Review Article - Interventional Cardiology (2023)
Fundamentals of coronary interventional equipment
- Corresponding Author:
- Jeffrey Cook
Department of Cardiology, Blessing Hospital, Illinois, USA,
E-mail: jrcook79@yahoo.com
Received date: 08-Nov-2023, Manuscript No. FMIC-23-119679; Editor assigned: 10-Nov-2023, PreQC No. FMIC-23-119679 (PQ); Reviewed date: 24-Nov-2023, QC No. FMIC-23-119679; Revised date: 01-Dec-2023, Manuscript No. FMIC-23-119679 (R); Published date: 08-Dec-2023, DOI: 10.37532/1755- 5310.2023.15(S20).492
Abstract
Interventional cardiology is an increasingly complex field, with new techniques and corresponding advances in equipment occurring annually. Keeping abreast of the expansive number of tools available can be daunting. A fundamental knowledge regarding the basic equipment that is employed in routine Percutaneous Coronary Intervention (PCI) cases is important for practitioners of interventional cardiology, as well as candidates for the interventional cardiology boards exams. Guide catheters share function with diagnostic coronary catheters, with many additional design features to facilitate smooth delivery of interventional devices. Appropriate choice of the guide catheter in a coronary case can be accomplished with attention to details of the diagnostic angiogram and fitment of the diagnostic catheter. Coronary guidewires are used to cross the coronary lesion, deliver devices, and, at times, navigate highly complex coronary anatomy. Guidewires are available with specialized characteristics altering cross-ability, track-ability, and device deliverability. Angioplasty balloons are also variable in design, and the choice of balloon often involves compromise of deliverability, lesion cross-ability, and compliance. In contemporary PCI practice, balloons are commonly used to pre and post-dilate lesions to be stented, as well as to deploy the stent itself. In this review, we examine guide catheters, coronary guidewires, and angioplasty balloons; as well as the basic evidence-based approach in PCI case. Appropriate choice and utilization of PCI equipment will improve procedural efficiency and safety. Awareness of the capabilities and, just as importantly, the limitations of our equipment will help us to improve patient care and outcomes on a day-to-day basis.
Keywords
Guide catheters • Coronary guidewires • Angioplasty balloons; Percutaneous coronary intervention • Boards review
Introduction
Coronary guides function similarly to diagnostic catheters, simultaneously allowing for angiography and pressure transduction, with additional engineering to facilitate delivery of interventional equipment. Guides are traditionally 3 layers; an outer nylon layer allowing for atraumatic delivery in the body, a middle layer of stainless-steel mesh providing structure and support, and an inner hydrophilic Polytetrafluoroethylene (PTFE) coated layer to allow for smooth passage of devices [1]. Guides typically have thinner walls than diagnostic catheters, a larger inner lumen, and higher rigidity; the outer 2 layers can also be combined to increase lumen size. Guides are more likely to obstruct the cannulated vessel due to lack of tapered tip. The guide catheter sizing in “French” describes the outer diameter in millimeters multiplied by 3 (2 mm OD=6 French). Conversely, the sheath size describes the inner diameter of sheath. Commonly, guide needs to be 2F size bigger than a comparable sheath to deliver a device, for example in carotid or renal stenting procedure, one can typically utilize a 6F sheath but would need an 8F guide to deliver the stent. “Passive” support refers to the degree of support provided by the basic guide shape and counter-force from opposing structure (aorta, sinus of Valsalva), while “active” support is achieved by advancement and pressure on the guide by the operator [2].
An ideal guide for a particular intervention balances coaxial fit, backup support, adequate lumen to deliver the necessary device, and lowest profile to prevent access complication and/or coronary hypoperfusion. Each of these may not be possible for a given case, and careful review of anatomy and potential challenges in a case informs guide choice. Attention to the fit of your previous diagnostic catheter can be helpful here as well. A good rule of thumb is to reduce curve length by 0.5 cm compared to the diagnostic catheter i.e., a JL4 diagnostic catheter may be well served by an EBU 3.5 or XB 3.5 guide. Workhorse guides are typically 6F, which allow for passage of up to 2 balloons simultaneously or even 1 balloon and 1 stent, and up to a 1.5 mm rotational atherectomy burr (although in the author’s experience 7F can allow for smoother passage). Procedural complexity should also influence the decision to choose a larger 7 or 8 F guide, including Chronic Total Occlusion (CTO), left main disease, bifurcation, planned atherectomy, calcification, and tortuosity of the vessel. It is beneficial in terms of procedural duration, contrast usage, and prevention of complication, to anticipate procedural complexities and choose appropriate equipment at the outset rather than partway through a case that is going “off the rails”. It is also important to be aware of the effect of guide size on coronary perfusion. For instance, an 8F guide may create a defacto stenosis of>75% of the cannulated vessel, a 7F~60%, and 6F~40%, without much augmentation of flow from side holes [3]. There is in fact a mortality signal with use of larger guides [4].
