Research Article - Neuropsychiatry (2020) Volume 10, Issue 4
Findings from a Neuroimaging & Early Diagnosis service for Dementia in East Kent
- Corresponding Author:
- Dalvi M
Honorary Senior Lecturer
Kings College London and Consultant
Old Age Psychiatrist Kent & Medway NHS and Social Care Partnership Trust
Email: madhusudan.dalvi@kcl.ac.uk
Abstract
ABSTRACT
- Introduction: A multidisciplinary neuroimaging service was developed in 2012 jointly by Old age Psychiatry consultants from Kent and Medway NHS & Social Care Partnership trust along with Radiologists from the Department of Radiology from East Kent University NHS Foundation Trust.We amimed to evaluate the following:
1)Whether the incorporation of the neuroimaging service helped in early diagnosis.
2) Evaluation of service in establishing diagnostic certainty / specificity .
3)Early initiation of cognitive enhancers
4)To evaluate if this service generated referrals to other specialities thereby improving patient care and experience
- Methods and Design: Data regaring the cases discussed in the Neuroimaging Service was collected from Sept 2012 till July 2013 from the radiology log book and Picture Archiving system( PACS).
- Results: The initial 100 cases were reviewed and evaluated showing improved diagnostic accuracy, decreased rates of unspecified cognitive conditions, increased specialist advice to GP’s and created new referrals to other teams such as stroke medicine improving patient care. Diagnosis for unspecified dementia before the Neuroimaging Early diagnosis Service was 71% and this dropped to 6% post neuroimaging MDT service.The diagnosis of Mild cognitive impairment increased from 8% to 16% and 12% to 24% for Alzheimer’s Disease and from 3% to 32% in Mixed dementias. The analysis of initial results demonstrated clinical efficiency of the service.
- Conclusion -It was found that the neuroimaging service has proved to be useful in improving early diagnosis and increased diagnostic certainty . From the data it clear that there were a number of clinical advantages after the scans were discussed in multidisciplinary service.
Keywords
Dementia, Neuroimaging
Introduction
Dementia is a progressive neurodegenerative illness and the prevalence of Alzheimer’s Disease (AD) is expected to increase to over 1 million by 2025 and over 2 million by 2051. Services need to be designed in such a way that dementia can be diagnosed early and accurately and the patients with dementia and their carers receive adequate care and support [1]. Currently there are many cases where diagnosis and contact often occurs late in illness or in crises [1]. There is a great value of making a timely and accurate diagnosis of dementia as it leads to early treatment initiation and support for the patients [1]. This will enable preparation of appropriate facilities and resources to manage the disease when dementia progresses and reduce unnecessary or inappropriate drug treatment e.g. neuroleptics in Lewy Body dementia (DLB). With timely and accurate diagnosis carers will also receive education and support which will give them a better understanding of challenging behaviours that may occur in Dementia. Furthermore, timely and accurate diagnosis may permit earlier access to care, potentially delaying nursing home placement [1].
Primary health care staff should consider referring people who show signs of mild cognitive impairment (MCI) for assessment to memory assessment services which will help aid early identification. It is possible to diagnose Alzheimer’s Disease at the Mild Cognitive Impairment stage. Positive predictive value of converting to Alzheimer’s Disease from Mild Cognitive Impairment with medial temporal atrophy (MTA) is 60% [2]. Those undertaking over 65 health checks in primary care or for special groups e.g. people with Down’s syndrome, stroke and those with Parkinson’s disease , Multiple Sclerosis should also be aware of the possibility of undiagnosed cognitive impairment.
The National Dementia Strategy 2009 speaks about Early Intervention teams but does not define them. Our neuroimaging service can be considered as an Early Intervention Service.
Magnetic resonance imaging (MRI) is the preferred modality to assist with early diagnosis and detect vascular changes. The FLAIR sequence is the most sensitive MRI sequence [3] for detection of white matter hyperintensities as a marker of small vessel disease. The Fazekas rating scale [4] is now used in clinical practice, and corresponds well with more detailed rating scales and histopathology. Imaging may not always be needed in those presenting with moderate and severe dementia if the diagnosis is already clear. FDG-PET and hexamethyl propylene amine oxime (HMPAO) SPECT should be used to help differentiate AD and frontotemporal dementia(FTD) if the diagnosis is in doubt [5-7]. Cerebrovascular changes on imaging are necessary for the application of standard diagnostic criteria for Vascular Dementia(VaD) [8]. A blinded study comparing FDG-PET with HMPAO SPECT in the diagnosis of degenerative conditions (Alzheimer’s Disease or Lewy Body dementia) from controls found a clear (20%) superiority for FDG-PET over HMPAO SPECT in diagnostic accuracy [9].
