Case Report - Interventional Cardiology (2014) Volume 6, Issue 3
Femoral arteriotomy closure using the Mynx vascular closure device: a profile of device efficacy and complications
- Corresponding Author:
- Tudor G Jovin
University of Pittsburgh School of Medicine
Pittsburgh, PA, USA
Tel: +1 412 647 8080
E-mail: jovitg@upmc.edu
Abstract
Vascular access for percutaneous coronary interventions (PCIs), peripheral vascular interventions and neuroendovascular procedures is established predominantly through percutaneous puncture of the femoral artery and, less commonly, via the radial artery. Arteriotomy closure is accomplished with either manual compression (MC) or with the use of a vascular closure device (VCD). While MC is considered the gold standard for mediating arteriotomy closure, numerous VCDs have been developed in recent years as alternatives to MC and extended bed rest.
Keywords
extravascular closure, femoral arteriotomy, Mynx, polyethylene glycol, vascular closure device
Vascular access for percutaneous coronary interventions (PCIs), peripheral vascular interventions and neuroendovascular procedures is established predominantly through percutaneous puncture of the femoral artery and, less commonly, via the radial artery. Arteriotomy closure is accomplished with either manual compression (MC) or with the use of a vascular closure device (VCD). While MC is considered the gold standard for mediating arteriotomy closure, numerous VCDs have been developed in recent years as alternatives to MC and extended bed rest. Initial studies posited VCD use as a means to increase closure efficacy and speed up time to ambulation and discharge [1–5]. Consequently, the putative benefits include maximized utilization of resources, increased patient throughput and reduced overall cost [6–8]. VCD-mediated arteriotomy closure has also been shown to increase patient comfort [6,9,10]. Given these considerations, over the past few years, there has been an increase in the utilization of VCDs; however, widespread adoption of VCDs for femoral arteriotomy closure, in lieu of standard manual closure, has been tempered by operator learning curve, procedure-related costs, impaired or delayed ability to reaccess the femoral artery after deployment, and the potential for complication development [11–14], more specifically, an increased frequency of groin hematomas [15–20], iatrogenic pseudoaneurysms [2,19,21–25], retroperitoneal hemorrhage [21,26–29] and limb ischemia [21,30–35].
Device overview & design
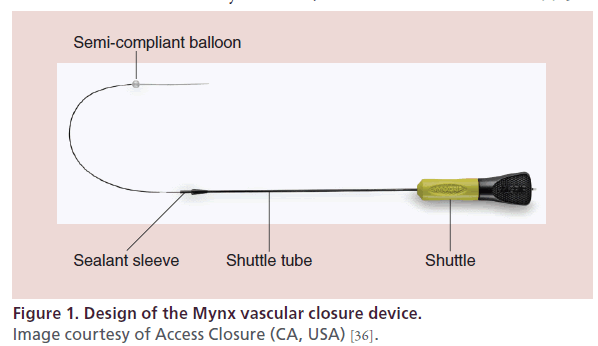
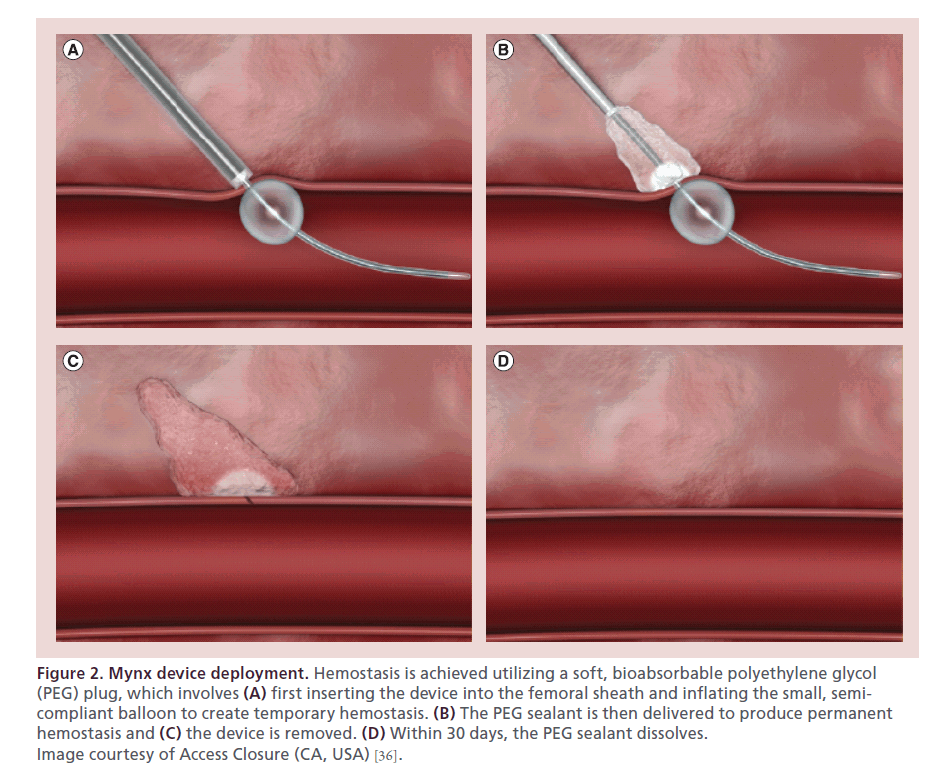
