Research Article - Interventional Cardiology (2023) Volume 15, Issue 4
Ferroptosis: A new perspective in the diagnosis and treatment of coronary atherosclerotic heart disease
- Corresponding Author:
- Hong Fang
Department of Cardiology, Tongji Hospital, School of Medicine, Tongji University, Shanghai 200065, China,
E-mail: de_61@aliyun.com
Received date: 28-Jun-2023, Manuscript No. FMIC-23-104327;Editor assigned: 30-Jun-2023, PreQC No. FMIC-23-104327 (PQ); Reviewed date: 14-Jul-2023, QC No. FMIC-23-104327;Revised date: 21-Jul-2023, Manuscript No. FMIC-23-104327 (R);Published date: 31-Jul-2023, DOI: 10.37532/1755-5310.2023.15(4).719
Abstract
Ferroptosis is an iron dependent lipid Reactive Oxygen Species (ROS) induced form of non-apoptotic cell death. It is governed by three antioxidant axes, such as the cyst(e)ine/GSH/GPX4 axis, the GCH1/BH4/DHFR axis, and the FSP1/CoQ10 axis. Emerging evidence has revealed that disorders of iron metabolism are the common denominator in the occurrence and development of diverse cardiovascular disease, including atherosclerosis, myocardial Ischemia Reperfusion and (I/R), and myocardial infarction. Some genes/proteins that are involved in lipid metabolism have been used as biomarkers of Ferroptosis. Those biomarkers have facilitated the development of potential methods for disease diagnosis. Iron chelators, antioxidants, Ferroptosis inhibitors, and genetic manipulations may alleviate Coronary Atherosclerotic Heart Disease (CHD) by blocking Ferroptosis pathways. Our found also suggest that Angiotensin Type1/2 Receptors (AT1/2R) blocker may inhibit Ferroptosis of vascular endothelial cells. Thus, suppression of Ferroptosis is a potential therapeutic option for CHD. Here, we aimed to highlight the effects of Ferroptosis in CHD and summarize new diagnoses and treatments based on Ferroptosis of CHD.
Keywords
Ferroptosis • Atherosclerosis • Coronary atherosclerotic heart disease • Potential therapeutic option
Introduction
Although cell death is an often necessary process in multicellular organisms during development, it can also play a crucial role in causing disease in many organs including the heart [1]. In the past 10 years, many studies support the notion that Ferroptosis has a pathophysiological role in the development of cardiovascular diseases [2]. CHD is one of the most common types of heart diseases and causes the highest mortality rate worldwide. Mounting evidence indicates that dysregulation of iron metabolism is associated with CHD [3,4]. Disordered iron metabolism runs through the whole pathological progression of CHD [5]. Ferroptosis is involved in vascular injury and cardiomyocytes death, promoting the development of Atherosclerosis (AS), myocardial I/R, and myocardial infarction. Some studies have directly or indirectly proved that Ferroptosis exists in ischemic heart disease and plays an essential role in the process of cardiomyocytes death [5]. Therefore, Ferroptosis, the form of regulated cell death characterized by iron-dependent lipid peroxidation, Glutathione (GSH) depletion, and Glutathione Peroxidase-4 (GPX4) inactivation, has received great attention in CHD [2,6]. However, the regulatory mechanisms of Ferroptosis death in CHD remain largely uncharacterized. Acquiring a fundamental understanding of the relevant molecular and pathological mechanisms may contribute to future therapeutic targets for CHD [7]. By gaining a deeper understanding of Ferroptosis and its role in CHD, we may uncover new therapeutic targets and strategies to combat this prevalent disease.
For this reason, this research aimed to highlight the effects of Ferroptosis in CHD, summarize new pathogenesis and treatments based on Ferroptosis of CHD, and identify research trends and hotspots in this field.
Materials and Methods
Characteristics and regulation of Ferroptosis
Basic properties: The morphological, biochemical, genetic, and metabolic features in Ferroptosis are unique in comparison with other known forms of cell death. The morphological transformation of cells that undergo Ferroptosis generally includes a loss of plasma membrane integrity, cytoplasmic swelling, cell membrane rupture, the release of intracellular content, and moderate chromatin condensation. The mitochondria in Ferroptosis cells usually present shrinkage, an increase in membrane density, reduced or absent cristae, and rupturing of the outer membrane [2,8-15].
The two main biochemical properties of Ferroptosis are iron accumulation and lipid peroxidation. Intracellular free ferrous iron accumulation generates excessive ROS through the Fenton reaction, thereby triggering lipid peroxidation and Ferroptosis [10,16-19]. Some genes related to Ferroptosis were overexpressed including Prostaglandin End Peroxide Synthase-2(PTGS2), Acyl-CoA synthetase Long-chain family member-4 (ACSL4), Transferrin Receptor-1 (TFR1), Nuclear Receptor Coactivator-4 (NCOA4). Correspondingly, many other genes are downregulated in Ferroptosis, such as Glutathione Peroxidase-4 (GPX4), Solute Carrier Family-7 Member-11 (SLC7A11), Nuclear factor elytroid 2-Related Factor-2 (NRF2), Ferroptosis Suppressor Protein-1 (FSP1) and Ferritin Heavy Chain-1 (FTH1). In addition, many noncoding RNAs are associated with Ferroptosis [10,20,21].
Regulation of Ferroptosis
Ferroptosis regulatory pathways: At present, a large number of studies have shown that the regulation mechanism of Ferroptosis involves multiple signaling and metabolic pathways. Those regulatory pathways can be roughly classifified into three types: The fifirst involve iron metabolism, including NCOA4 regulation of ferritin degradation, and the Nrf2-HO-1 pathway, which affects iron. The pathway is the key to Ferroptosis. Unstable iron pools, especially ferrous iron-induced Fenton reaction, produce many lipid-free radicals triggering Ferroptosis. The second is glutathione metabolism, the GSH/GPX4 pathway, including system Xc-(a cystine/glutamate antiporter system) inhibition, the transsulfuration pathway, the Mevalonate pathway (MVA pathway), the glutamine pathway, and p53. Amino acid metabolism is also closely related to Ferroptosis regulation. Cysteine availability restricts GSH biosynthesis, while cysteine starvation induces Ferroptosis. The third type is that of lipid metabolism, including ACSL4, P53/spermidine/Spermine N1-Acetyltransferase-1 (SAT1)/Arachidonate Lipoxygenase (ALOX15), and lipophagy, which are related to lipid regulation and Ferroptosis, as well as the FSP1-CoQ10-NAD(P)H pathway synergies with GPX4 and GSH, which reduce phospholipid peroxidation and Ferroptosis. In addition, Endoplasmic Reticulum (ER) stress facilitates Ferroptosis viaATF4-induced Glutathione Specific Gamma-Glutamyl Cyclo transferase-1 (CHAC1) expression. Mitochondria are the major source of ROS production. It hosts many important metabolism processes such as glutaminolysis and play pivotal roles in governing multiple forms of Regulated Cell Death (RCD). However, the role of mitochondria in Ferroptosis is still not fully clarified [8,10,14,22-39].