• Changes in terminology and definition
The diagnostic criteria and clinical definition of very early Alzheimer’s Disease has been proposed by the International Working Group Dubois et al in 2007 [10]. These will further improve early diagnosis. These criteria were later revised to include prodromal Alzheimer’s disease [11] and then amyloid imaging [12]. Whilst suggesting a new definition and new criteria for the diagnosis of Alzheimer’s disease,Dubois et al have incorporated various biomarkers to detect Alzheimer’sdisease in its early stages.
Alzheimer’s Disease has been defined as a clinical disease with different stages indicating a staged approach
• Preclinical Alzheimer’s Disease is the first stage when patient has no symptoms but there is a biomarker evidence of Alzheimer’s pathology.
• Prodromal Alzheimer’s disease is the second stage when patients present with early memory disturbances but other cognitive symptoms are not severe enough to cause dementia. Patients with prodromal Alzheimer’s disease also have biomarker evidence of disease pathology.
• Third stage is Alzheimer’s disease with dementia.
These diagnostic criteria and clinical definition of AD are likely to have huge impact on patient care. Using the current ICD10 criteria a patient has to present with clinical signs of dementia before being clinically diagnosed with it. This prerequisite has hindered patients with early Alzheimer’s Disease from receiving treatment with the currently available cholineesterase inhibitors or Memantine. This is an important issue as for treatment to be effective medications need to be initiated early in the disease process [13].
Various studies have highlighted the role of structural and functional imaging in early diagnosis of dementia. The study by Duara and colleagues highlighted the role of structural MRI in the early diagnosis by using visual rating scale to assess atrophy of medial temporal lobe structures which are involved in Alzheimer’s Disease. [14]. It concluded that medial temporal atrophy (MTA) scores provided significant additive predictive power to clinical assessment. This approach raised the diagnostic accuracy as high as 89% for distinguishing no cognitive impairment from Mild Cognitive Impairment and 99% for distinguishing no cognitive impairment from probable Alzheimer’s Disease. Early diagnosis will be even more important when diseasemodifying medications become available. As a result, it has been proposed that several biomarkers should be incorporated into the diagnostic criteria. The MRI biomarkers for Alzheimer’s Diseaseinclude presence of medial temporal atrophy on structural MRI [14]. Lewy Body Dementia is associated with hypoperfusion and hypometabolism of posterior parietal and occipitalareas on SPECT and FDG PET respectively [15]. Alzheimer’s Disease is associated with temporoparietal hypoperfusion on SPECT and hypometabolism on FDG-PET. Early onset AD is also associated with parietal and precuneus atrophy [15-20]. Alzheimer’s Disease is associated with raised total tau and decrease in amyloid-beta protein levels in CSF [21,22].
• Problems with current practice
1) Many patients suspected of cognitive impairment whatever the cause and type are requested a CT brain scan by the GP. This may not always be the right neuroimaging investigation. CT brain scan is more useful to exclude any pathologies like hydrocephalus, subdural collections but vascular changes are better seen on an MRI [23].
2) Medial Temporal Atrophy has consistently been shown to represent an early imaging feature of Alzheimer’s Disease [24,25].,Younger Patients with Rapidly progressive dementias will benefit from the gradient echo sequence to detect amyloid angiopathy. Patients with MCI may benefit from MRI sequences forearly diagnosis and follow up.
3) DAT scans should be used for patients with suspected Lewy body dementia (DLB) and Parkinson’s Disease Dementia (PDD) [26,27].
4) Disease specific Neuroimaging protocols need to be developed to improve accuracy of diagnosis.
