDG) positron emission tomography (PET). Anticancer Res. 21(5), 3609–3614 (2001).
- Schwarzbach M, Dimitrakopoulou-Strauss A, Willeke F. Clinical value of [18-F] fluorodeoxyglucose positron emission tomography imaging in soft tissue sarcomas. Ann. Surg. 231, 380–386 (2000).
- Gayed I, Vu T, Iyer R et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J. Nucl. Med. 45(1), 17–21 (2004).
- Warbey VS, Ferner RE, Dunn JT, Calonje E, O’Doherty MJ. [18F]FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur. J. Nucl. Med. Mol. Imaging 36(5), 751–757 (2009).
- Hawkins DS, Scheutze SM, Butrynski JE et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumours. J. Clin. Oncol. 23, 8828–8834 (2005).
- Baum SH, Frühwald M, Rahbar K, Wessling J, Schober O, Weckesser M. Contribution of PET/CT to prediction of outcome in children and young adults with rhabdomyosarcoma. J. Nucl. Med. 52(10), 1535–1540 (2011).
- Eary JF, Link JM, Muzi M et al. Multiagent PET for risk characterization in sarcoma. J. Nucl. Med. 52(4), 541–546 (2011). & Demonstrates that multi-agent PET with tracers, such as 18F-fluoromisonidazole for tissue hypoxia and 11C-verapamil for P-glycoprotein activity, are feasible and yield unique and potentially complementary biologic information on individual tumors.
- Mortensen LS, Buus S, Nordsmark M et al. Identifying hypoxia in human tumors: a correlation study between 18F-FMISO PET and the Eppendorf oxygen-sensitive electrode. Acta Oncol. 49(7), 934-940 (2010).