Review Article - Interventional Cardiology (2013) Volume 5, Issue 3
Fibronectin/fibrinogen/tropoelastin on a stent to promote CD34+ cell growth does not reduce neointima formation
- Corresponding Author:
- Gerard Pasterkamp
Laboratory of Experimental
Cardiology, Universitair Medisch
Centrum Utrecht, Utrecht,
The Netherlands
Tel: +31 88 755 7155
Fax: +31 88 755 2693
E-mail: g.pasterkamp@umcutrecht.nl
Abstract
Keywords
anti-CD34, endothelialization, intima hyperplasia, neointima formation, stent
Drug-eluting stents (DES) reduce neointima formation by inhibiting proliferation of vascular smooth muscle cells (SMCs). This beneficial effect of early-generation DES was accompanied by delayed vascular healing owing to the inhibitory effects of the drug on endothelial cells (ECs), leading to thrombotic complications [1–4]. Anti-proliferative drugs were nonspecific and influence the outgrowth of both SMCs and ECs, while proliferation of ECs is desired. New-generation DES showed a lower risk for stent thrombosis compared with bare-metal stents [5,6], owing to improvements of the stent design and drug coatings.
Besides DES, other promising strategies have been used to reduce in-stent thrombosis and restenosis. One of them is improving the endothelial cell coverage of the stent. A functionally intact endothelium prevents thrombotic complications, and also regulates the proliferation of the underlying SMCs. ECs can migrate from the injured vessel wall, but also circulating endothelial progenitor cells (EPCs) are capable of homing towards the exposed subendothelium, which subsequently promotes re-endothe-lialization [7,8]. Capturing circulating EPCs on a stent surface enhances stent endothelialization and is, therefore, a promising strategy to reduce in-stent thrombosis and restenosis [9–12]. The Genous™ (OrbusNeich Medical, FL, USA) stent was designed for capturing EPCs using a CD34 antibody immobilized on the stent surface. Unfortunately, clinical studies did not show improved outcomes compared with a bare-metal stent [10,13,14]. In the current study, we aimed to study the effect of combining new stent coatings with anti-CD34 antibodies on endothelial cell stent coverage and neointima formation.
In a previous study, we analyzed the properties of stent coatings in vitro. We found that a combination of tropoelastin, fibronectin and fibrinogen facilitated optimal EC growth, while vascular SMC outgrowth and endothelial inflammatory and procoagulant responses were kept minimal [15]. In this study, a rabbit model was used to study these specific stent coatings in vivo with and without the EPC capturing anti-CD34 antibody.
Materials & methods
▪ Experimental design
A total of 44 female New Zealand white rabbits (Charles River, MA, USA; 3.5–4 kg) were studied. Stents were implanted in the left and right iliac arteries. In each rabbit, two randomly chosen stents were placed in both iliac arteries, one in each artery. After 7 or 28 days, animals were sacrificed, whereupon stents were excised for histological analysis. All animal experiments were performed after receiving approval from the Ethical Committee on Animal Experimentation of the Universitair Medisch Centrum Utrecht (Utrecht, The Netherlands). Animal care followed established guidelines.
▪ Stent design
The stent coatings that were used are bare metal, anti-CD34, fibronectin/fibrinogen, fibronectin/fibrinogen/anti-CD34, fibronectin/fibrinogen/ tropoelastin and fibronectin/fibrinogen/tropoelastin/ anti-CD34. Bare metal and anti-CD34 were commercially available stents (R-Stent Evolution ™ 2, Genous Bio-engineered stent, Orbus- Neich Medical). The other coatings were custom- made, non-US FDA-approved devices using the same stent platform as that of the bare-metal stents (Ssens, Enschede, The Netherlands; and OrbusNeich Medical). Fibrinogen was purchased from Enzyme Research Laboratories (Swansea, UK), fibronectin from Millipore (MA, USA). Tropoelastin was purified as described before [15]. The proteins were covalently coupled to the stent surface on both sides of the stent.
▪ Anesthesia
Before operation and termination, rabbits were fasted overnight. As premedication, rabbits were injected intramuscularly with 0.5 ml vetranquil and 0.5 ml methadone. Etomidate (2 ml) was injected via the ear vein and after intubation rabbits were ventilated with a mixture of oxygen and air (1:2), and 1.5% isoflurane. An air vein was used for continuous administration of 4 μg/h sufentanil.
