Perspective - Interventional Cardiology (2012) Volume 4, Issue 1
Fractional flow reserve: a new paradigm for diagnosis and management of patients with coronary artery disease
- Corresponding Author:
- Massoud A Leesar
Division of Cardiovascular Diseases
University of Cincinnati, 231 Albert Sabin Way
ML 0542, Cincinnati, OH 45267, USA
E-mail:leesarma@uc.edu
Abstract
Keywords
fractional flow reserve, intravascular ultrasound
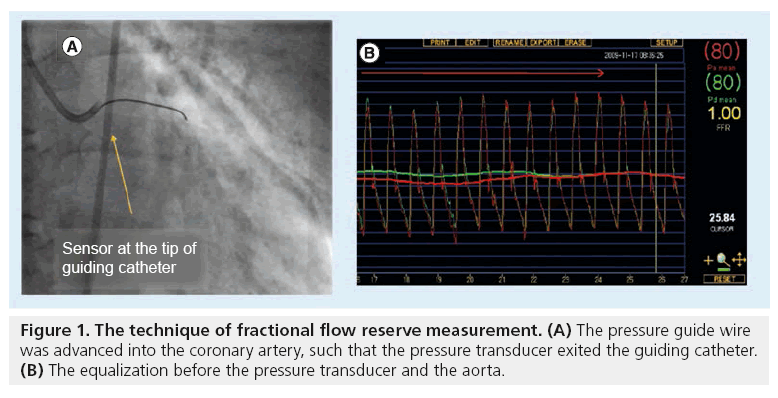
Coronary angiography is merely a ‘luminogram’ and does not provide much insight into the hemodynamic significance of a stenotic lesion and this view has been rigorously challenged for the past two decades [1–4]. This well recognized limitation has been documented repeatedly by intravascular ultrasound (IVUS) imaging and stress testing [2–4]. It is known that coronary angiography often leads to overestimation of the functional significance of the ostial sidebranch, more so than lesions in other segments of the coronary circulation [2,3]. This is in part due to difficulties in visualizing the ostial lesions in multiple orthogonal views and also due to the fact that such lesions are often very short, reducing the likelihood that they cause limitation of blood flow [2,4]. In this regard, fractional flow reserve (FFR) has emerged as a powerful catheter-based tool that provides robust information about the functional severity of the lesion. [3,5–8]. FFR, calculated from coronary pressure measurement, is a reliable, invasive index to indicate if a stenosis is ischemia-related and can be calculated in the catheterization laboratory using a pressure wire (Figures 1 & 2) with almost identical mechanical properties as a normal guide wire and that barely prolong the procedure, even when multiple vessels are interrogated [5–11].
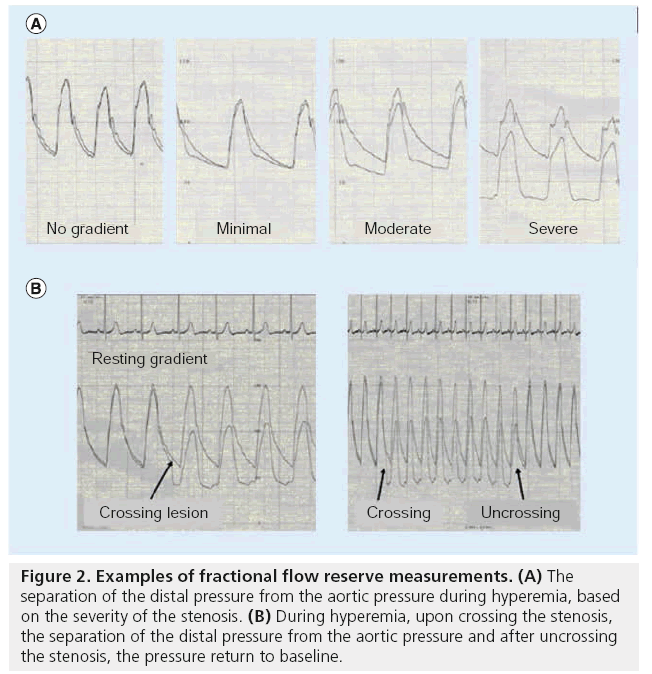
Figure 2: Examples of fractional flow reserve measurements. (A) The separation of the distal pressure from the aortic pressure during hyperemia, based on the severity of the stenosis. (B) During hyperemia, upon crossing the stenosis, the separation of the distal pressure from the aortic pressure and after uncrossing the stenosis, the pressure return to baseline.
FFR is calculated from coronary pressure measurement by taking the ratio of the coronary pressure, measured distal to the stenosis to aortic pressure (Pa), as the normal perfusion pressure (distal coronary pressure/Pa) and obtaining these measurements when the microvascular resistance was minimal and assumed to be constant (i.e., at maximal hyperemia), the percentage of normal coronary flow, or a fraction of normal flow (i.e., FFR), can be calculated [3–11]. Maximal hyperemia is usually achieved with intravenous adenosine administration at 140 μg/kg/min. Intracoronary adenosine boluses are also used, but continuous intracoronary adenosine infusion seems to be a safe and more accurate alternative [12]. FFR has a uniform normal value of 1.0 for every patient and every coronary artery; it is not dependent on changes in heart rate, blood pressure, or contractility; it accounts for collateral flow; and it has a sharp threshold value to indicate inducible ischemia: FFR <0.75 always indicates inducible ischemia; FFR >0.8 excludes ischemia in 90% of the cases [3–13]. The grey zone is very limited, which is important for clinical decision making in an individual patient. The ischemic threshold of FFR has been replicated independently with different noninvasive functional tests in numerous studies (including exercise electrocardiography, dobutamine stress echocardiography and myocardial perfusion imaging [MPI]) as well as alongside one another in the same population [3,13].
An FFR >0.75 identified coronary stenoses in patients with inducible myocardial ischemia with high sensitivity (88%), specificity (100%), positive predictive value (100%) and overall accuracy (93%). FFR has a high reproducibility and low intra-individual variability [3,5–8]. Moreover, FFR, unlike coronary flow reserve, is independent of gender and coronary artery disease (CAD) risk factors such as hypertension and diabetes [3,13].