Potential roles of noncoding RNA in Ferroptosis regulation: Noncoding RNA is widely expressed in the cardiovascular system and plays an important role in the occurrence and development of cardiovascular diseases. It can regulate the expression of genes associated with different Ferroptosis-related events, including iron homeostasis, cell protection, iron importation, and oxidative stress attenuation [40]. Numerous studies have confirmed that miRNAs and lncRNA participate in the process of cardiovascular disease by regulating Ferroptosis. Currently, many related miRNAs are reported, including miRNA-17-92, miR-15a-5p, miR-29b-3p, miR-190a-5p, miR-30d, miR-199a-5p and so on. Sun, et al., found that miR-135b-3p can promote Ferroptosis by targeting GPX4, then aggravating myocardial I/R injury. Similarly, miR-29b-3p can also aggravate cardiomyocyte I/R injury by down regulating the expression of PTX3 to exacerbate the inflammatory response. Known recently that the LncRNAs are involved in CHD include: lncRNA-KCNQ1OT1, lncRNA-XXYLT1-AS2, lncRNA-UCA1, lncRNA-p21, LncAABR07025387.1, LncRNA Mir9-3hg, etc. Some researchers have indicated that lncRNA PVT1 silencing signifificantly reduces infarct size and inhibits Ferroptosis [40,41-57].
Significantly, emerging studies have supported the vital role of circRNAs in CHDs, such as AS, and Myocardial Infarction (MI) [40,58]. Team Hou found that hsa_circ_0001558, hsa-circ_0021570, hsa_circ_0005941, hsa_circ_0002995, hsa-circ_0002665 and hsa_circ_0000530 may be closely related to the development of Ferroptosis in human coronary artery endothelial cells. The violated 6 circRNAs may function as circRNA-protein interactions to regulate the progression of Ferroptosis [59]. However, due to the lack of relevant research in this field, noncoding RNA regulates Ferroptosis and thus affects the pathophysiology of CHD remains to be further investigated.
Inducers and inhibitors of Ferroptosis: The known mechanisms of Ferroptosis inducers are as follows: Firstly, the inhibition of system Xc; and depletion of intracellular GSH; Secondly, the inhibition of GPX4; Thirdly, the degradation of GPX4 and exhaustion of antioxidant CoQ10; and Fourth, the direct oxidation of ferrous iron and lipidome, or indirect inactivation of GPX4. In contrast, there are mechanisms of Ferroptosis inhibitors: Inhibiting the accumulation of iron and lipid peroxidation [1,10,60].
Results
Implications of Ferroptosis in CHD
Current research indicates that Ferroptosis has a significant impact on many diseases. Numerous researchers report that Ferroptosis regulates the occurrence and progress of CHD [1-7]. A more precise understanding of the causes and mechanisms of CHD progression may be beneficial for the treatment of patients.
Ferroptosis and atherosclerosis
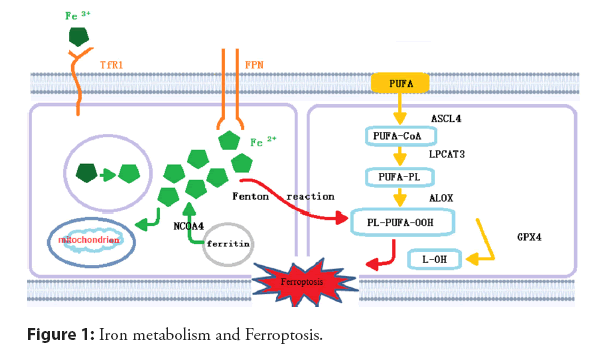
The primary pathological mechanism of CHD lies in the formation, growth, and even rupture of atherosclerotic plaques. AS is the pathological basis of CHD, which involves abnormal lipid metabolism, oxidative stress, and inflflammation, as well as other processes. Some researchers have confirmed the relationship between iron levels and AS [61]. Ferroptosis has been found to participate in atherogenesis and atherosclerotic progression through multiple physiological mechanisms, such as iron metabolism, lipid metabolism, and amino acid metabolism. The imbalance of iron metabolism may mediate Ferroptosis and promote the formation and development of AS. The relevant molecular mechanism is shown in Figure 1 [62-68].
Extracellular Fe3+ is transported into cells through TfR1 and reduced to Fe2+, which plays an effect in mitochondria or is stored in the form of ferritin. The remainder is transported out of cells through ferritin to maintain iron homeostasis. The reaction of PUFAs with excess reactive oxygen species occurs on the cell membrane, which is catalyzed viaACSL4 and LPCAT3, and mediated by ALOX, resulting in lipid peroxidation. GPX4 can reduce Ferroptosis via inhibiting lipid peroxidation. PUFAs: n‑-3 Polyunsaturated Fatty Acids; ACSL4: Acyl‑-CoA Synthetase Long chain family member-4; LPCAT3: Lysophosphatidylcholine Acyltransferase-3; ALOX: Arachidonate Lipoxygenase; PL: Phospholipid; GPX4: Glutathione Peroxidase-4; NCOA4: NuclearReceptor Coactivator-4; TfR1: Transferrin Receptor 1 [61,69].
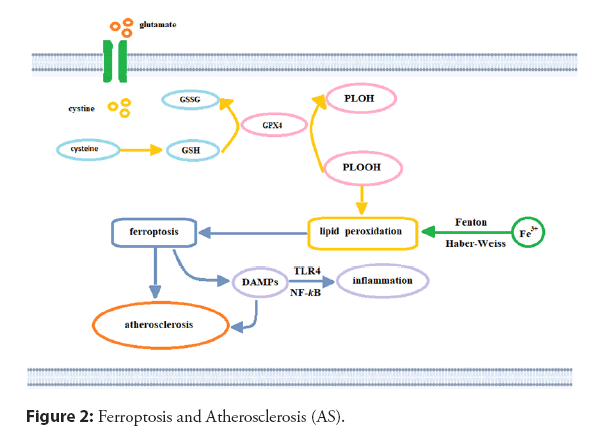
Lipid peroxidation is the core process of Ferroptosis. At the same time, iron is involved in various stages of lipid peroxide generation, including iron-catalyzed lipid oxidation and esterification, Polyunsaturated fatty acid oxidation, and lipid ROS generation through the fenton reaction. The Fenton reaction (Figure 2), and other peroxidation processes that incorporate iron can convert the mitochondrial oxidation respiration product, hydrogen peroxide, into hydroxyl radicals viathe catalysis of ferrous ions [61,70-73]. During the oxidative phosphorylation of the electron transport chain, which occurs on the inner mitochondrial membrane, electrons leak from the complex and oxygen forms ROS through a series of redox processes [71]. Excessive generation of ROS can lead to DNA damage, protein degeneration, and lipid peroxidation and can induce Ferroptosis. Nevertheless, ROS derived from mitochondria may also activate the Nucleotide-binding Oligomerization Domain (NOD)-Like Receptor NLR family pyrin domain containing-3 (NLRP3) inflflammatory bodies and cause the activation of the iron death signaling pathway degeneration, and lipid peroxidation and can induce Ferroptosis. Under normal physiological conditions [74], extracellular cystine is imported into cells in exchange for intracellular glutamate viathe cystine/glutamate antiporter system xc--, maintaining glutamate balance inside and outside the cell (Figure 2). Cysteine reductase promotes the conversion of intracellular Cystine to cysteine, and GSH is generated viaglutamate-cysteine ligase and GSH synthetase. The depletion of GSH also leads to glutamate-mediated iron and ROS generation and triggers oxidative cell death; specifically, Ferroptosis through the amino acid metabolic pathway [61,75].