▪ Stent implantation
Rabbits were injected intravenously with 150 U/kg heparin prior to vessel manipulation. Subcutaneous 1 mg/kg meloxicam was administered before surgery as analgesia. A 4F sheath was placed in the right common carotid artery and angiograms of the iliac arteries were obtained by contrast injection. No nitrates were used. A Fogarty® catheter (4.5 F) was inserted via the sheet and placed in the iliac arteries using angiography. The balloon was dilated and pulled through the iliac artery for approximately 3 cm to create endothelial damage. This denudation was performed twice in both iliac arteries. After endothelial denudation, the stents (3.5 × 13 mm) were inserted via the sheet. Pressure was applied to inflate the balloon and unfold the stent to a size of 3.4 mm. After stenting, a second angiogram was obtained. After surgery, rabbits daily received freshly prepared drinking water with 10 mg aspirin (Aspro, Bayer, Mijdrecht, The Netherlands) per 100 ml water. Rabbits consumed approximately 350 ml water a day and their drinking behavior was monitored.
▪ Quantitative angiography
Angiograms of the iliac arteries before and after stent placement were obtained during intervention and termination. The diameter of the lumen at maximal stenosis was measured using ImageJ before stenting, after stenting, and just before termination and calibrated using a ruler placed in the same image during angiography. The lumen loss ratio was defined as the angiographic diameter after stenting/angiographic diameter at termination.
▪ Tissue preparation & histological analysis
Animals were sacrificed after 7 (n = 23) and 28 (n = 21) days and stents were harvested. Heparin (intravenous 1000 U/kg) was administered prior to vessel manipulation. Catheters were placed in the aorta and vena cava under anesthesia (as described before). An angiogram was made to analyze the stents and surrounding arteries. Ringers lactate was perfused into the aorta to flush the stent to remove all blood cells. After perfusion with buffer, 100 ml 4% paraformaldehyde buffered