5) SPECT-HMPAO scan can be useful in differentiating Alzheimer’s disease from Fronto-temporal dementia where the diagnosis is in doubt but this may not be available in all centres [5-7]
6) There are no multidisciplinary team meetings with neuroradiology colleagues like in Stroke medicine or oncology which prevent a meaningful discussion for complex patients.
Materials and Methods
• Development of a new multidisciplinary service
Since there was no Early Diagnosis service in Kent, we developed a weekly neuroimaging service for early diagnosis of patients with dementia since 2012 where patients with Mild Cognitive Impairment, complex and atypical presentations are discussed and where neuroradiology and nuclear medicine advice is sought.
A multidisciplinary team (MDT) was developed with Old Age psychiatrists, radiologists, nuclear medicine physicians from Kent&Medway NHS and Social Care Partnership Trust and East Kent Hospitals University Foundation Trust. The team discussed, devised and adopted a common strategy which included clinical and imaging assessments and discussion of patients with cognitive disorders followed by further discussions at the weekly neuroimaging multidisciplinary Early diagnosis Service to reach a consensus regarding diagnosis,to consider treatment options including clinicaladvice for GP’s whenever needed regarding follow up of patients. This was achieved with no additional financial resources but maximised utilization of already existing services and facilities.
The Service included weekly discussion of diagnostically difficult, atypical, complex cases with their clinical assessment, cognitive tests(MMSE, ACE III), neuroimaging, preferably MRI or CT if former could not be done, further assessment with nuclear medicine scans if diagnosis was unclear, discussion of all relevant aspects at a devoted multidisciplinary team meeting and radiological advice for each patient. Multidisciplinary team discussion records from the meetings were uploaded on to the PACS system in each patient’s folder improving safety. GP’s were informed about clinical discussions by Psychiatry along with providing advice regarding adequate follow up. Neuroimaging care pathways were created and a CT/MRI, SPECT dementia protocol was developed.
The proposed basic protocol that was developed is stated below:
Axial T2 SE
Axial FLAIR
SPGR volume T1; isotropic 1mm voxels (1mm coronal reformats) - probably an ADNI protocol. DWI (b=0, 1000).
SWI or GE T2* - depending on timing and which works best.
(Short TE MRS optional).
A rapid protocol for those unable to tolerate it:
DWI, Axial FLAIR +/- T2-W SE (Blade), Coronal T1 (Blade)
This service involved integration across three secondary care services which is Old Age Psychiatry, Neuroradiology and Nuclear Medicine Services in the form of weekly multidisciplinary meeting where difficult cases with diagnostic conundrum, mild cognitive impairment, atypical cases referred to the memory clinics are discussed.
Composition of the service includes a Consultant Old Age Psychiatrist who chairs the meeting, Consultant Neuroradiologist, Clinical Lead for Nuclear Medicine, being attended by other psychiatrists and Neuropsychologist. The MDT meeting lasted for 1.5 hours.
Criteria for referral: The criteria for discussion includes all Young patients, atypical cases, patients presenting with rapid cognitive decline. Patients with normal scans, ACE-111 scores above the cut off range for the diagnosis of dementia, Patients with mild cognitive impairment but with clinical suspicion of dementia.
Each case is presented by the clinician. The neuroimaging studies are displayed on the screen using PACS and all the information discussed. Agreement is reached to quantitatively score degrees and patterns of atrophy, as well as postacute or chronic vascular lesions. Visual rating scales used at reporting and discussing studies were Global Cortical Atrophy, Schelten’s Medial temporal atrophy and Fazekas scales.
The neuroradiologist and the nuclear medicine specialist provide an advisory role and there is clinical reflection to consider diagnosis or change in the diagnosis. Further tests like MRI, DAT scan, SPECT-HMPAO are requested if needed. SPECT scans are reported by a dedicated Nuclear Medicine Consultant creating uniformity and the Chang’s attenuation correction of the images is done for depth artefacts before reporting to decrease false positive results.
• Aim of the project
A project was carried out to evaluatethe following:
1) Whether the incorporation of neuroimaging service helped in early diagnosis.
2) Evaluation of service in establishing diagnostic certainty/specificity.
3) Early initiation of cognitive enhancers.
4) To evaluate if this service generated referrals to other specialities thereby improving patient care and experience.