Intracellular free iron generates hydroxyl radicals through the Fenton reaction and participates in the synthesis of lipoxygenase to generate lipid peroxides. Cystine and intracellular glutamate may exchange with system xc as a transporter. After a series of reactions, Cystine is converted into GSH. GPX4 inhibits Ferroptosis by reducing lipid peroxide to its lipid alcohol with GSH. Inhibition of this process will lead to Ferroptosis, trigger the release of DAMP, and Atherosclerosis will be formed and aggravated. GSH: Glutathione; GPX4: GSH peroxidase-4; DAMPs: Danger-Associated Molecular Patterns; GSSG: Oxidized Glutathione [61].
Ferroptosis and cardiac I/R injury
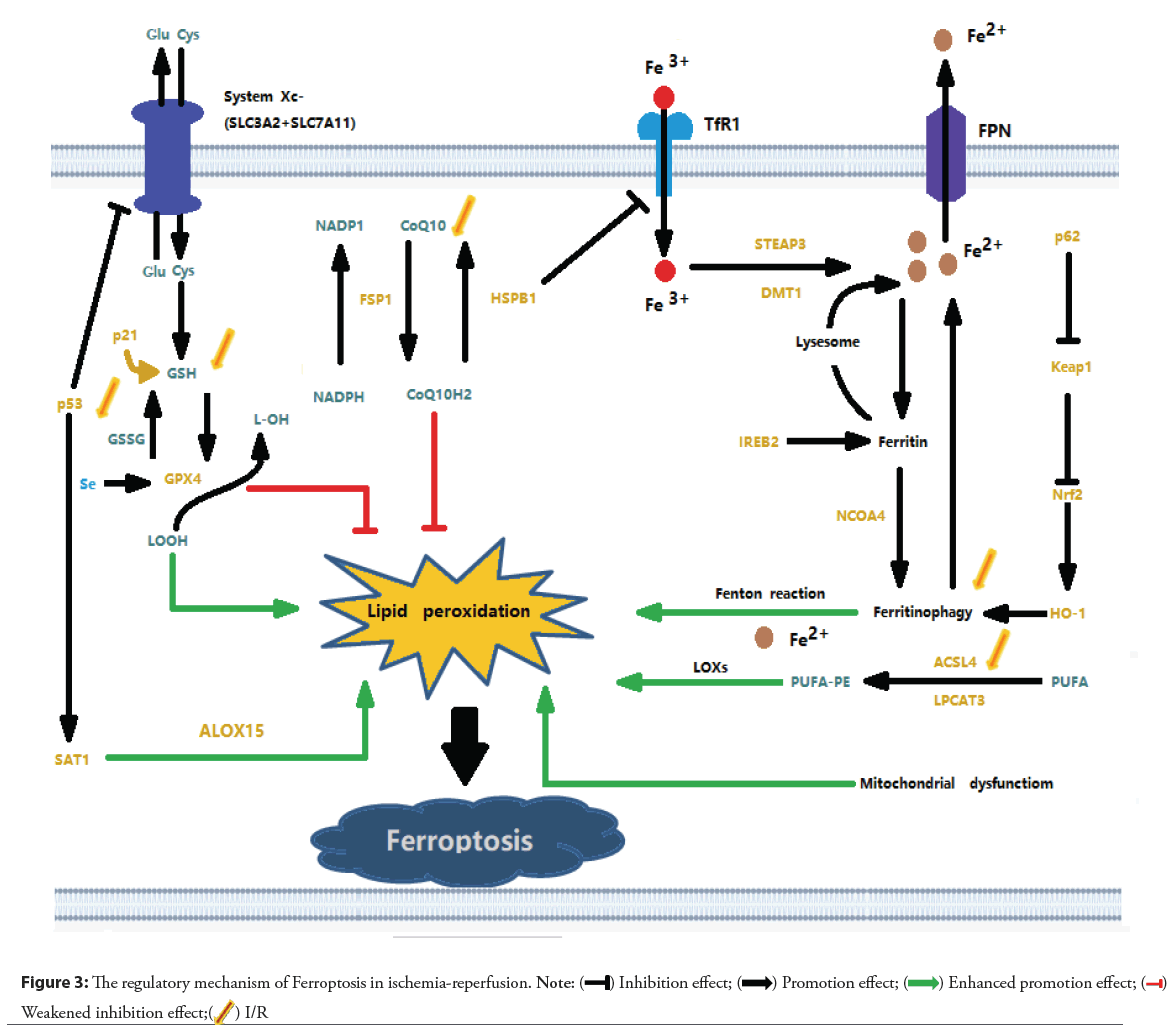
I/R is a pathological condition characterized by the initial restriction of the blood supply to the heart, followed by the restoration of blood flow and reoxygenation. Insuffificient blood supply leads to tissue hypoxia and cellular metabolic imbalance, and subsequent reperfusion and reoxygenation cause excessive inflammatory responses and exacerbate ischemic tissue damage, which is known as I/R injury [76]. Stable angina pectoris and acute coronary syndrome are mostly caused by myocardial cell ischemia. Timely reperfusion is now an effective treatment method [77-79]. However, myocardial I/R injury caused by reperfusion therapy can affect the prognosis of patients. Mounting evidence indicates that Ferroptosis is one of the key drivers of the onset and progression of ischemic heart injury. Ferroptosis primarily occurs during the period of myocardial reperfusion rather than ischemia [80]. After cardiac IR, the cardiomyocytes around the scar tissue appear iron deposition. An increase in intracellular iron concentration leads to lipid oxidation and then induces Ferroptosis [40]. Team Cai, suggests that 15-Lipoxygenase (Alox15)/15-Hydroperoxyeicosatetraenoic acid (15-HpETE)-mediated cardiomyocyte Ferroptosis plays an important role in prolonged I/R injury [81]. Team Cao reported that Lysine-specific Methyltransferase 2B (KMT2B)-dependent Riboflflavin Kinase (RFK) transcription activates the Tumor Necrosis Factor (TNF)-α/NADPH Oxidase- 2 (NOX2) pathway and enhances Ferroptosis caused by myocardial I/R as shown in the Figure 3 [82]. I/R, ischemia-reperfusion; Glu: Glutamate; Cys: Cystine; SLC3A2: Solute Carrier Family-3 Member-2; SLC7A11: Solute Carrier Family-7 Member-11; p21: Cyclin dependent kinase inhibitor 1A; p53: protein 53; GSH: Glutathione; GSSG: Glutathione Oxidized; Se: Selenium; GPX4: Glutathione Peroxidase-4; SAT1: Spermidine/Spermine N1-Acetyltransferase-1; ALOX-15: Arachidonate Lipoxygenase-15; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; CoQ10: Coenzyme Q10; FSP1: Ferroptosis Suppressor Protein-1; HSPB1: Heat Shock Factor-Binding Protein-1; TfR1: Transferrin Receptor-1; FPN: Ferroprotein; STEAP3: Six Transmembrane Epithelial Antigen of the Prostate-3; DMT1: Divalent Metal-Ion Transporter-1; IREB2: Iron Response Element-Binding Protein-2; NCOA4: Nuclear Receptor Coactivator-4; LOXs: Lipoxygenases; PUFA: Polyunsaturated Fatty Acids; PUFA-PE: Polyunsaturated Phosphatidylethanolamines; ACSL4: Acyl-CoA Synthetase Long-chain family member-4; LPCAT3: Lysophosphatidylcholine Acyltransferase-3; HO-1: Heme Oxygenase-1; Nrf2: Nuclear factor erythroid-2-related factor-2; Keap1: Kelch-like ECH-associated protein-1; p62: sequestosome-1 [83].