with phosphate-buffered saline was perfused via the aorta to pressure fix the tissue. Subsequently, the rabbits were sacrificed and the stents and adjacent arteries were dissected. The stents that were obtained after 7 days were cut axially in two equal parts. One part of this stent was used for morhpometric analyses and part was used for electron microscopical imaging. Morphometric analyses were executed on the stents obtained after 7 and 28 days rabbits which were fixed in formalin for at least 72 h followed by embedding in methyl metacrylate for further histological analysis. Sections were cut with a diamond- coated saw at the center of top, the center and the bottom of the stent. A hematoxylin and eosin staining was performed for morphometric analysis. Neointima formation was traced manually using pictures made at a 20× magnification using ImageJ.
Half of the stent from rabbits terminated after 7 days was used for scanning electron microscopy (sEM). For this purpose, stents were fixed in a 1.5% glutarealdehyde in 0.1 M cacodylate buffer (pH 7.2). A secondary fixation using 1% osmium tetroxide in 0.1 M cacodylate buffer was performed, followed by dehydration. Liquid was removed from the samples using critical point drying, sprayed with platinum and analyzed using sEM (Phenom desktop sEM, Phenom-World BV, Eindhoven, The Netherlands). Pictures were made at 380× magnification and stent endothelialization was quantified manually using ImageJ.
▪ Statistics
The differences between two coatings was compared with a Mann–Whitney test. Values are presented as mean ± standard error of the mean. A value of p < 0.05 was regarded significant. Statistical analysis was performed using SPSS version 20 software.
Results
▪ Quantitative angiography
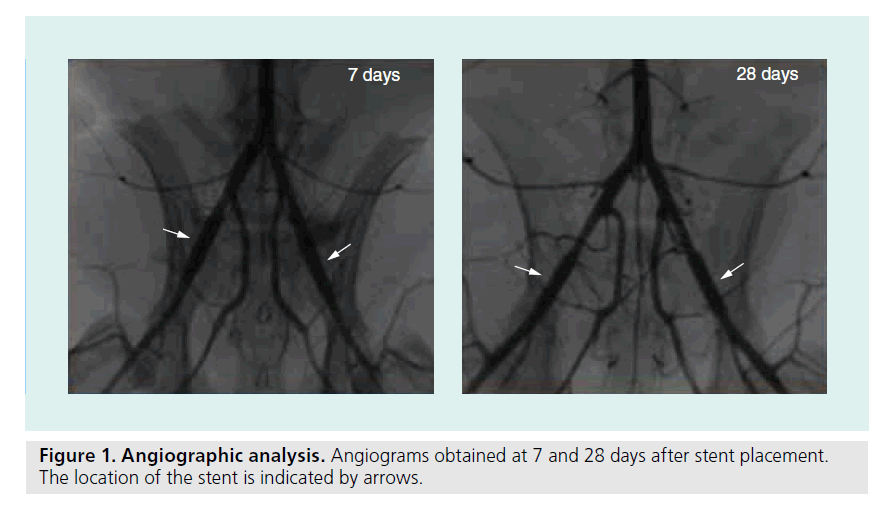
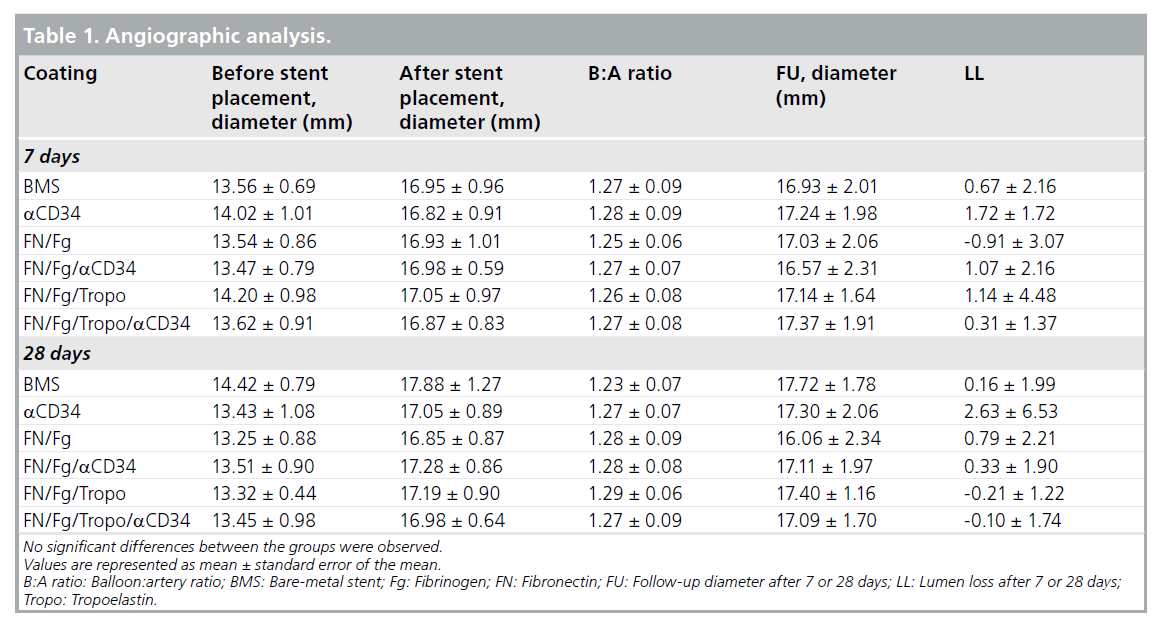
Stents were positioned in the iliac arteries of rabbits for a period of 7 or 28 days. Examples of angio-grams of stented arteries after 7 and 28 days, prior to termination are shown in Figure 1. Quantification of balloon–artery ratio and lumen loss are shown in Table 1, and no differences were observed for any stent type. No stent thrombosis or occlusion was observed directly after stent placement and after 7 or 28 days.
