Commentary - Interventional Cardiology (2024) Volume 16, Issue 4
Fragmented QRS in different clinical scenarios: A troubling red flag requiring appropriate differential diagnosis
- Corresponding Author:
- Francesco Chietera
Department of Medical and Surgical Sciences DIMEC, University of Bologna, Via Giuseppe Massarenti 9, 40138 Bologna, Italy,
E-mail: francesco.chietera3@unibo.it
Received date: 15-Jul-2024, Manuscript No. FMIC-24-141637; Editor assigned: 18-Jul-2024, PreQC No. FMIC-24-141637 (PQ); Reviewed date: 01-Aug-2024, QC No. FMIC-24-141637; Revised date: 08-Aug-2024, Manuscript No. FMIC-24-141637 (R); Published date: 15-Aug-2024, DOI: 10.37532/1755- 5310.2024.16(S23).603
Description
A fragmented QRS is an electrocardiographic finding which requires careful evaluation because of the possible ambiguity between the marker of a definite pathologic substrate and a benign finding, where minor delayed ventricular activation may ensue at the Purkinje-local myocardium level.
Diagnostic role of QRS fragmentation (QRSf)
QRSf has been described in the late 60s [1], as inhomogeneous ventricular activation due to myocardial scar/fibrotic tissue creating slow conduction and local block. Being first reported in patients with coronary artery disease-related scars, QRSf has been consistently observed also in other disease etiologies, such as Hypertrophic cardiomyopathy, Arrhythmogenic cardiomyopathy, sarcoidosis, infiltrative diseases and radiotherapy-related fibrosis [2-5]. It was observed that acute myocardial injury, as caused by transient ischemia or inflammatory response, may also be associated with the slowing of myocardial conduction velocity, hence to QRSf which may partially subside [6,7]. The pathophysiology of QRSf has thus broadened to include also acute myocardial ischemia and inflammation as potentially modifiable/reversible causes of conduction delay/functional block within the myocardium, as observed in our previously published case report (partial QRSf modification after steroids) [8]. The diagnostic role of QRSf for myocardial fibrosis underlying a structural heart disease has emerged as a simple and potential tool, triggering focused workflow/imaging for precise diagnosis in the individual patient.
Prognostic meaning of QRSf
QRSf has also proved as a prognostic indicator. QRSf is associated with cardiovascular events and with life-threatening ventricular arrhythmias in many clinical settings, owing to its close correlation with a myocardial substrate prone to reentry. Indeed, QRSf is a risk marker of the substrate for ventricular arrhythmias independently of disease etiology, being predictive of malignant ventricular arrhythmias in coronary artery disease patients as well as in other cardiomyopathies, and the broad population of heart failure patients [8-15]. These latter appear to have a significantly increased risk of both sudden cardiac death and all-cause mortality in the presence of QRSf [13].
One key issue of QRSf is its definition, the boundary between a benign variant and a pathologic finding being somewhat uncertain. While some extreme fragmentations, as reported in our clinical case [8], pose hardly any doubt of a pathologic finding, most frequently sensitivity and specificity of QRSf rely on experience, and the final diagnosis stands on multi-modality integration of other information sources rather than on precise metrics.
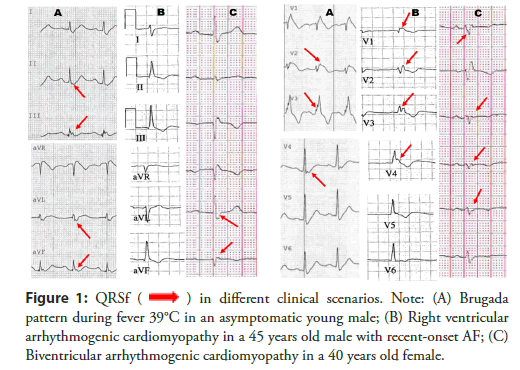
Indeed, a classification proposal different from Das, et al., [9], and Maheshwari, et al., [16], has been developed, which encompasses a broader range of QRSf morphologies and provides reference metrics for its appraisal. This proposal attempts to address an unmet clinical need, that is to discriminate benign variants from pathological ECGs. The manifold appearance of QRSf and the complexity of measuring peak amplitude, widths and peak-to-peak ratios on several leads requires an algorithmic approach to ECG reading and classification proposal. In other words, this is another potential application of artificial Intelligence to firstly screen suspected abnormal findings, and later to assist the differential diagnostic decision amongst the many patterns of QRSf as shown in Figure 1 [17].