Ferroptosis as a promising diagnosis and treatment target
With more and more intensive research on Ferroptosis, the important role of Ferroptosis in CHD has been gradually recognized. Ferroptosis plays a crucial role in the pathogenesis of AS and I/R injury in cardiomyocytes, and with the exploration of clinical feasibility, the targeting of Ferroptosis may provide novel insights into the diagnosis and treatment of CHD.
Diagnosis: The overexpression of some genes/proteins that are involved in lipid metabolism has been used as biomarkers of Ferroptosis. These biomarkers are Prostaglandin Endoperoxide Synthase-2 (PTGS2/COX2), a key enzyme in prostaglandin biosynthesis [34]; Acyl-CoA Synthetase Long-Chain family member-4 (ACSL4), an enzyme that enhances the PUFA content in membrane phospholipids [21,84]. The prevalence of CHD continues to increase, and clinical outcomes remain unsatisfactory. Although several studies have reported remarkable progress in the identifification of diagnostic biomarkers for CHD in the blood (including long noncoding RNAs, methylation, and mRNAs), only two studies have described clinical heterogeneity in patients with different CHD severities [85,86]. Clinical heterogeneity is an important characteristic of CHD. The identification of CHD molecular subtypes based on gene expression pattern can provide a new way for the diagnosis of CHD. Based on the expression of 156 iron removal related genes, Ding, et al., divided the CHD sample into two molecular subtypes and conducted a relevant analysis to prove that Ferroptosis-related genes have a high diagnostic value between the two subtypes of CHD [87]. Liu, et al., found that the high expression of CBS and TLR4 in CHD is associated with disease severity and may be promising diagnostic markers for CHD [88]. The identifification of genetic subgroups in patients with CHD has improved our understanding of the pathogenesis of CHD and has facilitated the development of potential methods for disease diagnosis, classifification, and prognosis evaluation.
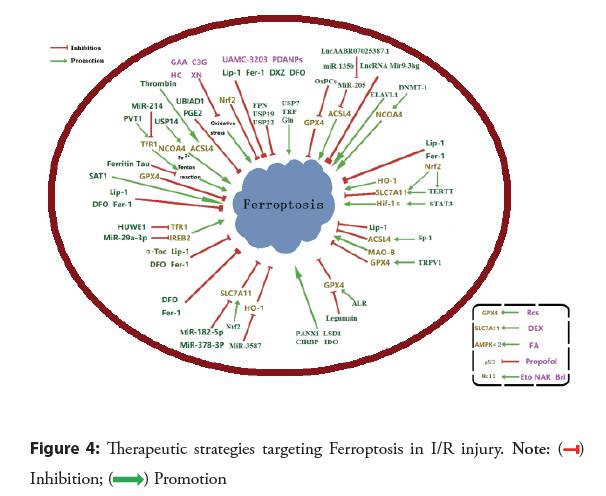
Treatment: At present, reperfusion is the preferred treatment for ACS. Unfortunately, the reperfusion procedure inevitably induces cardiomyocyte death and increases infarct size, worsening the condition. Ferroptosis occurred during the myocardial reperfusion phase but not ischemia. Hence, targeting Ferroptosis is a promising strategy to alleviate and cure myocardial I/R injury as shown in Figure 4. Among all types of organ I/R, Ferroptosis in myocardial I/R has been the most extensively investigated. Hence, pharmacological therapeutic strategies targeting Ferroptosis inhibition in myocardial I/R injury have been developed. The candidate agents for Ferroptosis suppressors include iron chelators and selective Ferroptosis inhibitors. In addition, genetic intervention that down-regulates TfR or up-regulates Ferroprotein, SLC7A11, or GPX4 expression may provide protection against Ferroptosis and multiple types of CHD [89].
Since Ferroptosis is an iron-dependent form of programmed cell death, it is no astonishment that iron chelation may suppress Ferroptosis. Iron chelators have been shown to alleviate myocardial I/R injury by inhibiting Ferroptosis. Also, Ferroptosis is characterized by iron-dependent accumulation of lipid peroxides, which causes oxidative damage to lipid bilayers. Thus, preventing oxidative stress is a common strategy to alleviate Ferroptotic injury, and a variety of Ferroptosis inhibitors that prevent oxidative stress have been developed and validated in I/R models. Suppression methods of Ferroptosis include chemical agents (such as iron chelators, synthetic compounds, natural monomers, etc.) and genetic interventions (Figure 4) [89-102].
Genetic interventions and single pharmacological therapies of Ferroptosis investigated in I/R injury are summarized in the Figure 4. Green capsules represent well-recognized Ferroptosis inhibitors and purple for myocardial. I/R: Ischemia-Reperfusion; GPX4: Glutathione Peroxidase-4; ACSL4: Acyl-CoA Synthetase Long-Chain family member-4; NCOA4: Nuclear Receptor Coactivator-4; SLC7A11: Solute Carrier Family-7 Member-11; Nrf2: Nuclear factor erythroid-2-related factor-2; HO-1: Heme Oxygenase-1; MAO-B: Monoamine Oxidase-B; IREB2: Iron Response Element-Binding protein-2; TfR1: Transferrin Receptor-1; AMPKα2: Adenosine 5′-Monophosphate-Activated Protein Kinase α2; p53: protein 53; FPN: Ferroprotein; USP7: Ubiquitin-Specifific Peptidase-7; USP14, Ubiquitin-Specifific Peptidase-14; USP19: Ubiquitin-Specifific Peptidase-19; USP22: Ubiquitin-Specifific Peptida-22; TRF: Transferrin; Gln: Glutamine; MiR: MicroRNA; OxPCs: Oxidized Phosphatidylcholines; LncRNA: Long non-coding RNA; ELAVL1: Embryonic Lethal-Abnormal Vision Like protein-1; DNMT-1: DNA (cytosine-5)-Methyltransferase-1; Nrf2: Nuclear factor erythroid-2-related factor-2; TERT: Telomerase Reverse Transcriptase; STAT3: Signal Transducer and Activator of Transcription-3; Sp1: Special protei-1; TRPV1: Transient Receptor Potential cation channel subfamily V member-1; LSD1: Lysine-Specifific Demethylase-1; Panx1: Pannexin-1; CIRBP: Cold-Inducible RNA-Binding Protein; IDO: Indoleamine 2,3-Dioxy-genase-1; ALR: Augmenter of Liver Regeneration; HUWE1: HECT domain-containing Ubiquitin E3 ligase; SAT1: Spermidine/Spermine N1-Acetyltransferase-1; PVT1: Plasmacytoma Variant-1; UBIAD1: UbiA prenyltransferase Domain Containing-1; PGE2: Prostaglandin E2; Fer-1: Ferrostatin-1; Lip-1: Liproxstatin-1; DFO: Deferoxamine; DXZ: Dexrazoxane; α-Toc: α-Tocopherol; GAA: Gossypol Acetic Acid; C3G: Cyanidin-3-Glucoside; XN: Xanthohumol; HC: Histochrome; PDA NPs: Polydopamine Nanoparticles; APG: Apigenin-7-O-β-D-(-6′′-p-coumaroyl)-Glucopyranoside; CAR: Carvacrol; Se: Selenium; Res: Resveratrol; CAT: Capsiate; CY: Carthamin Yellow; ROSI: Rosiglitazone; KF: Kaempferol; DEX.