▪ Stent endothelialization after 7 days of stent placement
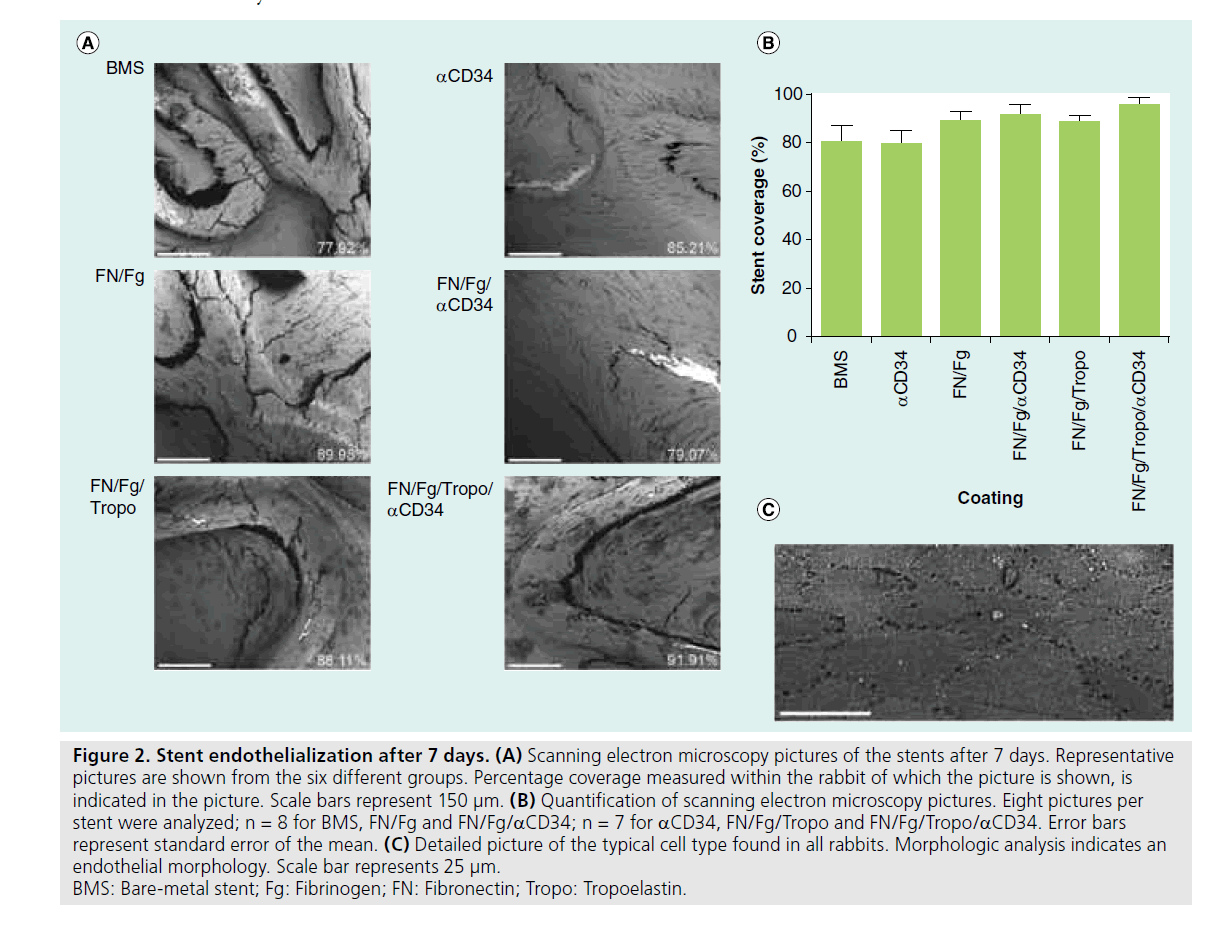
Stents were harvested after 7 days for endothelialization analysis. Endothelialized surface was measured with sEM analysis in eight single pictures per stent at a 350× magnification. A representative example for each coating is shown in Figure 2A. In each picture, stent outlines were determined and the percentage of coverage on the stent struts was measured. Coverage in the different groups was 74.3 ± 7.5% in bare-metal stents, 75.8 ± 6.1% in aCD34, 85.6 ± 3.7% in fibronectin/fibrinogen, 85.1 ± 4.5% infibronectin/fibrinogen/tropoelastin, 87.9 ± 4.0% in fibronectin/fibrinogen/ aCD34 and 89.1 ± 4.3% in fibronectin/fibrinogen/ tropoelastin/aCD34. No significant differences between the groups were found in endothelialization after 7 days (Figure 2B, p = 0.867 for bare-metal stents vs aCD34, p = 128 for aCD34 vs fibronectin/fibrinogen/tropoelastin/aCD34). A detailed picture of cells with endothelial morphology is shown in Figure 2C. This morphology was found in all samples.
Figure 2. Stent endothelialization after 7 days. (A) Scanning electron microscopy pictures of the stents after 7 days. Representative pictures are shown from the six different groups. Percentage coverage measured within the rabbit of which the picture is shown, is indicated in the picture. Scale bars represent 150 μm. (B) Quantification of scanning electron microscopy pictures. Eight pictures per stent were analyzed; n = 8 for BMS, FN/Fg and FN/Fg/aCD34; n = 7 for aCD34, FN/Fg/Tropo and FN/Fg/Tropo/aCD34. Error bars represent standard error of the mean. (C) Detailed picture of the typical cell type found in all rabbits. Morphologic analysis indicates an endothelial morphology. Scale bar represents 25 μm. BMS: Bare-metal stent; Fg: Fibrinogen; FN: Fibronectin; Tropo: Tropoelastin.