Clinical applications
The utility of FFR has been established in multiple settings of complex lesions, including discerning the hemodynamic significance of equivocal left main (LM) coronary artery lesions, multivessel disease and previous myocardial infarction (MI) [14–17]. FFR provides reliable information on any individual stenosis and therefore, can be used for immediate decision making in the catheterization laboratory regarding whether to stent or not. FFR has also been validated to correlate strongly with clinical outcomes in the short- [14–17] and long-term up to 5 years, as established by the DEFER trial investigators [18]. There was no difference in the outcomes of Defer group (FFR mn >0.75; no percutaneous coronary intervention [PCI]) and Perform group (FFR >0.75; PCI). They found that 5-year outcome, after deferral of PCI of an intermediate coronary stenosis, based on FFR ≥0.75 is excellent. The risk of cardiac death or MI related to this stenosis is <1% per year and is not decreased by stenting. An FFR <0.75 defines a physiologically significant stenosis. Examined by year, stented patients had a 1.5% risk of death or MI, whereas patients treated conservatively, with statins and aspirin, had only a 0.6% risk. The authors concluded that the 5-year outcome after deferral of PCI of an intermediate coronary stenosis, based on FFR of 0.75, was excellent. The risk of cardiac death or MI related to this stenosis was <1% per year and was not decreased by stenting [18]. In fact FFR has been unanimously considered as the ‘gold standard’ modality for assessing the physiological significance of ambiguous lesions [3,5–8].
According to the consensus statement by the American Heart Association (AHA)/ American College of Cardiology (ACC) ‘best clinical practice suggests that the addition of coronary physiological measurements complements traditional angiographic information and is essential for accurate clinical decision-making.’ In fact, physiological assessment (including FFR) of the effects of intermediate coronary stenoses (30–70% luminal narrowing) in patients with anginal symptoms, is a Class IIa recommendation by the ACC/AHA [3].
FFR & multivessel disease
The FAME study is the largest randomized, prospective, multicenter clinical trial that compared stenting guided by FFR with stenting guided by angiography alone, in 1005 patients with two or more diseased coronary arteries [19]. The primary end point (a composite of death, MI and repeat revascularization) occurred in 91 patients (18.3%) in the angiography group and in 67 (13.2%) in the FFR group (p = 0.02). All-cause mortality at 1 year was 3% (15 deaths, ten of which had cardiac causes) in the angiography group and 1.8% (nine deaths, seven of which had cardiac causes) in the FFR group (p = 0.19). MI occurred in 43 patients (8.7%) in the angiography group and in 29 (5.7%) in the FFR group (p = 0.07). The numbers of small, periprocedural infarctions were 16 and 12 in the two groups, respectively. A total of 47 patients (9.5%) in the angiography group and 33 (6.5%) in the FFR group required repeat revascularization (p = 0.08). In addition, an FFR-guided strategy reduced the number of stents used, decreased the amount of contrast agent used and resulted in a similar functional status with no decrease in health-related quality of life. Furthermore, the procedure-related costs were significantly lower with the FFR-guided strategy. The 2-year follow-up from FAME demonstrated sustained benefit, in that the 22.2% of patients randomized to angiography-guided PCI reached a primary end point, compared with 17.7% in the FRR-guided treatment arm, an absolute reduction of 4.5%. As in earlier analyses, the reduction was driven by a reduction in the rate of MI [20].
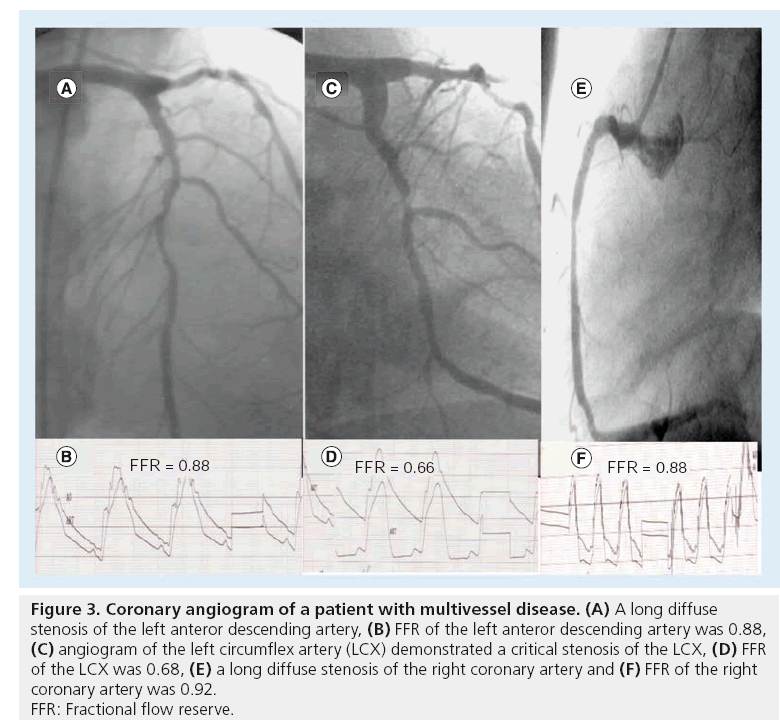
Tonino et al. examined the relationship between angiographic severity and FFR in the FFR arm of the FAME study [21]. In the FFR group, 44.1% had stenoses of 50–70% by visual estimate, 37.5% had stenoses of 71–90%, 14.3% had stenoses of 91–99% and 10.6% had stenoses that were totally occluded. In those with angiographic 3-vessel disease (approximately one-fourth of the FFR-guided group), only 14% had concordant 3-vessel functional disease (i.e., FFR <0.8 of all three vessels), 43% had functional 2-vessel disease, 34% had functional 1-vessel disease and 9% had no lesions, with an FFR < 0.8. Interestingly, in those with angiographic 2-vessel disease, the proportion of patients with functional 2-vessel disease was 43%, whereas 45% had functional 1-vessel disease and 12% had no lesions, with an FFR <0.8. Overall, in the FFR arm of the FAME trial, the subgroup with angiographic lesion severity of 50–70% by visual estimation, had an FFR < 0.8 in only 35% that increased to 80% in the group with angiographic lesion severity of 71–90% and to 96% in the group with angiographic severity of 91–99%. The authors inferred that only in the angiographic stenosis category >90%, visual lesion assessment corresponded well to a lesion’s capability of inducing myocardial ischemia because 96% of such lesions were functionally significant by FFR [21]. Figure 3 demonstrates a representative patient with multivessel disease. Despite the fact that angiography demonstrated that both the stenoses of the left anterior descending artery (LAD) and right coronary artery (RCA) were significant by angiography, however, neither FFR of the LAD or of the RCA was significant and stenting was deferred. Since FFR of the left circumflex artery (LCX) was 0.66, only LCX underwent stenting.