• Data collection
Data was collected from Sept 2012 till July 2013. A log book was kept by the Neuroradiologist regarding the cases discussed in the weekly MDT. We collected data from this log book and also from the PACS (Picture archiving system where a summary of the discussion including advice from radiology and Nuclear medicine. There is no comparable data prior to this. On an average roughly 3-5 patients were discussed during each meeting.
Results
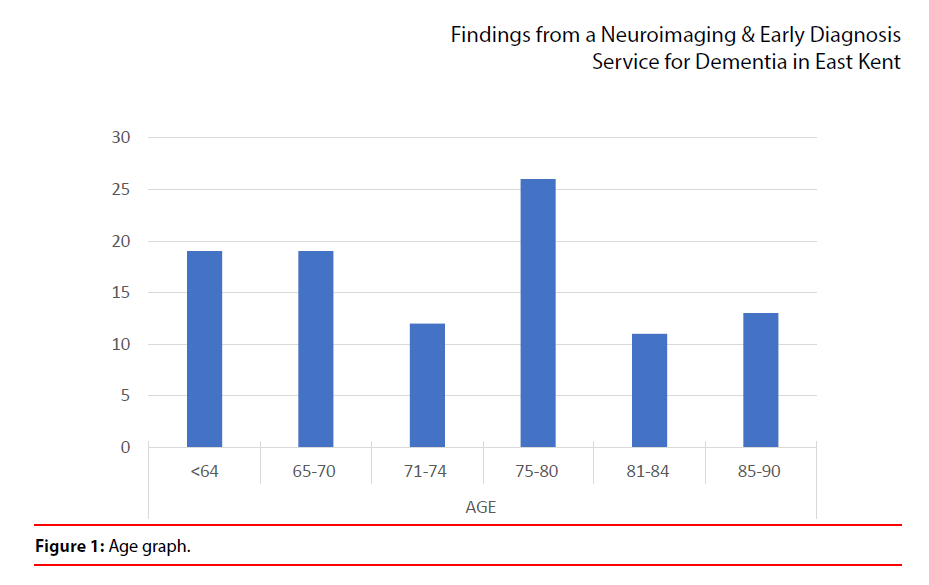
1. The sample contained 47% females and 53% males. It was found that the neuroimaging service has proved to be useful in improving early diagnosis and increased diagnostic certainty (Figure 1).
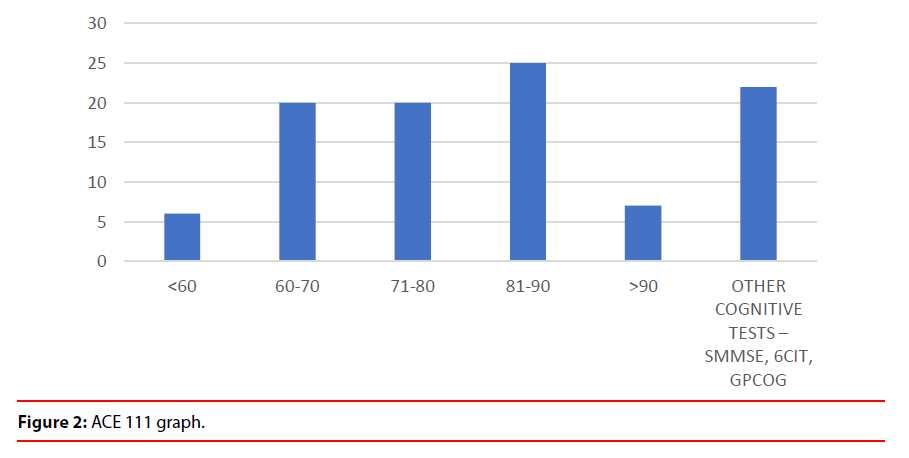
2. From the data it clear that there were a number of clinical advantages after the scans were discussed in multidisciplinary service (Figure 2).