Discussion
Pharmacological interventions
Iron chelators and lipid peroxidation inhibitors: Iron chelation may provide a novel disease-modifying treatment approach in alleviating AS [6]. They can bind to iron in the body to effectively increase iron excretion, thus blocking the redox reaction caused by iron overload [5]. Myocardial I/R injury can be alleviated when iron chelators block Ferroptosis. Further in vivo data provided additional evidence indicating that chelating iron during both acute and chronic myocardial I/R injury can provide cardio protective benefits, highlighting the potential of targeting Ferroptosis as a promising novel therapeutic strategy for I/R injury. Lipid peroxidation inhibitors (Such as Ferrostatin-1 and liproxstatin-1) may also pharmacologically inhibit Ferroptosis. Growing evidence shows that Ferrostatin-1 rescues lipid peroxidation and Liproxstatin-1 reverses Ferroptosis by scavenging free radicals to prevent PUFA oxidation, whose therapeutic effects were like Fer-1 [6,7,102,103].
Clinical drugs: Some clinical drugs have shown the potential to inhibit Ferroptosis [6]. The commonly prescribed heart medications might have previously unidentified anti-ferroptotic activity. A variety of agents with different biological activities have been shown to inhibit Ferroptosis in myocardial I/R injury [89]. For instance, carvedilol for the treatment of hypertension and heart failure has been shown to suppress Ferroptosis independent of its effect on β-adrenergic receptors, and the underlying mechanism might contribute to its capacity to scavenge lipid peroxides and chelate iron [104-106]. Propofol, a frequently used anesthetic agent, has been shown to protect against myocardial injury by suppressing I/R-induced Ferroptosis through the AKT/p53 signaling pathway [89,107]. Ferulic Acid (FA), the main active component of Angelica sinensis, was reported to alleviate myocardial I/R injury by enhancing AMPKα2 expression-mediated Ferroptosis inhibition [89,108]. Rapamycin also plays a crucial role in reducing excess iron and Ferroptosis in cardiomyocytes [89].
Natural products: With the rapid development of studies on Ferroptosis, increasing evidence has revealed that natural products can regulate Ferroptosis, and are related to cardiovascular disease. For example, the ethylacetate-extracted fraction of the total Ginkgo biloba flflower extract showed an anti-Ferroptosis effect in vascular endothelial cells. Compared with classical Ferroptosis inhibitors, natural products have the advantages of stable structure, high safety, low cost, and easy access, so it is a valuable and promising effort to explore natural products as Ferroptosis inhibitors [6].
Genetic manipulation
Noncoding RNA: The roles of several miRNAs and lncRNAs in regulating Ferroptosis have been found in myocardial I/R [40]. Zhang, et al., suggest that miR-199a-5p plays a central role in stimulating Ferroptosis-induced cardiomyocyte death during ischemic/hypoxic injury via inhibiting Akt/eNOS signaling pathway [48]. Wu, et al., confirmed the protective effect of lncRNA SEMA5A-IT1 carried by sEVs after CPB on cardiomyocytes after I/R [109,110]. Recently, miR-135b-3p was shown to exert an inhibitory effect on GPX4 expression, thereby exacerbating Ferroptosis during myocardial I/R injury [44]. Accumulating evidence suggests that lncRNAs can act as competing endogenous RNAs (ceRNAs) and sponge miRNAs to upregulate downstream gene expression [110]. LncAABR07025387.1 was shown to function as a ceRNA to sponge miR-205 (downregulating miR-205 expression) and consequently enhanced ACSL4 expression to exacerbate Ferroptosis during myocardial I/R [5]. In contrast, several lncRNAs are cardio protective against I/R injury. The Bone Marrow Mesenchymal Stem Cell (BMSC)-derived lncRNA Mir9-3hg has been reported to suppress I/R-induced cardiomyocyte Ferroptosis through regulating the Pumilio RNA binding family member-2 (Pum2)/Peroxiredoxin-6 (PRDX6) axis [55]. These fifindings highlight the therapeutic potential of using functional noncoding RNAs to treat myocardial I/R injury by suppressing Ferroptosis.
Although the role of Ferroptosis and circRNAs in the development of CHD pathology and the molecular mechanisms remains unclear, a few studies still provide some insights. Hou reveals that certain circRNAs may regulate Ferroptosis, which further affects the acceleration process of CHD. [59] These results provide new sight into the character of circRNAs in the progress of Human Coronary Artery Endothelial Cells (HCAECs) Ferroptosis and contribute signifificant data for further investigating the potential mechanisms of CHD. Protecting cells from Ferroptosis through circRNAs treatment may be an effective therapeutic strategy to prevent or treat CHD. The key circRNAs involved in Ferroptosis were identifified and the mechanisms of Ferroptosis in CHD were explored in this study. The results suggested that circRNAs may be a new target for the future treatment of Ferroptosis-involved CHD. Homson, et al., indicated that the circular antisense noncoding RNA in circANRIL slows down the development of atherosclerosis by regulating the maturation of rRNA and affects the formation of atherosclerosis, thus attenuating the development of atherosclerosis [40,111]. With the continuous deepening of circRNA and Ferroptosis research, whether circRNA can impact CHD by regulating Ferroptosis has become a focus of attention. Low-density Lipoprotein Receptor-related Protein-6 (LRP6) has a signifificant effect on the process of Ferroptosis. circRNA1615 regulated the expression of LRP6 through sponge adsorption of miR-152-3p. Then, it prevents Ferroptosis mediated by autophagy in cardiomyocytes [40,112]. Zheng, et al., showed that low expression of circSnx12 and high expression of miR-224-5p may downregulate the expression of FTH1 to make myocardial cells iron overloaded, then leading to Ferroptosis. The specifific mechanism is that circSnx12 promotes the expression of FTH1 viainhibiting the expression of miR-224-5p, thus maintaining an iron steady state in the cell [40,113,114].
Related gene: Ferroptosis has multiple regulatory genes, such as GPX4, ACSL4, Solute Carrier Family-7 Member-11 (SLC7A11), Nuclear factor erythroid-2-related factor-2 (Nrf2), Nuclear Receptor Coactivator-4 (NCOA4), and Heme Oxygenase-1 (HO-1). These genes are involved in multiple mechanisms related to Ferroptosis, such as iron metabolism, oxidative stress, and lipid peroxidation, and targeted therapeutic strategies associated with these genes have also been investigated in I/R injury [114].