Figure 3: Coronary angiogram of a patient with multivessel disease. (A) A long diffuse
stenosis of the left anteror descending artery, (B) FFR of the left anteror descending artery was 0.88,
(C) angiogram of the left circumflex artery (LCX) demonstrated a critical stenosis of the LCX, (D) FFR
of the LCX was 0.68, (E) a long diffuse stenosis of the right coronary artery and (F) FFR of the right
coronary artery was 0.92.
FFR: Fractional flow reserve.
Multivessel disease: FFR outperformed MPI
MPI is the most commonly used noninvasive modality for evaluation of CAD. It is based on the principle of differential flow in the vascular bed. In a recent study by Melikian et al., the performance of MPI was assessed against FFR in 67 patients (201 vascular territories) [22]. In 42% of patients, MPI and FFR detected identical ischemic territories, whereas in 36% MPI underestimated and in 22% overestimated the number of ischemic territories in comparison with FFR. There was poor concordance between the ability of the two methods to detect myocardial ischemia on both a per-patient (correlation coefficient (correlation coefficient [r] = 0.14; 95% CI: 0.1–0.39) and per-vessel (r = 0.28; 95% CI: 0.15–0.42) basis. As a functional index of epicardial vessel stenosis, FFR was unique to each and every vessel and not influenced by the presence and/or absence of stenoses in adjacent vessels. The authors concluded that FFR is ideally suited to the functional assessment of coronary stenoses in patients with multivessel CAD [22].
In a recent study, 497 patients enrolled in the FAME trial were followed over a 1-year period. A functional SYNTAX score (SS) was calculated by counting only the ischemia producing lesions with FFR < 0.8. The functional SS (FSS) was compared with the angiographic SS in predicting major adverse coronary events over a 1-year period. FSS moved 32% of the patients to a lower risk group. The FSS had a better predictive accuracy for major adverse coronary events as compared with SS [23].
FFR for the interrogation of equivocal LM coronary stenois
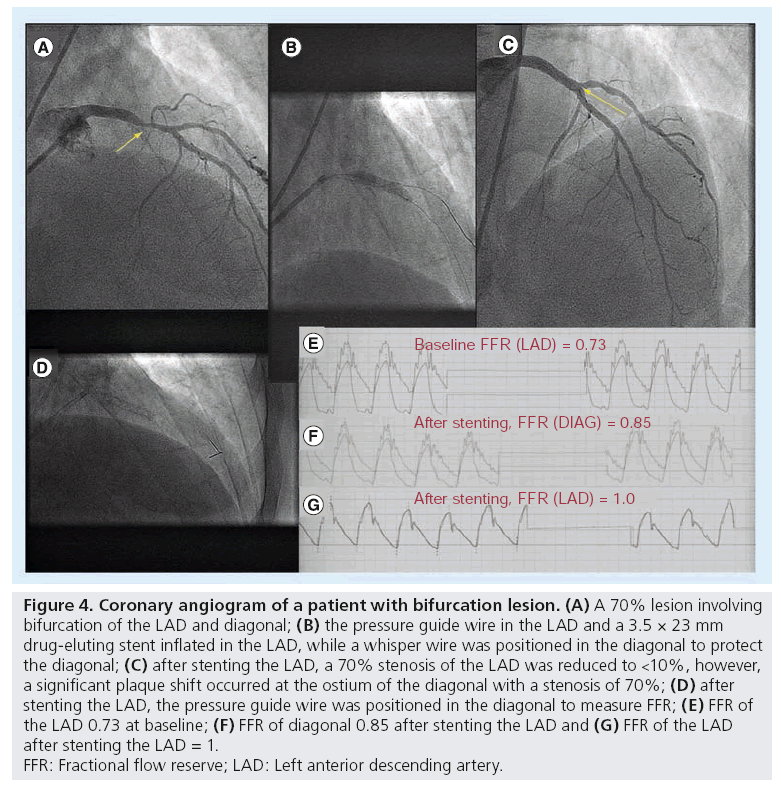
Significant LM disease has been traditionally defined as stenosis >50% luminal diameter and is a Class IA recommendation for surgical revascularization. It is also well known that grafting an insignificant lesion leads to a high rate of disease progression in the grafted native artery and is associated with a high rate of graft failure [24,25]. Given the inherent limitations with luminography and intraobserver variability, a more reliable form of evaluation is critical in determining the hemodynamic significance of LM stenosis. Hamilos et al. evaluated 213 patients with equivocal LM stenosis using an FFR guided strategy [26]. Patients with FFR <0.8 underwent surgical revascularization. There was poor correlation between coronary diameter stenosis and FFR (r = -0.38), with 23% of patients with <50% diameter stenosis angiography having FFR <0.8. The 5-year survival estimates were 89.8% in the nonsurgical group treated medically (FFR >0.8) and 85.4% in the surgical group (p = 0.48). Figure 4 demonstrates a representative case of the LM stenosis. Despite the fact the LM ostium appeared significantly stenosed by angiography, the FFR of the LM stenosis was 0.85 and the IVUS of the LM demonstrates that the ostium of the LM is not significant.