▪ Neointima formation in the stent
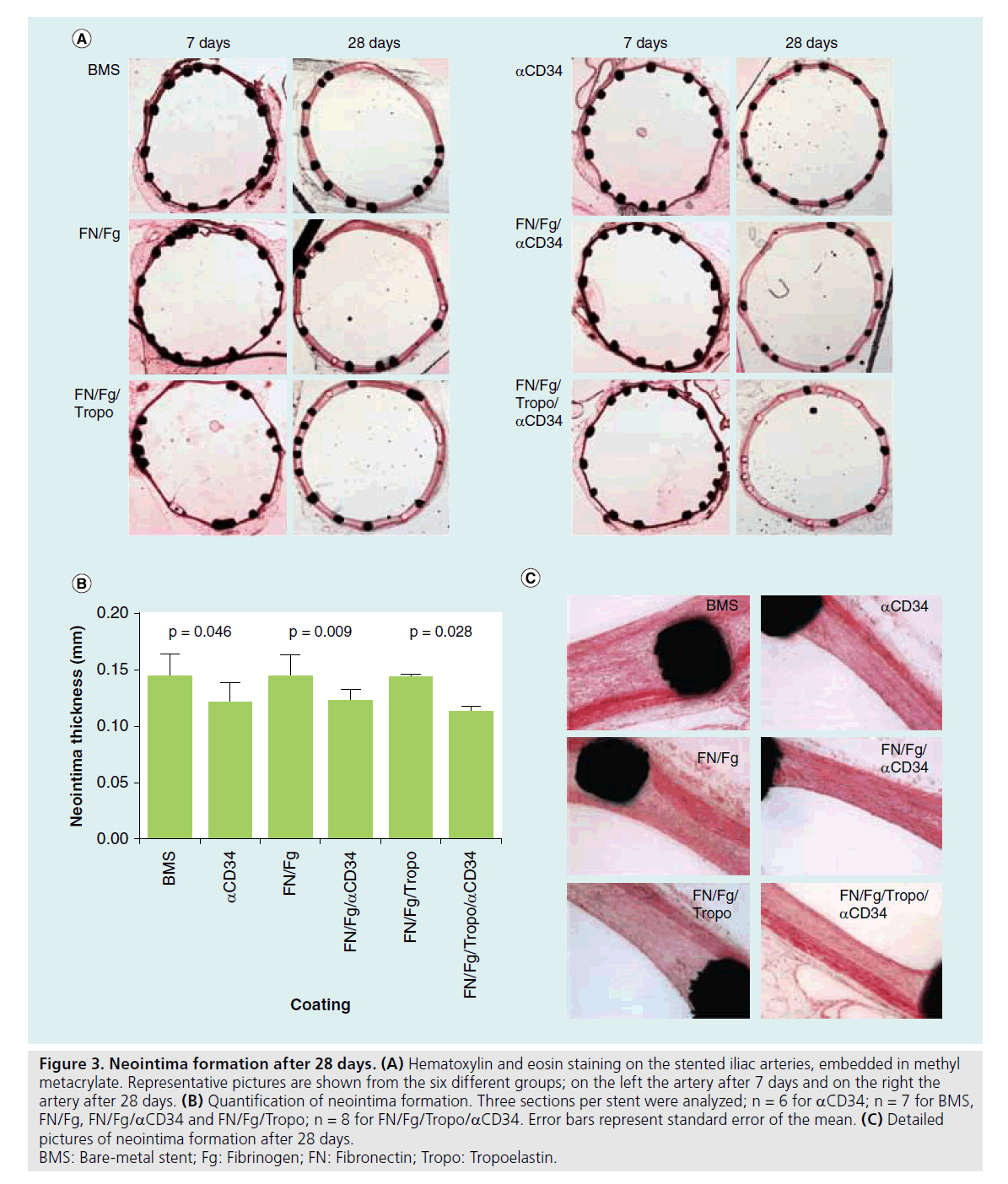
A hematoxilin eosin staining was performed on all stents and examples are shown in Figure 3A. After 7 days, no neointima formation could be observed. Stents harvested after 28 days did show intimal hyperplasia. Quantification revealed significantly less neointima in anti- CD34-coated stents compared with bare-metal stents as shown in Figure 3B (0.125 ± 0.010 mm vs 0.147 ± 0.006 mm in aCD34 and baremetal stents, respectively; p = 0.046). A coating with either fibronectin/fibrinogen or fibronectin/fibrinogen/tropoelastin results in a neointima comparable with a bare-metal stent (0.146 ± 0.006 mm [p = 0.902] and 0.139 ± 0.007 mm [p = 0.456]). When the CD34 antibody was present in these coatings, the amount of neointima was significantly decreased (p = 0.009 for fibronectin/fibrinogen with and without aCD34, p = 0.028 for fibronectin/fibrinogen/ tropoelastin with and without aCD34). The amount of neointima in these stents was equal compared with the stents coated with only aCD34 (0.122 ± 0.005 mm [p = 0.836] and 0.113 ± 0.006 mm [p = 0.282]). In Figure 3C, detailed representative examples are shown from fibronectin/fibrinogen/tropoelastin/aCD34 and bare-metal stents.
Figure 3. Neointima formation after 28 days. (A) Hematoxylin and eosin staining on the stented iliac arteries, embedded in methyl metacrylate. Representative pictures are shown from the six different groups; on the left the artery after 7 days and on the right the artery after 28 days. (B) Quantification of neointima formation. Three sections per stent were analyzed; n = 6 for aCD34; n = 7 for BMS, FN/Fg, FN/Fg/aCD34 and FN/Fg/Tropo; n = 8 for FN/Fg/Tropo/aCD34. Error bars represent standard error of the mean. (C) Detailed pictures of neointima formation after 28 days. BMS: Bare-metal stent; Fg: Fibrinogen; FN: Fibronectin; Tropo: Tropoelastin.