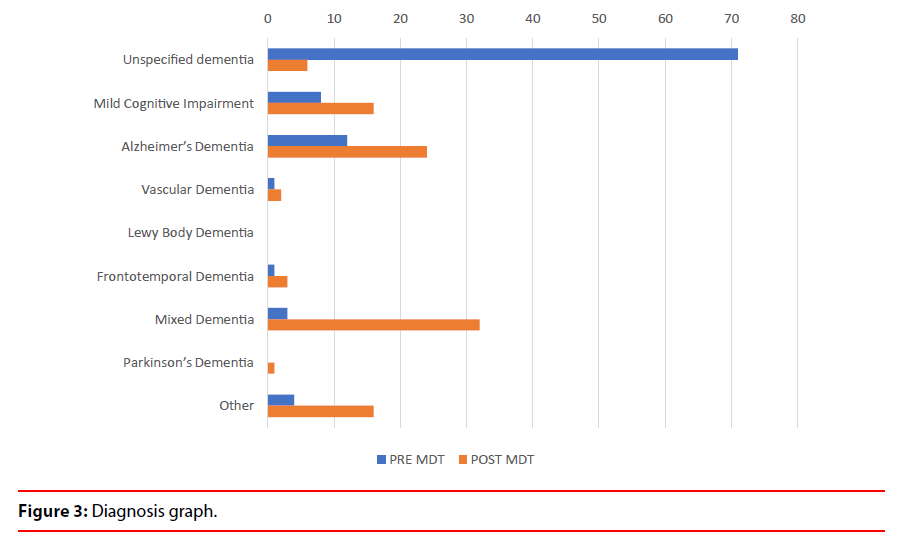
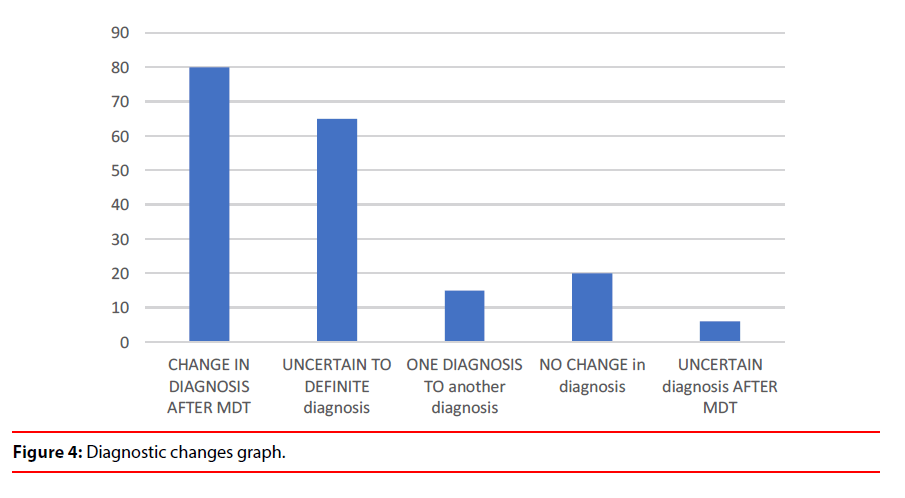
a) Uncertain diagnosis changed to more certain diagnosis in 65% cases (Table 1 and Figure 3). The diagnosis changed from one subtype to another subtype in 15% cases which depicts the value of service in establishing diagnostic accuracy (Figure 4).
Table 1: Change in diagnosis after MDT discussion.
| Diagnosis | Pre MDT | Post MDT | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Unspecified dementia | 71 | 71 | 6 | 6 |
| Mild Cognitive Impairment | 8 | 8 | 16 | 16 |
| Alzheimer’s Dementia | 12 | 12 | 24 | 24 |
| Vascular Dementia | 1 | 1 | 2 | 2 |
| Lewy Body Dementia | 0 | 0 | 0 | 0 |
| Frontotemporal Dementia | 1 | 1 | 3 | 3 |
| Mixed Dementia | 3 | 3 | 32 | 32 |
| Parkinson’s Dementia | 0 | 0 | 1 | 1 |
| Other | 4 | 4 | 16 | 16 |
Other diagnosis included: cognitive impairment in depression and schizophrenia, alcohol related dementia and post acute vascular disease.