Figure 4: Coronary angiogram of a patient with bifurcation lesion. (A) A 70% lesion involving
bifurcation of the LAD and diagonal; (B) the pressure guide wire in the LAD and a 3.5 × 23 mm
drug-eluting stent inflated in the LAD, while a whisper wire was positioned in the diagonal to protect
the diagonal; (C) after stenting the LAD, a 70% stenosis of the LAD was reduced to <10%, however,
a significant plaque shift occurred at the ostium of the diagonal with a stenosis of 70%; (D) after
stenting the LAD, the pressure guide wire was positioned in the diagonal to measure FFR; (E) FFR of
the LAD 0.73 at baseline; (F) FFR of diagonal 0.85 after stenting the LAD and (G) FFR of the LAD
after stenting the LAD = 1.
FFR: Fractional flow reserve; LAD: Left anterior descending artery.
FFR evaluation of coronary bifurcation lesions
The issue of performing angioplasty on a side branch (SB) after the main vessel (MV) has been stented across a bifurcation, continues to be a matter of debate. To date, there are no randomized studies to demonstrate the use of FFR-guided strategy for coronary bifurcation lesions (CBL). Koo et al. investigated the utility of FFR to determine the significance of SB stenosis after stenting the MV in 92 consecutive patients [27]. Patients with CBL with jailed SBs after successful stenting of the MV were prospectively enrolled. As part of the study protocol, jailed SB had to have thrombolysis in myocardial infarction (TIMI) III flow, with an ostial stenosis >50%, vessel size >2 mm and lesion length <10 mm. Important angiographic exclusion criteria included LM stenosis, totally occluded lesion, infarct-related artery or angiographically visible thrombus, diffuse or significant distal lesion at a SB, significant lesion at a main branch proximal to stented segment and predilation of the SB before the main-branch stent implantation FFR could successfully be measured in 97% of the patients. They reported that only 27% of the SB lesions with >75% stenosis by quantitative coronary angiography (QCA) were functionally significant, thereby highlighting the fact that visual assessment of jailed SB generally tends to overestimate the significance of a stenosis. Even amongst large SBs (>2.5 mm) with >75% stenosis, only 38% of lesions were hemodynamically significant (FFR < 0.75). Importantly, no lesion with <75% stenosis had FFR <0.75. Using receiver operator curve analysis, they estimated an optimal cut-off value of 85% stenosis by QCA in predicting functionally significant lesions (sensitivity 80%, specificity 76%) yielding an AUC of 0.85.
Sarno et al. investigated an improved three branch QCA analysis that has better correlation with FFR; however, the study included only 20 patients [28]. In a subsequent study by the same authors, 110 patients treated by provisional strategy were consecutively enrolled and SB FFR was measured in 91 patients [29]. SB intervention was guided by an FFR-based strategy (<0.75). After MV stent implantation, mean FFR was 0.81 ± 0.12 at the jailed SBs and 0.96 ± 0.04 at the MV. While the mean percent stenosis of jailed SB was 79 ± 11%, only 28 lesions (31%) turned out to be functionally significant and subsequently underwent intervention (all were treated by kissing balloon inflation over the pressure wire). Post-kissing balloon inflation, FFR significantly increased from 0.65 ± 0.08 to 0.85 ± 0.07 (n = 25; p < 0.001). FFR ≥0.75 was achieved in 92% of the lesions despite mean residual stenosis of 69 ± 10%.
At 6-months angiographic follow-up (85%), there were no changes in SB FFR in lesions with (0.86 ± 0.05 to 0.84 ± 0.01; p = 0.4) and without SB angioplasty (0.87 ± 0.06 to 0.89 ± 0.07; p = 0.1). Functional restenosis (FFR <0.75) rate was only 8%. When clinical outcomes of these patients were compared with 110 patients with similar bifurcation lesions, treated without FFR-guidance, there was no difference in 9-month cardiac event rates (4.6 vs 3.7%; p = 0.7) between the two groups. Clinical outcomes of these patients at 9 months (FFR-guided strategy) were then compared with 110 patients with similar bifurcation lesions treated without FFR-guidance (SB intervention was performed more often in the conventional group [45 vs 30%; p = 0.03]). There was no difference in the incidence of death, MI or revascularization between the two strategies (4.6 vs 3.7%; p = 0.7). As compared with angiographic criteria, FFR-guided SB intervention appeared to reduce what appeared as unnecessary interventions of SB by 55–80% [29].
In another small pilot study of 14 patients, Bellenger et al. demonstrated the efficacy of FFR-guided approach in patients with coronary CBL [30]. While QCA demonstrated a significant stenosis in the SB in 9/14 (64%) patients after stenting the MV, FFR showed that only 3/14 (21%) of these were hemodynamically significant. In all cases, TIMI III flow was seen in both the MV and SB after deployment of the MV stent. There was a poor correlation between the angiographic degree of stenosis by QCA and the FFR in the SB after stenting the MV.