Studying the inflfluence of genes on Ferroptosis can find a new therapeutic approach. Meng suggests that Ferroptosis-related genes GPX4 and TFRC were closely correlated with the identified overlapping genes CCNA2 and CDK1, which may serve as targeted therapies for the treatment of CHD [115]. The research results of Zhang, et al., show that Ferroptosis promotes neointima formation after arterial injury in mice and Vascular Smooth Muscle Cell (VMSC) phenotypic transition from a differentiated contractile to a dedifferentiated synthetic phenotype. Ferroptosis-induced VMSC phenotypic switching is associated with ROS accumulation. Thus, their findings support that inhibition of Ferroptosis or limiting ROS generation is a promising strategy to treat occlusive vascular diseases caused by restenosis of arteries following surgical interventions and injury [116,117]. Aybike Sena Ozuynuk reported that the genes related to oxidative stress and Ferroptosis were involved in biochemical parameters associated with CHD risk. P53 plays a dual role in Ferroptosis [117]. It reduces intracellular GSH by suppressing SLC7A11 expression to induce Ferroptosis [118]. Also, P53 enhances Ferroptosis viathe transcriptional induction of SAT1 or GSL2 [36]. On the other hand, p53 inhibits Ferroptosis by inducing CDKN1A/p21 (cyclin-dependent kinase inhibitor 1 A) expression [119]. P53 may also block Dipeptidyl Peptidase-4 (DPP4) activity through a transcriptional independent mechanism to limit erastin-induced Ferroptosis [120]. We also found that the renin-angiotensin-aldosterone system participates in Ferroptosis regulation through P53 [121]. These results may provide a new perspective to further exploration of the therapeutic method [122].
Conclusion
In conclusion, Ferroptosis represents a novel and promising therapeutic approach for the management of CHD, potentially revolutionizing current treatment paradigms. The unique features of Ferroptosis could provide valuable insights for the development of diagnostic and prognostic tools, enhancing our ability to detect and monitor CHD at both tissue and systemic levels. However, the application of Ferroptosis in CHD treatment is not without challenges. Further research is needed to fully understand the underlying mechanisms, optimize therapeutic strategies, and mitigate potential side effects. Nonetheless, the potential of Ferroptosis-based therapies to improve patient outcomes and quality of life makes it a compelling avenue for future research in CHD management.
References
- Xie LH, Fefelova N, Pamarthi SH, et al. Molecular mechanisms of ferroptosis and relevance to cardiovascular disease. Cells. 11(17): 2726 (2022).
- Fang X, Ardehali H, Min J, et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 20(1): 7-23 (2023).
- Lakhal-Littleton S, Wolna M, Carr CA, et al. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc Natl Acad Sci U S A. 112(10):3164-3169 (2015).
- Lakhal-Littleton S. Mechanisms of cardiac iron homeostasis and their importance to heart function. Free Radic Biol Med.133: 234-237 (2019).
- Fan X, Li A, Yan Z, et al. From iron metabolism to ferroptosis: Pathologic changes in coronary heart disease. Oxid Med Cell Longev. 2022: 6291889 (2022).
- Zhang H, Zhou S, Sun M, et al. Ferroptosis of endothelial cells in vascular diseases. Nutrients. 14(21): 4506 (2022).
- Zhou L, Han S, Guo J, et al. Ferroptosis-a new dawn in the treatment of organ ischemia-reperfusion injury. Cells. 11(22): 3653 (2022).
- Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 149(5): 1060-1072 (2012).
- Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 16(12): 1180-1191 (2014).
- Hu W, Liang K, Zhu H, et al. Ferroptosis and its role in chronic diseases. Cells. 11(13): 2040 (2022).
- Xie Y, Hou W, Song X, et al. Ferroptosis: Process and function. Cell Death Differ. 23(3): 369-379 (2016).
- Li J, Cao F, Yin HL, et al. Ferroptosis: Past, present and future. Cell Death Dis. 11(2): 88 (2020).
- Mou Y, Wang J, Wu J, et al. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J Hematol Oncol. 12(34) (2019).
- Tang D, Chen X, Kang R, et al. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 31:107-125 (2021).
- Chen X, Comish PB, Tang D, et al. Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol. 9: 637162 (2021).
- Li Y, Zeng X, Lu D, et al. Erastin induces ferroptosis viaferroportin-mediated iron accumulation in endometriosis. Hum Reprod. 36(4):951-964 (2021).
- Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 8: 22 (2019).
- Feng Z, Min L, Chen H, et al. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 43: 101984 (2021).
- Bai T, Li M, Liu Y, et al. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 160: 92-102 (2020).
- Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 156(1-2): 317-331 (2014).
- Yuan H, Li X, Zhang X, et al. Identifification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 478(3):1338-1343 (2016).
- Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 12(8): 1425-1428 (2016).
- Jiang M, Qiao M, Zhao C, et al. Targeting ferroptosis for cancer therapy: Exploring novel strategies from its mechanisms and role in cancers. Transl Lung Cancer Res. 9(4):1569-1584 (2020).
- Bai Y, Meng L, Han L, et al. Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys Res Commun. 508(4):997-1003 (2019).
- Chen X, Li J, Kang R, et al. Ferroptosis: Machinery and regulation. Autophagy 17(9):2054-2081 (2021).
- Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 171(2): 273-285 (2017).
- Wang L, Liu Y, Du T, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 27: 662-675 (2020).
- Song X, Zhu S, Chen P, et al. AMPK-mediated becn1 phosphorylation promotes ferroptosis by directly blocking system Xc - Activity. Curr Biol. 28(15): 2388-2399 (2018).
- Lang X, Green MD, Wang W, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 9(12): 1673-1685 (2019).
- Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 3: e02523 (2014).
- Chen L, Qiao L, Bian Y, et al. GDF15 knockdown promotes erastin-induced ferroptosis by decreasing SLC7A11 expression. Biochem Biophys Res Commun. 526(2): 293-299 (2020).
- Fang X, Cai Z, Wang H, et al. Loss of cardiac ferritin h facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res. 127(4): 486-501 (2020).
- Dong H, Qiang Z, Chai D, et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY) 12(13): 12943-12959 (2020).
- Hong T, Lei G, Chen X, et al. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-profificient ovarian cancer. Redox Biol. 42: 101928 (2021).
- Qiang Z, Dong H, Xia Y, et al. Nrf2 and STAT3 alleviates ferroptosis-mediated IIR-ALI by regulating SLC7A11. Oxid Med Cell Longev. 2020: 5146982 (2020).
- Kang R, Kroemer G, Tang D, et al. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 133:162-168 (2019).
- Gryzik M, Asperti M, Denardo A, et al. NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys Acta Mol Cell Res. 1868: 118913 (2021).
- Chen Y, Zhang P, Chen W, et al. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol Lett. 225: 9-15 (2020).
- Chen MS, Wang SF, Hsu CY, et al. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells viathe GCN2-eIF2α-ATF4 pathway. Oncotarget. 8(70):114588-114602 (2017).
[CrossRef][Google Scholar][PubMed]
- Zhang J, Liu X, Li X, et al. The emerging role of noncoding RNA regulation of the ferroptosis in cardiovascular diseases. Oxid Med Cell Longev. 2022: 3595745 (2022).
- Xiao FJ, Zhang D, Wu Y, et al. MiRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun. 515(3):448-454 (2019).