Figure 1: QRSf  in different clinical scenarios. Note: (A) Brugada
pattern during fever 39°C in an asymptomatic young male; (B) Right ventricular
arrhythmogenic cardiomyopathy in a 45 years old male with recent-onset AF; (C)
Biventricular arrhythmogenic cardiomyopathy in a 40 years old female.
in different clinical scenarios. Note: (A) Brugada
pattern during fever 39°C in an asymptomatic young male; (B) Right ventricular
arrhythmogenic cardiomyopathy in a 45 years old male with recent-onset AF; (C)
Biventricular arrhythmogenic cardiomyopathy in a 40 years old female.
QRSf is yet another red flag attracting the cardiologist’s attention to potential cardiac abnormalities and prompts a thorough investigation to precise diagnosis and targeted therapeutic interventions [18-20]
Conclusion
The clinical relevance of QRSf provides new insights into ECG as the first imaging technique of the myocardium and dictates a renovated approach at its acquisition and analysis:
• Immaculate skin preparation for maximize the informative content as for the Ultra High Frequency (UHF) ECG.
• Use of Artificial Intelligence to classify patterns of QRSf and of cardiac conduction.
• Algorithmic approach to pattern classification for a first screening in the differential diagnosis process.
References
- Flowers NC, Horan LG, Thomas JR, et al. The anatomic basis for high-frequency components in the electrocardiogram. Circulation. 39(4);531-539: (1969).
- Konno T, Hayashi K, Fujino N, et al. Electrocardiographic QRS fragmentation as a marker for myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 26(10);1081-1087: (2015).
- Fares H, Heist K, Lavie CJ, et al. Fragmented QRS complexes-A novel but underutilized electrocardiograhic marker of heart disease. Crit Pathw Cardiol. 12(4);181-183: (2013).
- Homsi M, Alsayed L, Safadi B, et al. Fragmented QRS complexes on 12-lead ECG: A marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol. 14(4);319-326: (2009).
- Adar A, Canyılmaz E, Kiris A, et al. Radiotherapy induces development of fragmented QRS in patients with breast cancer. Breast Care. 10(4):277-280: (2015).
- Das MK, Saha C, ElMasry H, et al. Fragmented QRS on a 12-lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 4;1385-1392: (2007).
- Jain R, Singh R, Yamini S, et al. Fragmented ECG as a risk marker in cardiovascular diseases. Curr Cardiol Rev. 10(3);277-286: (2014).
- Chietera F, Biffi M. Premature electrical activity or extreme QRS fragmentation? When early is too late! J Cardiovasc Med (Hagerstown). 24(3):213-216 (2023).
- Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 113(21);2495-2501: (2006).
- Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and non-ischemic cardiomyopathy. Heart Rhythm 7(1);74-80: (2010).
- Das MK, Michael MA, Suradi H, et al. Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 104(12):1631-1637: (2009).
- Sha J, Zhang S, Tang M, et al. Fragmented QRS is associated with all-cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol. 16(3);270-275: (2011).
- Engstrom N, Dobson G, Ng K, et al. Fragmented QRS is associated with ventricular arrhythmias in heart failure patients: A systematic review and meta-analysis. Ann Noninvasive Electrocardiol. 27(1):e12910; (2022).
- Terho HK, Tikkanen JT, Junttila JM, et al. Prevalence and prognostic significance of fragmented QRS complex in middle-aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol. 114(1);141-147: (2014).
- Haukilahti MA, Eranti A, Kenttä T, et al. QRS fragmentation patterns representing myocardial scar need to be separated from benign normal variants: Hypotheses and proposal for morphology based classification. Front Physiol. 7;653: (2016).
- Maheshwari S, Acharyya A, Puddu PE, et al. An automated algorithm for online detection of fragmented QRS and identification of its various morphologies. J R Soc Interface. 10(89):20130761: (2013).
- Saturi G, Caponetti AG, Leone O, et al. Cum grano salis: Cardiac sarcoidosis as a perfect mimic of arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 14(7);e012355: (2021).
- Jurak P, Curila K, Leinveber P, et al. Novel ultra-high-frequency electrocardiogram tool for the description of the ventricular depolarization pattern before and during cardiac resynchronization. J Cardiovasc Electrophysiol. 31(1);300-307: (2020).
- Leinveber P, Halamek J, Curila K, et al. Ultra-high-frequency ECG volumetric and negative derivative epicardial ventricular electrical activation pattern. Sci Rep. 14(1);5681 (2024).
- Nguyên UC, Rijks JHJ, Plesinger F, et al. Ultra-high-frequency ECG in cardiac pacing and cardiac resynchronization therapy: From technical concept to clinical application. J Cardiovasc Dev Dis. 11(3);76: (2024).