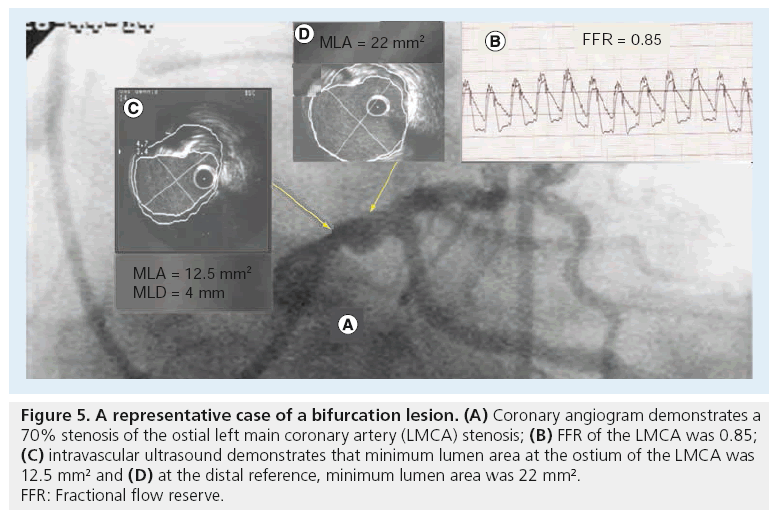
The utility of FFR assessment was investigated before stenting the MV and SB in patients with CBL and/or multivessel disease, and demonstrated that the event rate was significantly lower with FFR-guided strategy compared with the conventional strategy (6 vs 12% at 12 months) [16]. Furthermore, only 42% of lesions that were deemed significant by FFR underwent stenting, compared with 100% of the lesions assessed by angiography. However, in this pilot study, we did not perform FFR of the SB after stenting the MV [16]. Figure 5 demonstrates a representative case of a bifurcation lesion. After stenting of the LAD, a significant plaque was shifted to the ostium of the diagonal, despite a critical stenosis of the diagonal, the FFR of which was 0.85.
Figure 5: A representative case of a bifurcation lesion. (A) Coronary angiogram demonstrates a
70% stenosis of the ostial left main coronary artery (LMCA) stenosis; (B) FFR of the LMCA was 0.85;
(C) intravascular ultrasound demonstrates that minimum lumen area at the ostium of the LMCA was
12.5 mm2 and (D) at the distal reference, minimum lumen area was 22 mm2.
FFR: Fractional flow reserve.
FFR evaluation of myocardial bridging
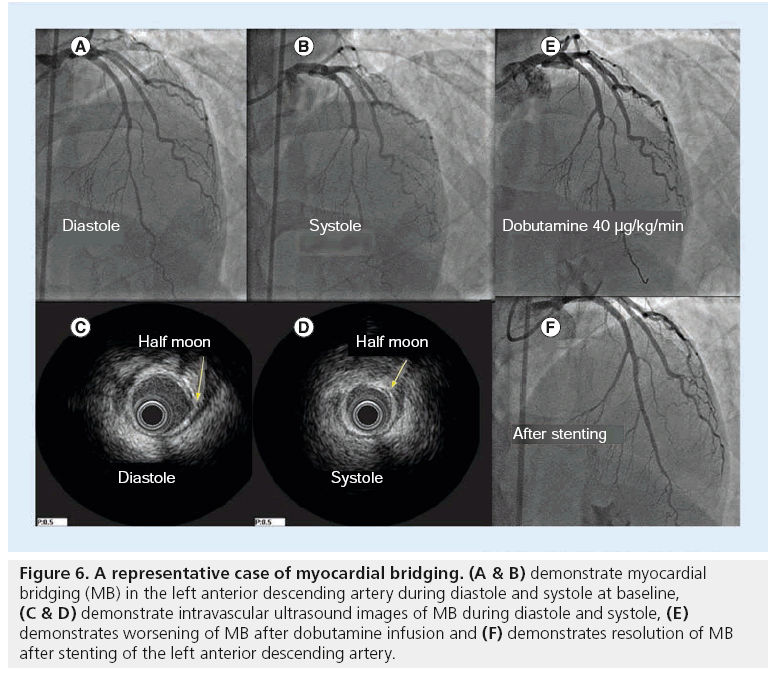
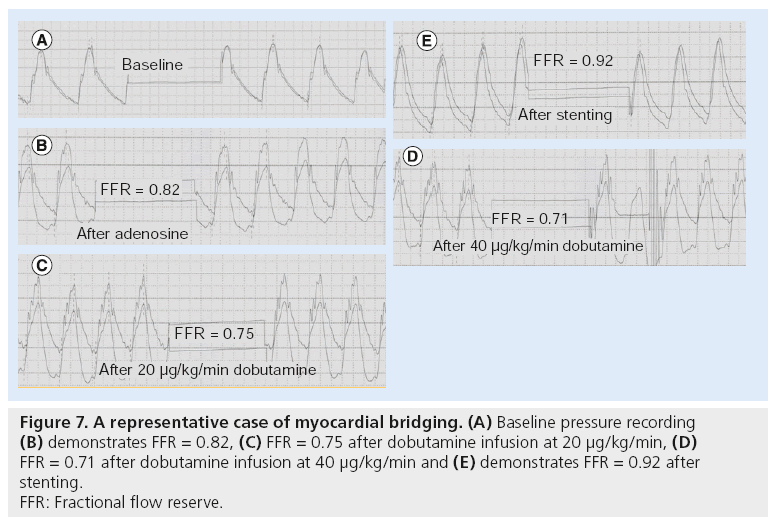
Myocardial bridging (MB) is a common finding and poses a diagnostic and therapeutic challenge. Distinguishing a bridge as the culprit of symptoms versus an innocent bystander needs to be firmly established to guide appropriate therapy. Data from the Mayo Clinic (MN, USA) have shown that MB is associated with a 36% increased risk of nonfatal-MI during a mean follow-up of 1 year [31]. In order to assess the physiological significance of MB, we have defined an ‘FFR paradox.’ We demonstrated that angiographic and functional severity of MB could be unmasked after inotropic stimulation by dobutamine infusion. After adenosine infusion, FFR was >0.82, indicating that MB was not hemodynamically significant, whereas after dobutamine infusion, FFR decreased to <0.75 [32]. The underlying mechanism of the ‘FFR paradox’, include a greater FFR drop with dobutamine than with adenosine among patients with MB, likely owing to increased contractility of muscle fibers overlying arterial segment in response to dobutamine and that, in turn, decreased minimum lumen diameter and increased the length of bridging segment, as previously reported [32]. In contrast, it has been reported that among patients with fixed coronary stenosis and no evidence for MB, both distal coronary pressure and Pa/distal pressure decreased to the same extent during intracoronary adenosine and high-dose dobutamine infusion [32]. Likewise, among patients with fixed coronary artery stenosis, minimum lumen diameter at the stenosis did not differ at baseline and after high-dose dobutamine infusion. FFR measurement, after a high-dose dobutamine infusion, is a promising strategy to unmask the significance of MB [32]. Figures 6 & 7 demonstrate a representative case of MB. After adenosine, FFR of the LAD was 0.82, but after dobutamine infusion at a rate of 40 μg/kg/min, FFR dropped to 0.71. After stenting, FFR increase to 0.92.