Diagnosis pre and post neuroimaging and multidisciplinary meetings dropped from 71% to 6% for uncertain diagnosis and increased from 8% to 16% for MCI, 12% to 24% in AD and 3% to 32% in Mixed Dementia.
b) The diagnosis of Mild Cognitive Impairment increased from 8% to 16%, Alzheimer’s Disease increased from 12% to 24%, Vascular Dementia increased from 1% to 2%, FrontoTemporal Dementia from 1% to 3%, Mixed Dementia from 3% to 32% and cognitive impairment due to other causes from 4% to 16%. (Table 1 and Figure 3).
c) Structural imaging in patients with suspected dementia helped to establish dementia subtype.
d) New neuroimaging pathways have been developed whereby the scans are carried out with dementia protocol using appropriate MRI and CT sequences to improve diagnostic accuracy.
e) Referrals to Nuclear medicine Service for (99mTcHMPAO SPECT) scans comprised a total of 24% of discussed patients, 7% were referred prior to MDT discussion and 17% after discussion. 6% of the patients were referred for DAT imaging, with 3% of these referred before MDT and further 3% after MDT discussion. 22% patients underwent HMPAO SPECT imaging of which 19(86%) had change in diagnosis or increased diagnostic certainty. 5 patients had a DAT scan, 3 of which had a change in diagnosis and in others it increased diagnostic certainty. Thus, a significant clinical impact is seen especially when the clinical assessment and initial MRI or CT imaging was already performed in these patients.
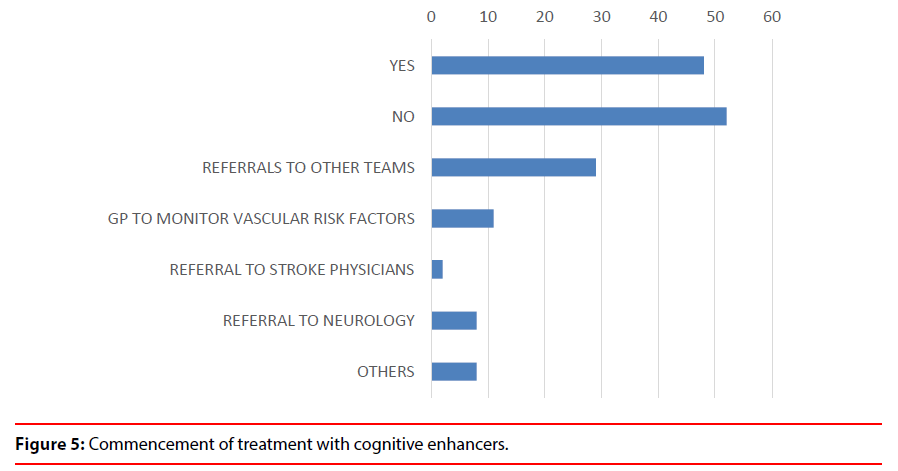
3. The Neuroimaging MDT service helped the Old Age Psychiatrists to take an early decision to commence patients on cognitive enhancers in 48% cases (Figure 5).
4. The MDT meetings have generated referrals to other teams e.g. Neurology,Neuropsychiatry, Neuropsychology, Psychology, Bereavement Counselling (8%), Stroke team (2%), advice given to GP’s (1%)mainly about vascular risk factors, thus improving patients’ safety (Figure 5).
Discussion
1) The incorporation of this neuroimaging Service has helped the clinicians arrive at earlier and clear diagnosis in patients suffering from cognitive conditions including dementia and particularly those with complex presentations.
2) It has helped and guided the decision process regarding the type of scan to request.
3) Functional neuroimaging has been helpful in differentiating Alzheimer’s Disease from Fronto-Temporal Dementia andLewy Body Dementia
4) The MDT meetings have generated referrals to other teams e.g. Neurology (8%), Stroke team (2%), advice given to GP’s (1%) mainly about vascular risk factors, thus improving patients’ safety.
5) These meetings have added educational value whereby on one hand the psychiatrists are gaining more knowledge about the brain scans and on the other hand neuroradiologists and nuclear physicians are able to gain more knowledge about theclinical presentation ofdementia related processes which in turn aids in interpretation of the scans. This has led to blended learning improving skills in both disciplines.
6) The advisory role of neuroradiologist was very helpful as rating the vascular lesions gave us a good feel of vascular burden which helped us both to inform the GP for control of vascular risk factors and in some cases direct referral to the stroke specialist.