The first iteration of the Mynx was approved by the US FDA in 2007. The next model, Mynx Cadence, approved in 2009, simplified the deployment system. Approved by the FDA in 2010, the MynxGrip VCD (Access Closure, CA, USA) is an active VCD that achieves arterial hemostasis by delivering a suture-free, conformable, water-soluble lyophilized polyethylene glycol (PEG) sealant into the extravascular space over the arteriotomy. The device consists of a catheter with PEG sealant and a 6-mm semicompliant balloon at the tip (Figure 1). PEG is a bioinert polymer that has been used extensively in medical devices, with an established record of biocompatibility and safety. There are two elements to the MynxGrip PEG sealant: a 14.5-mm porous matrix and a 1.5-mm distal tip with grip technology. The dual-action PEG sealant adheres to the artery by interlocking with the vessel wall and instantly absorbs bodily fluids, expanding up to three- to four-times its original size, providing a mechanical seal over the arteriotomy and within the tissue tract (Figure 2). Its matrix structure allows for infiltration of blood and serves as a scaffold for hemostasis. After deployment, the sealant undergoes steady hydrolysis into PEG monomers and is completely dissolved within 30 days.
Figure 1: Design of the Mynx vascular closure device.
Image courtesy of Access Closure (CA, USA) [36].
Figure 2: Mynx device deployment. Hemostasis is achieved utilizing a soft, bioabsorbable polyethylene glycol (PEG) plug, which involves (A) first inserting the device into the femoral sheath and inflating the small, semicompliant balloon to create temporary hemostasis. (B) The PEG sealant is then delivered to produce permanent hemostasis and (C) the device is removed. (D) Within 30 days, the PEG sealant dissolves. Image courtesy of Access Closure (CA, USA) [36].
The Mynx VCD is designed so that it can be inserted into 5–7 Fr procedural sheaths, thus avoiding the need for sheath exchange or tract dilation. Intra-arterial deployment of the sealant is prevented by the inclusion of the balloon and by the self-expanding nature of the sealant material. In addition, PEG is not known to interact with platelets or immune cells, theoretically making intravascular and inflammatory complications less likely.
Clinical profile
Device efficacy
Published in 2007, the first prospective clinical investigation of the Mynx VCD involved 190 patients undergoing percutaneous coronary procedures in five European centers [12]. The study population was split equally between patients who underwent interventional procedures and those who underwent diagnostic procedures. Aspirin and clopidogrel were used in 80.4 and 28% of patients, respectively, and median sheath size used in the study was 6 Fr, with a 6 or 7 Fr sheath in 99.5% of procedures. The average BMI among patients in the study was 27.54 kg/m2. The device had a success rate of 93.2% in the study population with a mean time-tohemostasis of 1.3 min and mean time-to-ambulation of 2.6 h. In addition, the authors found a negligible difference in the efficacy of the Mynx VCD between diagnostic and interventional groups.