Figure 6: A representative case of myocardial bridging. (A & B) demonstrate myocardial bridging (MB) in the left anterior descending artery during diastole and systole at baseline, (C & D) demonstrate intravascular ultrasound images of MB during diastole and systole, (E) demonstrates worsening of MB after dobutamine infusion and (F) demonstrates resolution of MB after stenting of the left anterior descending artery.
Figure 7: A representative case of myocardial bridging. (A) Baseline pressure recording
(B) demonstrates FFR = 0.82, (C) FFR = 0.75 after dobutamine infusion at 20 μg/kg/min, (D)
FFR = 0.71 after dobutamine infusion at 40 μg/kg/min and (E) demonstrates FFR = 0.92 after
stenting.
FFR: Fractional flow reserve.
FFR & viability
Assessment of myocardial viability and physiological significance of coronary artery stenoses are essential for appropriate guidance of revascularization. Samady et al. studied 48 patients within 4 days following acute MI [33]. An FFR of 0.78 was found to provide optimal discriminatory power for detecting reversibility on single photon emission computed tomography (SPECT) or myocardial perfusion echocardiography, with no significant loss in sensitivity to detect ischemia in large infarct sizes. In addition, patients with irreversible defects on MPI had a mean FFR of 0.93. The ability of FFR to discriminate ischemic tissue from scar tissue in post MI period may further guide clinical decision making when considering revascularization in acute MI patients.
In patients with prior MI, a FFR cut-off value of 0.75 can be used to distinguish patients with positive from patients with negative SPECT. There is an 85% concordance between FFR and SPECT results. Patients with positive SPECT imaging have better preserved left ventricular ejection fraction and lower FFR compared with patients with negative SPECT imaging. This suggests that lower FFR values indicate more viable myocardial mass [34].
The therapeutic implications of FFR in predicting myocardial recovery were further tested clinically. Beleseline et al. concluded that increased coronary flow following PCI – as measured by increased FFR – is a significant predictor for improvement of left ventricular function on echocardiography [35]. FFR <0.71 before angioplasty and improvement in FFR by 0.21 were significant predictors of improved left ventricular function following PCI.
FFR & IVUS
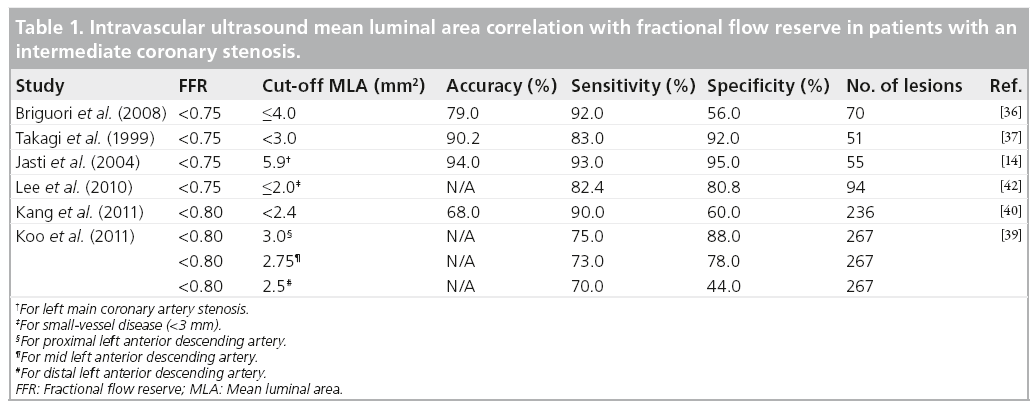
The summary of correlations between IVUS cutoff points and FFR is depicted in Table 1. Brigouri et al. reported comparative data on FFR and IVUS in evaluating the hemodynamic significance of coronary stenoses [36]. For stenoses with FFR <0.75, the best cut-off values for corresponding IVUS measurements were mean luminal areas (MLA) <4 mm2, mean luminal diameter <1.8 mm and lesion length greater than 10 mm. A MLA of <4 mm2 yielded a sensitivity of 92% in detecting significant coronary stenoses, but specificity was limited to 56%. A MLA <3 mm2 by IVUS measurement resulted in significant improvement in specificity of IVUS in detecting hemodynamically significant lesions [37].
The diagnostic accuracy of IVUS MLA is highly variable, depending on lesion location. In 267 lesions of indeterminate significance, 33% had FFR <0.8 [38]. The best cut-off value of MLA to define the functional significance was 3 mm2 for proximal LAD lesions and 2.75 mm2 for mid-LAD lesions located before the second diagonal branch. However, the functional significance of MLA could not be determined for other lesion locations [39]. Kang et al. further questioned the specificity of IVUS measurements as compared with FFR; an IVUS MLA >2.4 mm2 ruled out FFR <0.8 in 96% of cases, but an IVUS MLA <2.4 mm2 was associated with an FFR <0.8 in only 37% of the cases [40]. IVUS measurements are sensitive in ruling out hemodynamically significant stenoses. However, the specificity of IVUS measurements remains in question.
Brugaletta et al. investigated correlations between IVUS virtual histology and FFR in patients with an intermediate coronary stenosis [41]. They demonstrated that, although the stenoses with an FFR ≤0.8 had larger plaque size compared with an FFR >0.8, plaque composition was different between the groups. Lee et al. demonstrated that a small-vessel disease, an IVUS MLA <2.00mm2 yielded a sensitivity and specificity of 82.4 and 80%, respectively, for detection of significant stenosis by FFR [42].