Discussion
Here, we investigated whether additional protein coating of anti-CD34 Genous stents could improve patency in an in vivo rabbit model. The goal of this study was to identify coating conditions that stimulate the outgrowth of captured EPCs without subsequent increase in neointima formation. The protein combinations that were applied have previously been shown to facilitate optimal EC growth in vitro, while SMC outgrowth and endothelial inf lammatory and procoagulant responses were kept minimal [15]. In this in vivo study, the stent coatings fibronectin/fibrinogen and fibronectin/fibrinogen/tropoelastin did not show a significantly improved stent endothelialization 7 days after stent placement compared with a bare-metal stent. Surprisingly, the presence of the anti-CD34 antibody did not show a significant effect on endothelialization as visualized by sEM. A decreased neointima formation was observed after 28 days when the anti- CD34 antibody was present on the stent, while no additional effect of the coated proteins was observed. When the proteins were coated on the stent without the antibody, the neointima formation was comparable with the bare-metal stent. These results indicate that the coating of fibronectin/ fibrinogen and/or tropoelastinon on the stent did not improve stent endothelialization or intimal hyperplasia in vivo compared with the anti-CD34-coated Genous control stent.
The Genous stent is designed to capture CD34+ cells from the circulation to improve endothelialization of the stent struts. Capturing circulating EPCs on a stent surface was shown to increase endothelialization and is, therefore, a promising strategy to reduce in-stent thrombosis [9–12]. Improved endothelialization has been implied to result in decreased restenosis; however, this was shown not to be necessarily true [16]. Clinical studies using the Genous showed no improvement in outcome and late loss was similar compared with bare-metal stents [10,13,14]. CD34 is used as a marker for EPCs. Despite an enormous quantity of studies on EPCs, the exact definition of EPCs remains unclear and not consistent. Most commonly, EPCs are characterized by the marker combinations CD133+CD34+VEGFR2+ and CD34+VEGFR2+ on the cell surface [17,18]. Capturing EPCs with only an antibody against CD34 may, therefore, not elicit a capturing of specifically EPCs. de Boer et al. suggested that captured CD34+ cells exposed to shear and activated platelets result in differentiation towards mature endothelium [19,20]. Progenitor cells may be highly plastic in their ultimate phenotypic preference, and environmental and local cytokine factors on circulating cells may be more important than a specific EPC marker in determining phenotype differentiation.
To overcome this problem, we coated different proteins on the stent surface together with the anti-CD34 antibody, namely fibronectin, fibrinogen and tropoelastin. Fibronectin and fibrinogen were chosen for their capacity to improve EC adhesion, migration and differentiation [15,21–24]. Since these proteins were also able to promote SMC growth, tropoelastin was added to the mixture to inhibit SMC proliferation and migration [25–27]. The effect of the coated proteins did not show any additional effect on intima hyperplasia. This was unexpected since we previously obtained in vitro results showing the beneficial effects of our stent coatings on EC growth and SMC inhibition [15]. These unexpected findings may possibly be due to the ‘Vroman effect’, which exhibits the covering of foreign surfaces placed in the bloodstream by plasma proteins. Fibrinogen is one of the first proteins that will adhere to a surface, followed by replacement with high molecular weight kininogen [28]. Furthermore, our in vitro setup was performed in an isolated setting, using a single cell type in each experiment and without taking the effect of blood cells and proteins into account. In the in vivo model, stents were placed after denudation where they were exposed to an artery without an endothelial layer, and with a necrotic medial layer due to the Fogarty denudation. This leads to the adhesion of platelets and inflammatory cells, which can induce local conditions that are very difficult to simulate in a cell-culture model; however, that can activate the surrounding cells in such a way that the proteins coated on the stent cannot overcome this problem.
In our results, no difference was observed in stent coverage between the bare-metal and anti-CD34 coated stents after 7 days. Our study could not confirm the earlier reported accelerated endothelialization that was observed with the anti-CD34-coated stents in animal experiments [16]. Iliac arteries were pressure-fixed under flow and preparation was performed using standardized protocols; we do not expect that the obtained results are explained by wrong tissue preparation. The time points for endothelialization analysis in this study was 7 days. In a recent study by van Beusekom et al., the authors observed improved re-endothelialization using an anti-CD34 coated stent after 2 and 5 days [16]. After 7 days, all of our stents were almost completely covered, indicating that 7 days might have been too late to observe differences in stent coverage. Furthermore, the 28 days time point shows minor differences of which the clinical relevance is questionable. It might be possible that larger differences would have been found if the stents were removed at a later time point. Even though 28 days is commonly used in literature to study neointima formation in rabbits, investigating the results at a later time point might show interesting differences [29,30]. Another study limitation is the lack of stainings for endothelial cell markers to test the viability of the endothe-lialized surface [16], which can give more details regarding the capacity of the proteins to improve endothelialization. Owing to the embedding of our stents in methyl metacrylate, our antibodies were not able to recognize the correct epitopes, and we, therefore, cannot rely on the quality of our stainings.