A more recent study from the cardiac literature of 238 patients who had undergone PCI and subsequent Mynx closure demonstrated successful closure in 90.8% of cases, a particularly impressive rate given that all patients received preprocedural aspirin and clopidogrel loads, as well as intraprocedural intravenous heparin boluses to achieve activated clotting times between 200 and 300 s [16].
Within the neuroendovascular realm, the Mynx VCD has demonstrated similar efficacy. A publication from our group involving a cohort of 766 patients undergoing diagnostic cerebral arteriograms or neuro-interventions detailed a device success rate of 92%, in-line with the previously published results. Of note, compared with the previous studies in cardiac patients, our patients overall had less risk factors for bleeding [37].
Complications
Prior studies of complications associated with the Mynx VCD have cited rates ranging from 0 to 4.2% [16,37–43], with rates varying based on length of followup, consistency of follow-up, and definition of major and minor complications. Scheinert and colleagues reported eight complications (4.2%) in 190 patients, with one major complication involving access-site bleeding, which required a blood transfusion [38]. The seven minor complications included six groin hematomas and one femoral artery pseudoaneurysm, none of which required subsequent treatment. Despite the large number of patients on antithrombotic medications, the authors were unable to find a correlation between activated clotting time and complication development [38]. Azmoon et al. encountered five major complications (2.1%) and no minor complications: two cases of retroperitoneal bleeding requiring surgical intervention, two pseudoaneurysms requiring surgical intervention, and one patient who experienced accesssite bleeding and required a blood transfusion [16]. Interestingly, their study also showed a statistically significant difference in device failure between Mynx and AngioSeal (St Jude Medical, MN, USA; 9.2 vs 3.7%; p = 0.033) [16]. Among our 766 patients, we had 23 complications (2.45%); there were 13 major complications (1.39%), including seven patients who needed an operation for treatment of a femoral artery dissection or pseudoaneurysm and six patients who required a blood transfusion following the development of a groin hematoma. Of the ten patients with minor complications (1.07%), four had groin hematomas that did not require transfusions, three developed infections at the femoral access site, one experienced puncture site pain, and one had a nonflow limiting femoral artery dissection. We found that older age, lower BMI, higher number of antithrombotic medications used and device failure conferred a statistically significant increased risk of complication development. Our data corroborated findings from previous studies involving VCDs, which demonstrated a correlation between low BMI and increased rate of complications [20,44,45].
Inadvertent intravascular deployment of sealant can lead to embolization of the sealant and arterial occlusion. Although the Mynx VCD has features that are designed to minimize the likelihood of intravascular sealant deployment, there have been cases of arterial occlusion caused by embolized sealant. Of further note is the fact that intravascular sealant deployment does not always result in clinically symptomatic complications, which could lead to a potential under-reporting of this phenomenon. In a retrospective study in which patients who received repeat femoral arteriograms approximately 1 week after an initial diagnostic procedure in which a Mynx VCD was used, Fields and colleagues observed five instances (18%) of intravascular sealant on follow-up vascular imaging [39]. Of the five patients, one had a near occlusion of the superficial femoral artery, which required a surgical intervention, while the remaining four were asymptomatic [39]. By contrast, a separate study by Fargen and colleagues demonstrated no cases of intraluminal filling defects on follow-up angiography in 31 patients who underwent repeat arteriography after prior closure with Mynx. The average time to repeat angiography among the patients was 6.2 days, with a median of 5.5 days [42].
Alternative devices
Given the increasing number of patients undergoing diagnostic and interventional coronary, peripheral vascular and neuroendovascular procedures, improved patient comfort associated with VCD use, and systems-based concerns relating to patient throughput and resource utilization, an increasing number of VCDs are being used in the clinical setting. VCDs currently on the market can be classified according to their mechanism of action into three categories: suture devices (Perclose and Prostar; both Abbott Laboratories, IL, USA), vessel plugs (Mynx, AngioSeal, EXOSEAL [Cordis Corporation, NJ, USA] and Femo- Seal [St Jude Medical]), and vascular clips (StarClose [Abbott Laboratories]). Suture devices and vascular clips achieve hemostasis through direct closure of the defect in the external arterial wall. Vessel plugs achieve closure through extravascular filling of the defect with biomaterial. Plugs are made with a number of different materials and vary by brand. Commonly used materials include PEG (Mynx), collagen (AngioSeal), and polyglycolic acid (EXOSEAL). FemSeal utilizes an intravascular and an extravascular plug that are held together with a suture.