Economic rationale
While the cost of obtaining FFR adds apparent operational expense, the net benefit of objective and timely decision-making can provide a significant overall savings to the healthcare delivery system. The use of physiological lesion assessment is associated with favorable medical economics for the strategy of risk assessment in the catheterization laboratory. Leesar et al. found comparable results for patients admitted with acute coronary syndrome who were randomized either to angiography with measurement of FFR and interventional or medical treatment as indicated by FFR, or to nuclear stress imaging with angiography if results were abnormal [43]. They found that a FFR strategy significantly reduced both the duration and cost of hospitalization, with identical cardiac event rates in the two groups at 1-year follow-up. A decision analysis by Fearon et al. demonstrated that an FFR based PCI strategy saved US$1795 per patient as compared with nuclear stress based PCI strategy and $3830 per patient as compared with stent implantation for all intermediate lesions without measuring FFR [44]. Most recently, a costeffective analysis based on 1-year results from the FAME study showed that the mean cost of PCI was reduced by almost $2400 when decisionmaking was guided by FFR. The cost-savings come initially from the reduction in use of stents (1/3 fewer stents were used with FFR guided decision-making) but continued through the first year, due to less need for repeat procedures, rehospitalization and adverse events [45].
Limitations of FFR measurements
Both the DEFER and FAME trial did not report an increased incidence of complications during FFR evaluation [18,19]. However, FFR may be associated with an increase in radiation dose (4 mSv), contrast dose (50 ml) and procedure time (9 min) [46]. In addition, FFR evaluation confers risk of coronary complications associated with wiring, specifically in anatomically complex, bifurcation and calcified lesions. Heparin administration with FFR may confer a slightly higher risk of bleeding complications, despite a lack of clinical data to validate this theory.
False-positive FFR is rare. One potential explanation is a drift in the pressure wire. When FFR is low a pullback across the lesion with normalization of FFR is evidence of significant stenosis. Wire induced coronary spasm is another pitfall which is avoided by injection of intracoronary nitroglycerin [47].
False-negative FFR may occur due to technical factors such as insufficient hyperemia, deep guide-catheter engagement, or electrical drift in the system [45]. Actual false-negative FFR may occur in the setting of severe microvascular dysfunction, which can theoretically occur with LV hypertrophy and ST-elevation MI [48].
Unlike IVUS, FFR does not provide morphological information about coronary stenoses. Information on healthy reference segment, stent sizing and accurate stent placement is therefore lacking.
Future perspective
FFR is more routinely used in evaluation of lesions of indeterminate significance. The major pitfall of FFR is lack of anatomical data and lesion morphology. The development of remote consoles in the near future will cut down on added time to FFR procedures and will likely facilitate the use of this modality. Combining FFR wires with IVUS or optical coherence tomography catheters in the future, will fill one of the major gaps of FFR. The combination of coronary flow reserve calculation with FFR wires is an additional enhancement that is already available for use and will likely grow in the near future. As more stringent rules are applied to appropriate PCI, the use of ‘functional proof of significance’ should become a cornerstone in evaluating coronary stenosis.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Fractional flow reserve:
– Provides functional data that can safely reduce the need for percutaneous coronary interventions;
– Provides more accurate risk assessment in patients with multivessel disease as compared with coronary angiography;
– Reduces the need for bifurcation stenting;
– Is more specific than intravascular ultrasound in assessing coronary stenoses;
– Is not associated with an increased risk of complications in larger clinical trials.
References
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 92, 2333-2342 (1995).
- Nissen SE, Gurley JC. Assessment of the functional significance of coronary stenoses. Circulation 81, 1431-1435 (1990).
- Kern MJ, Lerman A et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on diagnostic and interventional cardiac catheterization, Council on Clinical Cardiology. Circulation 114, 1321-1341 (2006).
- Mintz GS, Popma JJ, Pichard AD et al. Limitations of angiography in the assessment of plaque distribution in coronary artery disease: a systematic study of target lesion eccentricity in 1446 lesions. Circulation 93, 924-931 (1996).
- Pijls NH, De Bruyne B et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 334, 1703-1708 (1996).
- De Bruyne B, Hersbach F, Pijls NHJ et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but ‘normal’ coronary angiography. Circulation 104, 2401-2406 (2001).
- Pijls NHJ, De Bruyne B, Bech GJW et al. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery: validations in humans. Circulation 102, 2371-2377 (2000).
- Pijls NHJ, Klauss V, Siebert U et al. Coronary pressure measurement after stenting predicts adverse events at follow-up: a multicenter registry. Circulation 105, 2950-2954 (2002).
- De Bruyne B, Bartunek J, Sys SU, Hendrickx GR. Relation between myocardial fractional flow reserve calculated from coronary pressure measurements and exercise-induced myocardial ischemia. Circulation 92, 39-46 (1995).
- Murtagh B, Higano ST, Lennon R et al. Role of incremental doses of intracoronary adenosine for fractional flow reserve assessment. Am. Heart J. 146, 99-105 (2003).
- Kern MJ. Coronary physiology revisited: practical insights from the cardiac catheterization laboratory. Circulation 101, 1344 -1351 (2000).
- Koo BK, Kim CH, Na SH et al. Intracoronary continuous adenosine infusion. Circ. J. 69(8), 908-912 (2005).
- Kern MJ, De Bruyne B, Pijls NH. From research to clinical practice: current role of intracoronary physiologically based decision making in the cardiac catheterization laboratory. J. Am. Coll. Cardiol. 30, 613-620 (1997).
- Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation 110(18), 2831-2836 (2004).
- Bech GJW, De Bruyne B, Pijls NHJ et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 103, 2928-2934 (2001).
- Wongpraparut N, Yalamonchili V, Pasnoori V et al. Thirty-month outcome after fractional flow reserve-guided versus conventional multivessel percutaneous coronary intervention. Am. J. Cardiol. 96, 877-884 (2005).
- Legalery P, Schiele F, Seronde MF et al. One-year outcome of patients submitted to routine fractional flow reserve assessment to determine the need for angioplasty. Eur. Heart J. 26, 2623-2629 (2005).