Dual-stent coatings that are coated with anti-proliferative drugs on the intimal side, and the anti-CD34 antibody on the luminal side of the artery, have shown promising results [31]. The anti-CD34-coated stents were able to capture EPCs, as well as inhibit SMCs. Next to this new-generation stent coating, stents coated with more specific markers for EPCs would be a more suitable clinical application. Stents coated with antibodies against vascular endothelial cadherin show better endothelialization and less neointima formation compared with stents coated with anti-bodies against CD34 [11,12]. Similar improvements were obtained using stents coating with cyclic RGD peptides (against RGD sequence Arg-Gly-Asp) and GPVI/CD133 [32,33].
Conclusion
In conclusion, a stent coated with fibronectin, fibrinogen and/or tropoelastin does not improve stent endothelialization or decrease intima hyperplasia. The anti-CD34-covered stent slightly reduced intimal hyperplasia. Alternative coatings with fibronectin, fibrinogen and/or tropoelastin do not further accelerate endothelialization or inhibit neointima formation.
Future perspective
With the use of the new-generation DES, the risk for in-stent thrombosis and neointima formation has become lower compared with bare-metal stents [5,6]. Much improvement has been made with these stent types, and it will be difficult to improve these outcomes with EC capturing stents. New stent coatings for future clinical use do not comprise coatings with fibronectin/fibrinogen/ tropoelastin, combined with the CD34+ cell-capturing antibodies. Promising results are obtained with dual-stent coatings with antipro-liferative drugs on the intimal side and the anti- CD34 antibody on the luminal side. These stents are able to capture EPCs from the circulation, as well as inhibit SMCs from the vascular wall. It remains to be proven whether these stents are superior to the new-generation DES, and have good potential for future clinical use.
Acknowledgements
The authors thank J Visser, M Schurink and C Verlaan for technical assistance concerning the animal experiments and C Sneijdenberg for assistance with scanning electron microscopy.
Financial & competing interests disclosure
This research was performed within the framework of the Center for Translational Molecular Medicine, project CIRCULATING CELLS (grant 01C-102), and supported by the Dutch Heart Foundation. E Ligtenberg and S Rowland are employees of OrbusNeich Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Executive summary
Background
▪ Endothelial progenitor cell (EPC) capturing is an attractive method to improve re-endothelialization on a stent.
▪ Combining the capturing of EPCs with the inhibition of smooth muscle cells proliferation using different protein coatings might result in decreased restenosis.
Stent endothelialization after 7 days of stent placement
▪ Optimizing the outgrowth of EPCs with a coating of fibronectin/fibrinogen/tropoelastin does not improve endothelialization in a rabbit model after 7 days.
Neointima formation in the stent
▪ A coating with anti-CD34 to capture EPCs results in a modest decrease of intima hyperplasia; however, coating additional fibronectin/fibrinogen/tropoelastin on the surface did not show any effect in a rabbit model after 28 days.
Conclusion
▪ Coating additional proteins on a stent surface to optimize captured-cell outgrowth shows no promising results for future clinical use.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Caixeta A, Leon MB, Lansky AJ et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J. Am. Coll. Cardiol. 54(10), 894–902 (2009).
- Kastrati A, Mehilli J, Pache J et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 1030–1039 (2007).
- Kastrati A, Dibra A, Spaulding C et al. Meta-analysis of randomized trials on drugeluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur. Heart J. 28(22), 2706–2713 (2007).
- Bangalore S, Kumar S, Fusaro M et al. Shortand long-term outcomes with drug-eluting and bare-metal coronary stents: a mixedtreatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 125(23), 2873–2891 (2012).
- Palmerini T, Biondi-Zoccai G, Riva Della D et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 379(9824), 1393–1402 (2012).
- Werner N, Junk S, Laufs U et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ. Res. 93(2), e17–e24 (2003).
- Jiang S, Walker L, Afentoulis M et al. Transplanted human bone marrow contributes to vascular endothelium. Proc. Natl Acad. Sci. USA 101(48), 16891–16896 (2004).
- Rotmans JI, Heyligers JM, Verhagen HJ et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation 112(1), 12–18 (2005).
- Aoki J, Serruys PW, van Beusekom H et al. Endothelial progenitor cell capture by stents coated with antibody against CD34. J. Am. Coll. Cardiol. 45(10), 1574–1579 (2005).
- Lee JM, Choe W, Kim BK et al. Comparison of endothelialization and neointimal formation with stents coated with antibodies against CD34 and vascular endothelial–cadherin. Biomaterials 33(35), 8917–8927 (2012).
- Lim WH, Seo WW, Choe W et al. Stent coated with antibody against vascular endothelial– cadherin captures endothelial progenitor cells, accelerates re-endothelialization, and reduces neointimal formation. Arterioscler. Thromb. Vasc. Biol. 31(12), 2798–2805 (2011).