As previously mentioned, MC remains the gold standard for achieving arteriotomy closure. Koreny et al. merged 30 randomized controlled trials of VCDs versus MC and found similar risks for hematoma, local bleeding and pseudoaneurysm between VCD and MC cohorts [19]. Patients who underwent VCD-mediated arteriotomy closure experienced shorter times to hemostasis, duration of bed rest and earlier hospital discharge [19]. Noor and colleagues performed a retrospective study of rates of surgical repair following arteriotomy closure with Myxn, AngioSeal and MC in 11,006 diagnostic and interventional transfemoral cardiac and peripheral 6/7 Fr catheterization arteriotomies. In that study, Mynx had a lower surgical repair rate than both AngioSeal (0.06 vs 0.61; p < 0.0001) and MC (0.19; p < 0.14) suggesting that Mynx is superior to AngioSeal and comparable to MC with regard to complications requiring surgical intervention [40]. Although Mynx has lower rates of surgical repair than AngioSeal, its device success rate of 91–93% is lower than 97–99.7% with AngioSeal and comparable to 94% with EXOSEAL [16,38,46,47].
Conclusion
MC remains the gold standard for arteriotomy closure because of its extensive record of safety and efficacy. The dramatic growth seen in the utilization of Mynx and other VCDs in recent years is in part due to the belief that VCDs are more cost effective than MC, due to the reduction in postprocedural time to ambulation, and, as a consequence, decreasing the duration of hospital stay and resources needed for patient monitoring.
The concept of analyzing VCD cost utility is complex, with many variables, including device cost, postprocedural nursing care and length of time required to monitor the patient after the angiogram. Wagenbach and colleagues reported results from the Mayo Clinic subsequent to the institution of an ultra-fast, early ambulation proctocol among patients who underwent neuroendovascular procedures and experienced MC for arteriotomy closure [48]. Remarkably, 142 out of 214 patients (66.4%) who underwent a diagnostic neuroendovascular procedure and 21 out of 81 patients (25.9%) who underwent a neurointervention were able to ambulate within 3 h. Only 14 out of 295 patients (4.7%) required delayed ambulation due to local oozing, hematoma or pseudoaneurysm [48]. The feasibility, efficacy and safety of a similar, ultra-fast ambulation protocol following MC is currently underway at our institution. In turn, if this protocol can be further validated, the potential cost utility of Mynx and other VCDs could be questioned; however, given the lack of uniformity in postprocedural protocols throughout various hospital systems, as well as site-specific policies and resources, a true determination of whether VCD use is associated with cost savings is rather difficult. Several studies have found that a radial approach, which does not require a VCD, reduces global bleeding risk in patients undergoing PCI as compared with the femoral approach [49]. Increasing popularity and validation of radial approach for PCI can decrease the need for femoral access and VCD use.
Over the last several years, the manufacturers of the Mynx VCD have made improvements to its design and have recently released the latest two iterations of the device: the MynxGrip and the MynxAce. Included in both of the new devices is a separate PEG sealant called grip technology, which is attached to the typical Mynx PEG sealant; this grip sealant is specifically designed to adhere to the outside of the artery for improved closure. New studies are needed to determine whether these design improvements will translate to improved clinical performance.
The Mynx VCD is one of the most common closure devices used today. It is well suited for arteriotomy closure in most patients, and its design avoids the need for sheath exchange or tract dilation. In addition, in comparison with other leading VCDs, it is associated with less pain [43]. Given recent clinical data showing a high success rate of over 90%, low complication rates, as well as decreased time to hemostasis, ambulation and hospital discharge, the Mynx VCD offers an efficacious and safe alternative to the traditional approach of MC and extended bed rest, as well as other currently marketed VCDs.