7) The neuroimaging is done using a dementia protocol with appropriate sequences. Gradient Echo Sequence helped us to know that there was a prevalence of congophilic amyloid angiopathy and chronic hypertensive encephalopathy. This added further safety implications on those patients who were on Warfarin and hence triggered stroke referrals and helped targeting patients in whom a better control of hypertension was needed.
• Recommendations and Action plan
1) To continue with the neuroimaging service to enhance early diagnosis and diagnostic accuracy. Diagnosis rates of dementia in Kent are around 44%. One of the key objectives within the Kent and Medway strategic plan is to increase these rates to 60%.
2) Nuclear Medicine Department is in the process of making a business case for SPECTCT, PET scans.
3) To make a business case for statistical parametric mapping software which will help in interpreting the scans using normative values improving diagnostic accuracy.
4) To develop Vascular and Parkinson’s disease cognitive pathways into stroke and Parkinson’s disease clinics.
5) To try and develop a unique weighting system based on: Lab investigations, MRI, SPECT scan for diagnostic accuracy. MRI will include Visual rating scale, Global atrophy scale and Fazekas Grading.
6) To agree on following standards as a benchmark
• Recommended Standards for a Neuroimaging Service for Dementia
These are summarized in the following table and advised as a need to be reached in all patients with cognitive -related conditions as a matter of adequate clinical governance in the best interest of the subjects under our care (Table 2).
Table 2: Proposed standardsfor a Neuroimaging & Early Diagnosis Service for Dementia.
| Criteria | Required standard |
|---|---|
| All patients need to have at least an appropriate scan i.e.CT/ MRI Brain scan at baseline | 100% |
| All scans to be done with a Dementia protocol | 100% |
| Use of Visual rating scales when interpreting all the scans | 100% |
| All patients with Mild Cognitive Impairment/Atypical presentations to be discussed in the meetings | 100% |
| Essential information to provided when requesting scans: brief clinical history, cognitive scores using standardised cognitive tests, Vascular risk factors, family history of dementia, any history of Alcohol abuse and Head injury. | 100% |
| Use of SPECT scans when results of MRI are inconclusive and when diagnosis is debated between AD and FTD | 100% |
| FDG-PET scans, Use of Statistical Parametric mapping, Video-conferencing where available | To be kept flexible |
Conclusion
Health Care systems for complex multimorbid illnesses should have integrated care to improve patient care and safety as such illness often cross different specialties and disciplines. Such complex systems need to be benchmarked which may be difficult to achieve. The quality of such aservice should be defined by integration as well as safety. In East Kent we have been able to achieve this integration which has directly led to improved patient care. The incorporation of Neuroimaging service has helped to achieve both high quality care and safety by aiding the clinicians to arrive at early and clear diagnosis in patients suffering from cognitive conditions including dementia and particularly those with complex presentation. Functional neuroimaging has been helpful in differentiating Alzheimer’s Disease from Fronto-temporal Dementia and Lewy Body Dementia. The Early diagnosis Service has generated referrals to other teams like Neurology, Stroke Services improving patient safety by the process of triangulation i.e. a robust clinical discussion and analysis of scans of complex cognitive presentations between Psychiatrists, Neuroradiologists and Nuclear Physicians leading to a very powerful clinical outcome. The scans are done using a specific Dementia protocol with appropriate sequences when needed like Gradient Echo T2 Sequences, this has helped us to know if there was a presence of congophilic amyloid angiopathy, chronic hypertensive encephalopathy including microbleeds, lacunae, vascular changes of varying degree and Neuroradiologists have been rating these on Neuroradiological Visual Rating scales further improving quality and safety. After the analysis of the data we have been able to set clear Service standards and benchmarks for establishing a Early Diagnosis Neuroimaging Service which will be useful for other services in the United Kingdom and elsewhere and in our opinion is a main strength of this paper as the authors are not aware of any other available benchmarks.
Conflict of Interest
The Authors declare that there is no conflict of interest. This research was conducted in the absence of any financial, commercial, or other conflicts of interest.
Acknowledgement
To Dr Sheeba Hakeem Consultant Old Age Psychiatrist Kent and Medway NHS and Social Care Partnership Trust for helping to collect data. Data Availability statement - The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Living Well with Dementia: A National Dementia Strategy -2009.