Once in place, note coaxial fit of the catheter that has been selected, and change immediately if roofing on the artery, diving deeply, decannulating with patient respiration, etc. Note dampening of the pressure and recognize this as a warning sign for potential ischemia during case as well as hydrostatic dissection. Consider changing to a smaller caliber or adding side holes. A “roofed” and/or non-coaxial guide can increase risk of stent dislodgement upon removal of a “no-cross” as well. The Right Coronary Artery (RCA) is particularly prone to dissection by the guide, which happens with deep diving of an aggressive shape or “sucking in” of the catheter tip while balloons (often stent balloons) are being withdrawn hence ensure the guide tip is coaxial and in direct view as you remove any device. Counteract any diving motion by applying traction on the guide with the left hand as you remove devices, using a staccato pulling motion on the balloon.
Radial access has the clear advantage for prevention of access site complication and is the default approach for many operators. In a meta-analysis of PCI, radial access was found to reduce the risk of access site complications by about 10-fold, from 2.8% to 0.3% [5]. In acute coronary syndrome, radial access has been shown to reduce mortality as well [6]. Nonetheless, it is also important to recognize coronary anatomy that may be better served from a groin approach as it pertains to guide support, guide stability (such as ostial lesions), and upsizing. In the case of aorto-ostial coronary lesions, visible coronary calcium, tortuous vessels, and CTO cases, strongly consider transitioning these cases to femoral. In excessive chest wall movement (which can occur in pulmonary patients), femoral can also provide more stability. In extreme iliac tortuosity, guide catheter manipulation can become quite difficult–this can be overcome by using a longer, braided sheath (such as Arrow by Teleflex), and even potentially upsizing.
Literature Review
Choosing the guide catheter
Left sided lesions: Extra-Backup type guides (EBU, XB, Voda, Q-curve) all provide support off contralateral aortic wall, and should generally be the first choice for left lesions. Judkins guides often provide coaxial/ atraumatic fitment but notoriously poor delivery support as the case progresses.
To cannulate the left-sided guide catheter:
• Advance the atraumatic table wire to the left coronary sinus, always leading the guide catheter with the wire and monitoring for proximity to the coronaries. The image intensifier should be in slight LAO position 15-20 degrees.
• Mindfully deliver the guide catheter the left sinus over the wire, then remove the wire. Attaining the left sinus is especially important from the radial position.
• The guided is actively aspirated with a syringe to remove 2 mL-4 mL of blood and attached to the manifold or power injector to allow for pressure transduction and test injections.
• To cannulate the vessel, the catheter is usually slightly withdrawn with counter-clockwise torque, then re-advanced toward the left main. Instructing the patient to inspire can bring the aorta down and facilitate engagement as well.
• High take off left main can sometimes be cannulated by advancing the guide to “ride” up the left sinus until it “pops” into left main, and subsequently pulling back on catheter to achieve coaxial position.
• If a catheter is repetitively “roofing” on the vessel, it is likely undersized. If the tip continually sits below the vessel, it is likely oversized. Change to an appropriately sized catheter up front to facilitate a smoother PCI procedure.
From the radial approach, extra-backup guides provide lesser degree of support, because a shorter portion of the catheter is abutting the vertical aortic wall [7]. Nevertheless, EBU 3.75, EBU 4.0, JCL 3.5-4.0, Ikari left can work well. A C-shaped (JCL) guide from the radial approach balances coaxial shape, moderate backup, and less proclivity for deep-diving. Always remove these highly curved catheters over the J-wire to avoid shearing of aortic plaques and potential for embolism. The highest degree of support can be achieved (from either radial or femoral approach) with appropriately sized Amplatz-Left (AL) guides, as passive support is provided by both the contralateral aortic wall as well as the non-coronary sinus. AL guides are commonly employed in CTO intervention in 7 or 8 F size-in a standard root this would be sized as AL-2. A Judkins-Left (JL) 3.5 or 4.0 guide can be considered in aorto-ostial left main disease, straightforward non-calcified lesions, or if a longer catheter is selectively engaging Left-Anterior Descending (LAD) or Circumflex (Cx). Guide extensions have utility to improve backup support as well.