- Zhou X, Zhuo M, Zhang Y, et al. MiR190a-5p regulates cardiomyocytes response to ferroptosis via directly targeting GLS2. Biochem Biophys Res Commun. 566: 9-15 (2021).
- Sun W, Shi R, Guo J, et al. MiR-135b-3p promotes cardiomyocyte ferroptosis by targeting GPX4 and aggravates myocardial ischemia/reperfusion injury. Front Cardiovasc Med. 8:663832 (2021).
- Fan K, Huang W, Qi H, et al. The Egr-1/miR-15a-5p/GPX4 axis regulates ferroptosis in acute myocardial infarction. Eur J Pharmacol. 909: 174403 (2021).
- Song Y, Cai W, Wang J, et al. Upregulation of miR-143-3pattenuates oxidative stress-mediated cell ferroptosis in cardiomyocytes with atrial fifibrillation by degrading glutamicoxaloacetic transaminase. Biocell. 45(3):733-744 (2021).
- Tang S, Wang Y, Ma T, et al. MiR-30d inhibits cardiomyocytes autophagy promoting ferroptosis after myocardial infarction. Panminerva Medica. 64(2) (2020).
- He D, Yan L. MiR-29b-3p aggrav2ates cardiac hypoxia/reoxygenation injury via targeting PTX3. Cytotechnology. 73(1):91-100 (2021).
- Zhang GY, Gao Y, Guo XY, et al. MiR-199a-5p promotes ferroptosis-induced cardiomyocyte death responding to oxygen-glucose deprivation/reperfusion injury via inhibiting Akt/eNOS signaling pathway. Kaohsiung J Med Sci. 38(11):1093-1102 (2022).
- Zhang L, Meng X, Zhu XW, et al. Long non-coding RNAs in oral squamous cell carcinoma: Biologic function, mechanisms and clinical implications. Mol Cancer. 18(1):102 (2019).
- Akerman I, Tu Z, Beucher A, et al. Human pancreatic β cell lncRNAs control cell-specifific regulatory networks. Cell Metab. 25(2):400-411 (2017).
- Wu G, Cai J, Han Y, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 130(17):1452-1465 (2014).
- Wang Q, Yang Y, Fu X, et al. Long noncoding RNA _XXYLT1-AS2_ regulates proliferation and adhesion by targeting the RNA binding protein FUS in HUVEC. Atherosclerosis.298:58-69 (2020).
- Sun L, Zhu W, Zhao P, et al. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: A novel molecular target for cardio protection through miR-873-5p/XIAP axis. Cell Death Dis. 11(8):696 (2020).
- Sun W, Wu X, Yu P, et al. Lncaabr07025387.1 enhances myocardial ischemia/reperfusion injury via mir-205/acsl4-mediated ferroptosis. Front Cell Dev Biol. 10:672391 (2022).
- Zhang JK, Zhang Z, Guo ZA, et al. The bmsc-derived exosomal lncrna mir9-3hg suppresses cardiomyocyte ferroptosis in ischemia-reperfusion mice via the pum2/prdx6 axis. Nutr Meta Cardiovasc Dis. 32(2):515-527 (2022).
- Lu J, Xu F, Lu H, et al. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 260:118305 (2020).
- Zhuang S, Ma Y, Zeng Y, et al. METTL14 promotes doxorubicin-induced cardiomyocyte ferroptosis by regulating the KCNQ1OT1-miR-7-5p-TFRC axis. Cell Biol Toxicol. (2021).
- Ding C, Zhou Y. Insights into circular RNAs: Biogenesis, function and their regulatory roles in cardiovascular disease. J Cell Mol Med. 27(10):1299-1314 (2023).
- Hou C, Wang Y, Wang Y, et al. Circular RNA expression profile of H2O2 induced ferroptosis model of human coronary artery endothelial cells. Atheroscler Plus. 49:1-11 (2022).
- Gaschler MM, Andia AA, Liu H, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 14(5):507-515 (2018).
- Li J, Xu L, Zuo YX, et al. Potential intervention target of atherosclerosis. Mol Med Rep. 26(5):343 (2022).
- Gong L, Tian X, Zhou J, et al. Iron dyshomeostasis induces binding of APP to BACE1 for amyloid pathology, and impairs APP/Fpn1 complex in microglia: Implication in pathogenesis of cerebral microbleeds. Cell Transplant. 28(8):1009-1017 (2019).
- Zhang C. Essential functions of iron‑requiring proteins in DNA replication, repair and cell cycle control. Protein Cell. 5(10):750-760 (2014).
- Pisano G, Lombardi R, Fracanzani AL, et al. Vascular damage in patients with nonalcoholic fatty liver disease: Possible role of iron and ferritin. Int J Mol Sci. 17(5):675 (2016).
- Valenti L, Dongiovanni P, Motta BM, et al. Serum hepcidin and macrophage iron correlate with MCP-1 release and Serum hepcidin and macrophage iron correlate with MCP‑1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler Thromb Vasc Biol. 31(3):683-690 (2011).
- Liu J, Kuang F, Kroemer G, et al. Autophagy‑dependent ferroptosis: Machinery and regulation. Cell Chem Biol. 27(4):420-435 (2020).
- Liu J, Kuang F, Kroemer G, et al. Apolipoprotein E deficiency induces a progressive increase in tissue iron contents with age in mice. Redox Biol. 64:102779 (2023).
- Fuhrmann DC, Mondorf A, Beifuß J, et al. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 36:101670 (2020).
- Shan X, Lv ZY, Yin MJ, et al. The protective effect of cyanidin‑3‑glucoside on myocardial ischemia‑reperfusion injury through ferroptosis. Oxid Med Cell Longev. 2021:8880141 (2021).
- Gan B. Mitochondrial regulation of ferroptosis. J Cell Biol. 220(9):e202105043 (2021). [CrossRef][Google Scholar][PubMed]
- Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 289(22):7038-7050 (2022).
- Mishra SR, Mahapatra KK, Behera BP, et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int J Biochem Cell Biol. 136:106013 (2021).
- Jelinek A, Heyder L, Daude M, et al. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol Med. 117:45-57 (2018).
- Jiang X, Stockwell BR, Conrad M, et al. Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 22(4):266-282 (2021).
- Chen X, Yu C, Kang R, et al. Cellular degradation systems in ferroptosis. Cell Death Differ. 28(4):1135-1148 (2021).
- Eltzschig HK, Eckle T. Ischemia and reperfusion-From mechanism to translation. Nat Med. 17(11):1391-401 (2011).
- Liu C, Li Z, Li B, et al. Relationship between ferroptosis and mitophagy in cardiac ischemia reperfusion injury: A mini-review. PeerJ. 11:e14952 (2023).
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 123(1):92-100 (2013).
- Zhao T, Wu W, Sui L, et al. Reactive oxygen species based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 7:47-72 (2021).
- Tang LJ, Luo XJ, Tu H, et al. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn Schmiedebergs Arch. Pharm.394:401-410 (2021).
- Cai W, Liu L, Shi X, et al. Alox15/15-HpETE aggravates myocardial ischemia-reperfusion injury by promoting cardiomyocyte ferroptosis. Circulation. 147(19):1444-1460 (2023).