- Visser P, Verhey FR, Hofman PA, et al. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. Neurol. Neurosurg. Psychiatry 72(4), 491-497 (2002).
- Van Straaten EC, Scheltens P, Barkhof F. MRI and CT in the diagnosis of vascular dementia. J. Neurol. Sci 226(1), 9-12 (2004).
- Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR. Am. J. Roentgenol 149(2), 351-356 (1987).
- Talbot PR, Lloyd JJ, Snowden JS, et al. A clinical role for 99m Tc-HMPAO SPECT in the investigation of dementia. J. Neurol. Neurosurg. Psychiatry 64(1), 306-313 (1998).
- Charpentier P, Lavenu I, Defebvre L, et al. Alzheimer’s disease and frontotemporal dementia are differentiated by discriminant analysis applied to 99mTc HMPAOSPECT data. J. Neurol. Neurosurg. Psychiatry 69(1), 661-663 (2000).
- Dane Rayment. Neuroimaging in dementia: an update for the general clinician. Progress. In. Neurology. And. Psychiatry 1-20 (2016).
- Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43(2), 250–260 (1993).
- O’Brien JT, Firbank MJ, Davison C, et al. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J. Nucl .Med 55(12), 1959–1965 (2014).
- Dubois B, Feldman HH, Jacova C et al. Research Criteria for the diagnosis of Alzheimer’s disease ; Revising the NINCDS-ADRDA criteria. Lancet. Neurol 6(8), 734-746 (2007).
- Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet. Neurol 9(11), 1118–1127 (2010).
- Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet. Neurol 13(6), 614–629 (2014).
- Practice Guidelines Dementia: assessment, management and support: summary of updated NICE guidance. BMJ 361(1), k2438 (2018).
- Duara R, Loewenstein DA, Potter E, et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer’s disease. Neurology 71(24), 1986-1992 (2008).
- Colloby SJ, Firbank MJ, Pakrasi S, et al. A comparison of 99mTc-exametazime and 123I-FP-CIT SPECT imaging in the differential diagnosis of Alzheimer’s disease and dementia with Lewy bodies. Int. Psychogeriatr 20(6), 1124–1140 (2008).
- Foundas AL, Leonard CM, Mahoney SM, Agee et al. Atrophy of the hippocampus, parietal cortex and insula in Alzheimer’s disease: a volumetric magnetic resonance imaging study. Neuropsychiatry Neuropsychol. Behav. Neurol 10(1), 81-89 (1997).
- Pantel J, Schonknecht P, Essig M, et al. Distribution of cerebral atrophy assessed by magnetic resonance imaging reflects patterns of neuropsychological deficits in Alzheimer’s dementia. Neurosci. Lett 361(1),17-20 (2004).
- Ahmed S, Irish M, Loane C, et al. Association between precuneus volume and autobiographical memory impairment in posterior cortical atrophy: Beyond the visual syndrome. Neuroimage. Clin; 18(1), 822-834 (2018).
- Bailly M, Destrieux C, Hommet C, et al. Precuneus and Cingulate Cortex Atrophy and Hypometabolism in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: MRI and(18)F-FDG PET Quantitative Analysis Using FreeSurfer. Biomed. Res. Int 583931 (2015).
- Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology 49(1), 967-976 (2007).
- Blennow K, Hampel H, Weiner M, et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol 6(3), 131-44 (2010).
- Mattsson N, Zetterberg H, Hansson O et al. CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment. JAMA 302(4), 385-93 (2009).
- Mijajlovic MD, Pavlovic A, Brainin M, et al. Post-stroke dementia-a comprehensive review. BMC. Med 15(1), 11 (2017).
- Frisoni GB, Fox NC, Clifford R Jack Jr et al. The clinical use of structural MRI in Alzheimer’s disease. Nature. Reviews. Neurology 6 (2), 67 (2010)
- Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease fromdementia with Lewy bodies and vascular cognitive impairment: Aprospective study with pathological verification of diagnosis. Brain 34(1), 195–203 (2009).
- Booij J, Habraken JB, Bergmans P et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson's disease. J. Nucl. Med 39(11), 1879-1884 (1998).
- Dementia: assessment, management and support for people living with dementia and their carers NICE guideline nice.org.uk/guidance/ng97.