- Duckers HJ, Silber S, de Winter R et al. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the HEALING-II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention 3(1), 67–75 (2007).
- Beijk MA, Damman P, Klomp M et al. Twelve-month clinical outcomes after coronary stenting with the Genous Bioengineered R Stent in patients with a bifurcation lesion: from the e-HEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) registry. Coron. Artery Dis. 23(3), 201–207 (2012).
- Tersteeg C, Roest M, Mak-Nienhuis EM et al. A fibronectin–fibrinogen–tropoelastin coating reduces smooth muscle cell growth but improves endothelial cell function. J. Cell. Mol. Med. 16(9), 2117–2126 (2012).
- van Beusekom HM, Ertaş G, Sorop O, Serruys PW, van der Giessen WJ. The Genous™ endothelial progenitor cell capture stent accelerates stent re-endothelialization but does not affect intimal hyperplasia in porcine coronary arteries. Catheter Cardiovasc. Interv. 79(2), 231–242 (2012).
- Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302), 964–967 (1997).
- Peichev M, Naiyer AJ, Pereira D et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95(3), 952–958 (2000).
- de Boer HC, Verseyden C, Ulfman LH et al. Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler. Thromb. Vasc. Biol. 26(7), 1653–1659 (2006).
- de Boer H, Hovens M, van Oeveren A et al. Human CD34+/KDR+ cells are generated from circulating CD34+ Cells after immobilization on activated platelets. Arterioscler. Thromb. Vasc. Biol. 31(2), 408–415 (2010).
- Dejana E, Lampugnani MG, Giorgi M, Gaboli M, Marchisio PC. Fibrinogen induces endothelial cell adhesion and spreading via the release of endogenous matrix proteins and the recruitment of more than one integrin receptor. Blood 75(7), 1509–1517 (1990).
- Naito M, Hayashi T, Kuzuya M et al. Effects of fibrinogen and fibrin on the migration of vascular smooth muscle cells in vitro. Atherosclerosis 83(1), 9–14 (1990).
- Bramfeldt H, Vermette P. Enhanced smooth muscle cell adhesion and proliferation on protein-modified polycaprolactone-based copolymers. J. Biomed. Mater. Res. A 88(2), 520–530 (2009).
- Yamamoto M, Yamamoto K, Noumura T. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp. Cell Res. 204(1), 121 (1993).
- Yamamoto K, Aoyagi M, Yamamoto M. Changes in elastin-binding proteins during the phenotypic transition of rabbit arterial smooth muscle cells in primary culture. Exp. Cell Res. 218(1), 339–345 (1995).
- Ito S, Ishimaru S, Wilson SE. Inhibitory effect of type 1 collagen gel containing [alpha]-elastin on proliferation and migration of vascular smooth muscle and endothelial cells. Cardiovasc. Surg. 5(2), 176 (1997).
- Karnik SK, Brooke BS, Bayes-Genis A et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130(2), 411–423 (2003).
- Vroman L, Adams AL, Fischer GC, Munoz PC. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 55(1), 156–159 (1980).
- Yang W, Ge J, Liu H et al. Arsenic trioxide eluting stent reduces neointima formation in a rabbit iliac artery injury model. Cardiovasc. Res. 72(3), 483–493 (2006).
- Farb A, John M, Acampado E et al. Oral everolimus inhibits in-stent neointimal growth. Circulation 106(18), 2379–2384 (2002).
- Nakazawa G, Granada JF, Alviar CL et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc. Interv. 3(1), 68–75 (2010).
- Kammerer PW, Heller M, Brieger J et al. Immobilisation of linear and cyclic RGD-peptides on titanium surfaces and their impact on endothelial cell adhesion and proliferation. Eur. Cell Mater. 21, 364–372 (2011).
- Langer HF, Ruhr JW, Daub K et al. Capture of endothelial progenitor cells by a bispecific protein/monoclonal antibody molecule induces reendothelialization of vascular lesions. J. Mol. Med. 88(7), 687–699 (2010).
•• Demonstrates the idea behind the anti-CD34- coated stent: using an antibody against CD34 for the capturing of endothelial progenitor cells from the circulation.
• Clinical outcomes with Genous™ (OrbusNeich Medical, FL, USA) stent: patients suffering from restenosis after Genous stent implantation were still observed.
•• Demonstrates a decreased growth of smooth muscle cells and improved endothelial cell proliferation, while inflammatory responses are kept minimal using fibronectin/fibrinogen and tropoelastin.
• Genous stents used on animal model did not show improved neointima formation, while re-endothelialization was accelerated.
• Characterization of CD34+ endothelial progenitor cells.
• Combining the anti-CD34-coated stent with sirolimus-coated stents improves endothelialization.