- Pijls NH, van Schaardenburgh P, Manoharan G et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 49, 2105-2111 (2007).
- Tonino PA, De Bruyne B, Pijls NH et al. FAME study investigators fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 360, 213-224 (2009).
- Pijls NH, Fearon WF, Tonino PA et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME study. J. Am. Coll. Cardiol. 56(3), 177-184 (2010).
- 21 Tonino PAL, Fearon WF, De Bruyne B et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study: fractional flow reserve versus angiography for multivessel evaluation. J. Am. Coll. Cardiol. 55, 2816-2821 (2010).
- Melikian N, De Bondt P, Tonino P et al. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc. Interv. 3(3), 307-314 (2010).
- Nam CW, Mangiacapra F, Entjes R et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J. Am. Coll. Cardiol. 58(12), 1211-1218 (2011).
- Kroncke GM, Kosolcharoen P, Clayman JA, Peduzzi PN, Detre K, Takaro T. Five-year changes in coronary arteries of medical and surgical patients of the Veterans Administration Randomized Study of Bypass Surgery. Circulation 78(3 Pt. 2), I144-I150 (1988).
- Berger A, MacCarthy PA, Siebert U et al. Long-term patency of internal mammary artery bypass grafts: relationship with preoperative severity of the native coronary artery stenosis. Circulation 110(Suppl. 1), 36-40 (2004).
- Hamilos M, Muller O, Cuisset T et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation 120(15), 1505-1512 (2009).
- Koo BK, Kang HJ, Youn TJ et al. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J. Am. Coll. Cardiol. 46, 633-637 (2005).
- Sarno G, Garg S, Onuma Y et al. Bifurcation lesions: functional assessment by fractional flow reserve vs. anatomical assessment using conventional and dedicated bifurcation quantitative coronary angiogram. Catheter. Cardiovasc. Interv. 76(6), 817-823 (2010).
- Koo BK, Park KW, Kang HJ et al. Physiological evaluation of the provisional side branch intervention strategy for bifurcation lesions using fractional flow reserve. Eur. Heart J. 29, 726-732 (2008).
- Bellenger NG, Swallow R et al. Hemodynamic significance of ostial side branch nipping following percutaneous intervention at bifurcations: a pressure wire pilot study. Heart 93, 249-250 (2007).
- Mookadam F, Green J, Holmes D, Moustafa SE, Rihal C. Clinical relevance of myocardial bridging severity: single center experience. Eur. J. Clin. Invest. 39, 110-115 (2009).
- Hakeem A, Cilingiroglu M, Leesar MA. Hemodynamic and intravascular ultrasound assessment of myocardial bridging: fractional flow reserve paradox with dobutamine versus adenosine. Catheter. Cardiovasc. Interv. 75(2), 229-236 (2010).
- Samady H, Lepper W, Powers ER et al. Fractional flow reserve of infarct-related arteries identifies reversible defects on noninvasive myocardial perfusion imaging early after myocardial infarction. J. Am. Coll. Cardiol. 47, 2187-2193 (2006).
- De Bruyne B, Pijls NH, Bartunek J et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation 104, 157-162 (2001).
- Beleslin B, Ostojic M, Djordjevic-Dikic A et al. The value of fractional and coronary flow reserve in predicting myocardial recovery in patients with previous myocardial infarction. Eur. Heart J. 29(21), 2617-2624 (2008).
- Briguori C, Anzuini A, Airoldi F et al. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve. Am. J. Cardiol. 87, 136-141 (2001).
- Takagi A, Tsurumi Y, Ishii Y, Suzuki K, Kawana M, Kasanuki H. Clinical potential of intravascular ultrasound for physiological assessment of coronary stenosis relationship between quantitative ultrasound tomography and pressure-derived fractional flow reserve. Circulation 100, 250-255 (1999).
- Nam CW, Yoon HJ, Cho YK et al. Outcomes of percutaneous coronary intervention in intermediate coronary artery disease fractional flow reserve-guided versus intravascular ultrasound-guided. J. Am. Coll. Cardiol. Interv. 3, 812-817 (2010).
- Koo BK, Yang HM, Doh JH et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. J. Am. Coll. Cardiol. Interv. 4, 803-811 (2011).
- Kang SJ, Lee JY, Ahn JM et al. Validation of intravascular ultrasound derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circ. Cardiovasc. Interv. 4, 65-71 (2011).
- Brugaletta S, Garcia-Garcia HM, Shen ZJ et al. Morphology of coronary artery lesions assessed by virtual histology intravascular ultrasound tissue characterization and fractional flow reserve. Int. J. Cardiovasc. Imaging doi:10.1007/s10554-011-9816-3 (2011) (Epub ahead of print).
- Lee CH, Tai BC, Soon CY et al. New set of intravascular ultrasound-derived anatomic criteria for defining functionally significant stenoses in small coronary arteries (results from IDEAS study). Am. J. Cardiol. 105, 1378-1384 (2010).
- Leesar MA, Abdul-Baki T, Akkus NI, Sharma A, Kannan T, Bolli R. Use of fractional flow reserve versus stress perfusion scintigraphy after unstable angina: effect on duration of hospitalization, cost, procedural characteristics and clinical outcome. J. Am. Coll. Cardiol. 41, 1115-1121 (2003).
- Fearon WF, Yeung AC, Lee DP, Yock PG, Heidenreich PA. Cost-effectiveness of measuring fractional flow reserve to guide coronary interventions. Am. Heart J. 145, 882-887 (2003).
- Fearon WF, Bornschein B, Tonino PA et al. Fractional flow reserve versus angiography for multivessel evaluation (FAME) study investigators. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 122(24), 2545-2550 (2010).
- Ntalianis A, Trana C, Muller O et al. Effective radiation dose, time, and contrast medium to measure fractional flow reserve. JACC Cardiovasc. Interv. 3(8), 821-827 (2010).
- Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc. Interv. 49(1), 1-16 (2000).
- Meuwissen M, Chamuleau SA, Siebes M et al. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation 103(2), 184-187 (2001).