Executive summary
Clinical rationale
• Numerous vascular closure devices (VCDs) have been developed in recent years as alternatives to manual compression and extended bed rest.
• VCDs have been shown to increase closure efficacy, speed up time to ambulation and discharge, and improve patient comfort. Consequently, the putative benefits of their use include maximized utilization of resources, increased patient throughput and reduced overall healthcare costs.
Device description
• The Mynx (Access Closure, CA, USA) VCD is an active VCD that achieves arterial hemostasis by delivering a suture-free, conformable, water-soluble lyophilized polyethylene glycol (PEG) sealant into the extravascular space over the arteriotomy.
• Studies of device efficacy within the cardiovascular and neuroendovascular realm have demonstrated ≥90% clinical efficacy of achieving hemostasis, including patients on multiple antithrombotic medications.
• Complications associated with Mynx use include femoral artery dissection, pseudoaneurysm formation, arterial occlusion and limb ischemia, infection and groin hematoma formation.
Alternative devices
• Many other VCDs are currently available on the market. These devices can be classified according to their mechanism of action: suture-mediated devices (Perclose and Prostar); vessel plugs (Mynx, AngioSeal, EXOSEAL and FemoSeal); and vascular clips (StarClose).
Future perspective
• Manual compression remains the gold standard for arteriotomy closure because of its extensive record of safety and efficacy. The dramatic growth seen in the utilization of Mynx and other VCDs in recent years is in part due to the belief that VCDs are more cost effective, due to the reduction in postprocedural time to ambulation and, as a consequence, decreasing the duration of hospital stay and resources needed for patient monitoring.
• Ultra-fast ambulation protocols following transfemoral angiographic procedures are being developed and implemented in hospital systems. If these protocols prove to be efficacious in achieving hemostasis, the potential cost utility of VCD use could be in jeopardy; however, this must be taken into consideration with site-specific policies and resources.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- Juergens CP, Leung DY, Crozier JA et al. Patient tolerance and resource utilization associated with an arterial closure versus an external compression device after percutaneous coronary intervention. Catheter Cardiovasc. Interv. 63(2), 166–170 (2004).
- Mlekusch W, Minar E, Dick P et al. Access site management after peripheral percutaneous transluminal procedures: Neptune pad compared with conventional manual compression. Radiology 249(3), 1058–1063 (2008).
- Chevalier B, Lancelin B, Koning R et al. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc. Interv. 58(3), 285–291 (2003).
- Nasu K, Tsuchikane E, Sumitsuji S et al. The safety and efficacy of ‘pre-closure’ utilizing the Closer suture-mediated vascular closure device for achievement of hemostasis in patients following coronary interventions: results of the second Perclose Accelerated Ambulation and Discharge (PARADISE II) Trial. J. Invasive Cardiol. 17(1), 30–33 (2005).
- Noguchi T, Miyazaki S, Yasuda S et al. A randomised controlled trial of Prostar Plus for haemostasis in patients after coronary angioplasty. Eur. J. Vasc. Endovasc. Surg. 19(5), 451–455 (2000).
- Rickli H, Unterweger M, Sutsch G et al. Comparison of costs and safety of a suture-mediated closure device with conventional manual compression after coronary artery interventions. Catheter Cardiovasc. Interv. 57(3), 297–302 (2002).
- Carere RG, Webb JG, Buller CE et al. Suture closure of femoral arterial puncture sites after coronary angioplasty followed by same-day discharge. Am. Heart J. 139(1 Pt 1), 52–58 (2000).
- Resnic FS, Arora N, Matheny M et al. A cost-minimization analysis of the AngioSeal vascular closure device following percutaneous coronary intervention. Am. J. Cardiol. 99(6), 766–770 (2007).
- Rogers EW Jr, Doty WD, J Stewart. Significant improvements in patient care and cost savings resulting from percutaneous vascular surgery (PVS). J. Cardiovasc. Manag. 10(2), 13–17 (1999).
- Deuling JH, Vermeulen RP, Anthonio RA et al. Closure of the femoral artery after cardiac catheterization: a comparison of AngioSeal, StarClose, and manual compression. Catheter Cardiovasc. Interv. 71(4), 518–523 (2008).