- Cao Y, Luo F, Peng J, et al. KMT2B-dependent RFK transcription activates the TNF-α/NOX2 pathway and enhances ferroptosis caused by myocardial ischemia-reperfusion. J Mol Cell Cardiol. 173:75-91 (2022).
- Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 13(1):81-90 (2017).
- Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 13(1):91-98 (2017).
- Zhang X, Xiang Y, He D, et al. Identification of potential biomarkers for CHD using integrated expression and methylation data. Front Genet. 9(11):778 (2020).
- Zhang B, Zeng K, Li R, et al. Construction of the gene expression subgroups of patients with coronary artery disease through bioinformatics approach. Math Biosci Eng. 18(6):8622-8640 (2021).
- Ding L, Long F, An D, et al. Construction and validation of molecular subtypes of coronary artery disease based on ferroptosis-related genes. BMC Cardiovasc Disord. 22(1):283 (2022).
- Liu WP, Li P, Zhan X, et al. Identification of molecular subtypes of coronary artery disease based on ferroptosis-and necroptosis-related genes. Front Genet. 20(13):870222 (2022).
- Pan Y, Wang X, Liu X, et al. Targeting ferroptosis as a promising therapeutic strategy for ischemia-reperfusion injury. Antioxidants (Basel). 11(11):2196 (2022).
- Gao M, Monian P, Quadri N, et al. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 59(2):298-308 (2015).
- Ma S, Sun L, Wu W, et al. Usp22 protects against myocardial ischemia-reperfusion injury via the sirt1-p53/slc7a11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Front Physiol. 11:551318 (2020).
- Tang LJ, Zhou YJ, Xiong XM, et al. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/tfr1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med. 162:339-352 (2021).
- Tian H, Xiong Y, Zhang Y, et al. Activation of nrf2/fpn1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones. 27(2):149-164 (2021).
- Li W, Wang Y, Leng Y, et al. Inhibition of dnmt-1 alleviates ferroptosis through ncoa4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death Discov. 7(1):267 (2021).
- Stamenkovic A, O'Hara KA, Nelson DC, et al. Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 320(3):H1170-H1184 (2021).
- Chen HY, Xiao ZZ, Ling X, et al. Elavl1 is transcriptionally activated by foxc1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med. 27(1):14(2021).
- Li C, Sun G, Chen B, et al. Nuclear receptor coactivato 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res. 174:105933 (2021).
- Chen J, Yang L, Geng L, et al. Inhibition of Acyl-CoA synthetase long-chain family member 4 facilitates neurological recovery after stroke by regulation ferroptosis. Front Cell Neurosci. 15:632354 (2021).
- Lu H, Wang B, Cui N, et al. Artesunate suppresses oxidative and inflammatory processes by activating Nrf2 and ROS dependent p38 MAPK and protects against cerebral ischemia-reperfusion injury. Mol Med Rep.17(5):6639-6646 (2018).
- Liu Z, Lv X, Song E, et al. Fostered Nrf2 expression antagonizes iron overload and glutathione depletion to promote resistance of neuron-like cells to ferroptosis. Toxicol Appl Pharmacol.2020;407:115241 (2020).
- Wang H, Liu C, Zhao Y, et al. Mitochondria regulation in ferroptosis. Eur J Cell Biol. 99(1):151058 (2020).
- Ratan RR. The chemical biology of ferroptosis in the central nervous system. Cell Chem Biol. 27(5):479-498 (2020).
- Zilka O, Shah R, Li B, et al. On the mechanism of cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. 3:23-243 (2017).
- Dayani PN, Bishop MC, Black K, et al. Desferoxamine (DFO)-mediated iron chelation: Rationale for a novel approach to therapy for brain cancer. J Neuro-Oncol. 67:367-377 (2004).
- Mishima E, Sato E, Ito J, et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J Am Soc Nephrol. 31(2):280-296 (2020).
- Conlon M, Poltorack CD, Forcina GC, et al. A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat Chem Biol. 17(6):665-674 (2021).
- Oettl J, Greilberger K, Zangger E, et al. Radical- scavenging and iron-chelating properties of carvedilol, an antihypertensive drug with antioxidative activity. Biochem. Pharmacol. 62(2):241-248 (2001).
- Li S, Lei Z, Yang X, et al. Propofol protects myocardium from ischemia/reperfusion injury by inhibiting ferroptosis through the akt/p53 signaling pathway. Front Pharmacol. 13:841410 (2022).
- Liu X, Qi K, Gong Y, et al. Ferulic acid alleviates myocardial ischemia reperfusion injury via upregulating ampkα2 expression-mediated ferroptosis depression. J Cardiovasc Pharmacol. 79(4):489-500 (2021).
- Wu T, Shi G, Ji Z, et al. Circulating small extracellular vesicle‑encapsulated SEMA5A‑IT1 attenuates myocardial ischemia-reperfusion injury after cardiac surgery with cardiopulmonary bypass. Cell Mol Biol Lett. 27(1):95 (2022).
- Homson DW, Dinger ME. Endogenous microRNA sponges: Evidence and controversy. Nat Rev Genet. 17(5):272-283 (2016).
- Liang ZX, Liu HS, Xiong L, et al. A novel NF-κB regulator encoded by circPLCE1 inhibits colorectal carcinoma progression by promoting RPS3 ubiquitin-dependent degradation. Mol Cancer. 20(1):103 (2021).
- Li RL, Fan CH, Gong SY, et al. CircRNA1615 inhibits ferroptosis via modulation of autophagy by the miRNA152-3p/LRP6 axis in cardiomyocytes of myocardial infarction. Oxid Med Cell Longev. 2021:8963987 (2021).
- Zheng H, Shi L, Tong C, et al. CircSnx12 is involved in ferroptosis during heart failure by targeting miR224-5p. Front Cardiovasc Med. 8:656093 (2021).
- Chen Y, Fan H, Wang S, et al. Ferroptosis: A novel therapeutic target for ischemia-reperfusion injury. Front Cell Dev Biol. 9:688605 (2021).
- Meng Q, Xu Y, Ling X, et al. Role of ferroptosis-related genes in coronary atherosclerosis and identification of key genes: integration of bioinformatics analysis and experimental validation. BMC Cardiovasc Disord. 22(1):339 (2022).
- Zhang S, Bei Y, Huang Y, et al. Induction of ferroptosis promotes vascular smooth muscle cell phenotypic switching and aggravates neointimal hyperplasia in mice. Mol Med. 28(1):121 (2022).
- Ozuynuk AS, Erkan AF, Coban N, et al. Examining the expression levels of ferroptosis-related genes in angiographically determined coronary artery disease patients. Mol Biol Rep. 49(8):7677-7686 (2022).
- Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 520(7545):57-62 (2015).
- Tarangelo A, Magtanong L, Bieging-Rolett KT, et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 22(3):569-575 (2018).
- Xie Y, Zhu S, Song X, et al. The tumor suppressor p53 limits ferroptosis by blocking dpp4 activity. Cell Rep. 20(7):1692-1704 (2001).
- Liu C, Shen Y, Cavdar O, et al. Angiotensin II-induced vascular endothelial cells ferroptosis via P53-ALOX12 signal axis. Clin Exp Hypertens. 45(1):2180019 (2023).