- Ansel G, Yakubov S, Neilsen C et al. Safety and efficacy of staple-mediated femoral arteriotomy closure: results from a randomized multicenter study. Catheter Cardiovasc. Interv. 67(4), 546–553 (2006).
- Sanborn TA, Ebrahimi R, Manoukian SV et al. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ. Cardiovasc. Interv. 3(1), 57–62 (2010).
- Schwartz BG, Burstein S, Economides C et al. Review of vascular closure devices. J. Invasive Cardiol. 22(12), 599–607 (2010).
- Turi ZG. An evidence-based approach to femoral arterial access and closure. Rev. Cardiovasc. Med. 9(1), 7–18 (2008).
- Aziz EF, Pulimi S, Coleman C et al. Increased vascular access complications in patients with renal dysfunction undergoing percutaneous coronary procedures using arteriotomy closure devices. J. Invasive Cardiol. 22(1), 8–13 (2010).
- Azmoon S, Pucillo AL, Aronow WS et al. Vascular complications after percutaneous coronary intervention following hemostasis with the Mynx vascular closure device versus the AngioSeal vascular closure device. J. Invasive Cardiol. 22(4), 175–178 (2010).
- Dangas G, Mehran R, Kokolis S et al. Vascular complications after percutaneous coronary interventions following hemostasis with manual compression versus arteriotomy closure devices. J. Am. Coll. Cardiol. 38(3), 638–641 (2001).
- Exaire JE, Dauerman HL, Topol EJ et al. Triple antiplatelet therapy does not increase femoral access bleeding with vascular closure devices. Am. Heart J. 147(1), 31–34 (2004).
- Koreny M, Riedmuller E, Nikfardjam M et al. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA 291(3), 350–357 (2004).
- Yatskar L, Selzer F, Feit F et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc. Interv. 69(7), 961–966 (2007).
- Branzan D, Sixt S, Rastan A et al. Safety and efficacy of the StarClose vascular closure system using 7-F and 8-F sheath sizes: a consecutive single-center analysis. J. Endovasc. Ther. 16(4), 475–482 (2009).
- Katzenschlager R, Ugurluoglu A, Ahmadi A et al. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology 195(2), 463–466 (1995).
- Kresowik TF, Khoury MD, Miller BV et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J. Vasc. Surg. 13(2), 328–333; discussion 333–335 (1991).
- Sohail MR, Khan AH, Holmes DR Jr. et al. Infectious complications of percutaneous vascular closure devices. Mayo Clin. Proc. 80(8), 1011–1015 (2005).
- Stone PA, AbuRahma AF, Flaherty SK et al. Femoral pseudoaneurysms. Vasc. Endovascular Surg. 40(2), 109–117 (2006).
- Farouque HM, Tremmel JA, Raissi F Shabari et al. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J. Am. Coll. Cardiol. 45(3), 363–368 (2005).
- Foley P, A retrospective study of the management of retroperitoneal bleeding after percutaneous coronary intervention in the era of combination anti-platelet therapy. J. Cardiovasc. Med. (Hagerstown) 9(1), 56–58 (2008).
- Fram DB, Giri S, Jamil G et al. Suture closure of the femoral arteriotomy following invasive cardiac procedures: a detailed analysis of efficacy, complications, and the impact of early ambulation in 1200 consecutive, unselected cases. Catheter Cardiovasc. Interv. 53(2), 163–173 (2001).
- Peters SA, Yazar A, Lemburg SP et al. Renal perforation and retroperitoneal hematoma: an unusual complication following cardiac catheterization. Int. J. Cardiovasc. Imaging 23(6), 805–808 (2007).
- Baim DS, Knopf WD, Hinohara T et al. Suture-mediated closure of the femoral access site after cardiac catheterization: results of the suture to ambulate and discharge (STAND I and STAND II) trials. Am. J. Cardiol. 85(7), 864–869 (2000).
- Brachmann J, Ansah M, Kosinski EJ et al. Improved clinical effectiveness with a collagen vascular hemostasis device for shortened immobilization time following diagnostic angiography and percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 81(12), 1502–1505 (1998).
- Chaturvedi P, Prospective nonrandomized trial of manual compression and AngioSeal and StarClose arterial closure devices in common femoral punctures. Cardiovasc. Intervent. Radiol. 30(4), 812 (2007).
- Derham C, Davies JF, Shahbazi R et al. Iatrogenic limb ischemia caused by angiography closure devices. Vasc. Endovascular Surg. 40(6), 492–494 (2006).
- Fifi JT, Meyers PM, Lavine SD et al. Complications of modern diagnostic cerebral angiography in an academic medical center. J. Vasc. Interv. Radiol. 20(4), 442–447 (2009).
- 35 Schumacher PM, Ross CB, Wu YC et al. Ischemic complications of percutaneous femoral artery catheterization. Ann. Vasc. Surg. 21(6), 704–712 (2007).
- Access Closure. www.accessclosure.com
- Grandhi R, Kanaan H, Shah A et al. Safety and efficacy of percutaneous femoral artery access followed by Mynx closure in cerebral neurovascular procedures: a single center analysis. J. Neurointerv. Surg. doi:10.1136/neurintsurg-2013-010749 (2013) (Epub ahead of print).
- Scheinert D, Sievert H, Turco MA et al. The safety and efficacy of an extravascular, water-soluble sealant for vascular closure: initial clinical results for Mynx. Catheter Cardiovasc. Interv. 70(5), 627–633 (2007).
- Fields JD, Liu KC, Lee DS et al. Femoral artery complications associated with the Mynx closure device. Am. J. Neuroradiol. 31(9), 1737–1740 (2010).
- Noor S, Meyers S, Curl R. Successful reduction of surgeries secondary to arterial access site complications: a retrospective review at a single center with an extravascular closure device. Vasc. Endovascular Surg. 44(5), 345–349 (2010).
- Brown C. The Mynx vascular closure device: the Piedmont Heart Institute experience. Cath Lab Digest (1999). www.cathlabdigest.com/articles/The-Mynx%C2%AEVascular- Closure-Device-The-Piedmont-Heart-Institute- Experience
- Fargen KM, Velat GJ, Lawson MF et al. Occurrence of angiographic femoral artery complications after vascular closure with Mynx and AngioSeal. J. Neurointerv. Surg. 5(2), 161–164 (2013).
- Fargen KM, Hoh BL, Mocco J. A prospective randomized single-blind trial of patient comfort following vessel closure: extravascular synthetic sealant closure provides less pain than a self-tightening suture vascular compression device. J. Neurointerv. Surg. 3(3), 219–223 (2011).
- Ratnam LA, Raja J, Munneke GJ et al. Prospective nonrandomized trial of manual compression and AngioSeal and StarClose arterial closure devices in common femoral punctures. Cardiovasc. Intervent. Radiol. 30(2), 182–188 (2007).
- Satler L. Reducing complications of femoral access. Catheter Cardiovasc. Interv. 71(4), 524–525 (2008).
- Applegate RJ, Grabarczyk MA, Little WC et al. Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J. Am. Coll. Cardiol. 40, 78–83 (2002).
- Wong SC, Bachinsky W, Cambier P et al. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures: the ECLIPSE. JACC Cardiovasc. Interv. 2, 785–793 (2009).
- 48 Wagenbach A, Saladino A, Daugherty WP et al. Safety of early ambulation after diagnostic and therapeutic neuroendovascular procedures without use of closure devices. Neurosurgery, 66(3), 493–496; discussion 496–497 (2010).
- Vorobcsuk A, Konyi A, Aradi D et al. Tranradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction: systematic overview and metaanalysis. Am. Heart J. 109, 813–818 (2009).
• Direct comparison of Mynx and AngioSeal devices in cardiovascular procedures.
•• Our experience with the Mynx closure device in cerebral neurovascular procedures.
•• First large-scale study on the safety and efficacy of Mynx vascular closure devices.
•• Retrospective comparison of Mynx, AngioSeal and manual compression.
• First study to demonstrate increased patient comfort with the Mynx device.
•• First large study demonstrating safety of early ambulation without use of closure devices.