Right sided lesions: The Judkins-Right 4 (JR4) is the workhorse RCA guide in straightforward cases with horizontal takeoff of the vessel, from both the femoral and radial approach. Radial Tig guides work quite well and can be helpful in a STEMI scenario, as the left-sided angiogram can be done with the same catheter. Given the heightened risk for dissection in the RCA with more aggressive guide shapes, the choice to ramp-up to another guide must not be taken lightly. If higher level of support necessary, a less traumatic choice can be an Ikari LEFT which provides moderate backup. Ikari RIGHT can dive exceptionally deep, and if non-coaxial create prohibitive dissection risk. Otherwise, Amplatz LEFT shapes (AL 0.75 or AL1), and Right Back-Up (RBU) provide the most substantial RCA backup, often used with side holes. Anterior and/or high takeoff RCA can be approached with Amplatz right or left shapes. Upgoing RCA can be approached with AL shape, Internal Mammary (IMA), Williams/ 3DRC, Hockey stick (HS), and Amplatz modified right. Downgoing RCA once again AL shape, AR, Right Coronary Bypass (RCB), Multi-Purpose (MP), and Hockey Stick (HS) [8].
To cannulate the right-sided guide catheter:
• Advance the atraumatic table wire to the right coronary sinus, always leading the guide catheter and monitoring for proximity to the coronaries. The image intensifier should be in the LAO position, 20-25 degrees.
• Mindfully deliver the guide catheter to the right sinus over the wire, then remove the wire..
• The guided is actively aspirated with a syringe to remove 2-4 mL of blood and attached to the manifold or power injector to allow for pressure transduction and test injections.
• To cannulate the vessel, the catheter is usually torqued clockwise to direct towards the RCA ostium to engage.
• Small “in-and-out” movements of the catheter can transmit torque to the guide tip.
• If the catheter is pointing slightly above or below the vessel, remove some but not all of the torque and correct the position before retouring into place.
• For ease and efficiency, the coronary guidewire should already be within the guide and immediately advanced into proximal RCA once the guide is cannulated.
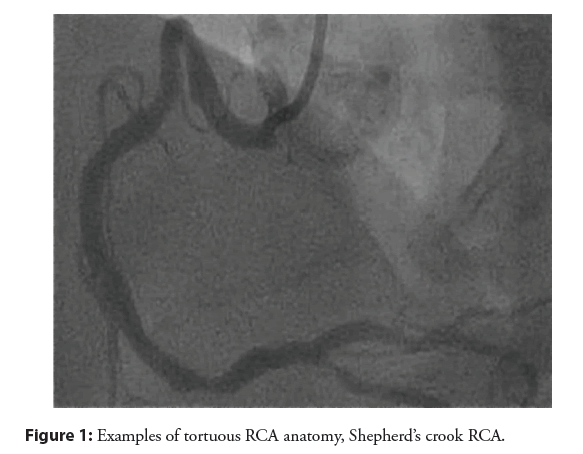
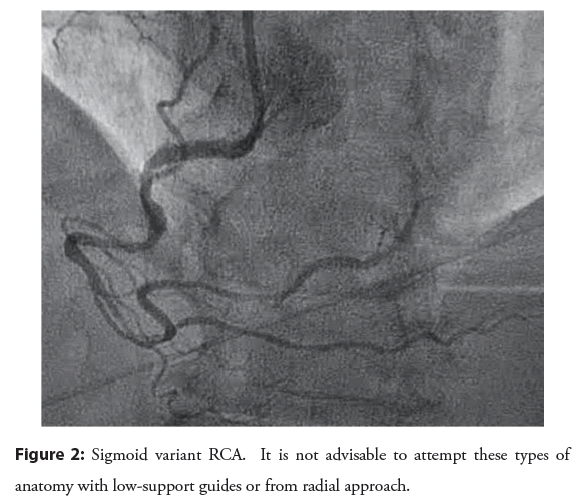
The RCA shepherd’s crook and sigmoid variants need to be given their due course: These are not easily approached from the radial access. Tortuous RCA vessels can create difficulty wiring, difficulty in advancement of devices, and vessel straightening can create pseudolesion and/or reduction in coronary flow. Shepherd’s crook has association with procedural complication. AL1 will offer the best support [9]. Commonly stocked guides that can be employed included AL1, AR1, HS, IMA. If you have taken up a JR4 and recognize a “crook” or sigmoid vessel, replace it before proceeding with the case as shown in the Figures 1 and 2. Wiring of these vessels should be done very slowly and cautiously with use of coil- tipped/atraumatic wires.
Figure 2: Sigmoid variant RCA. It is not advisable to attempt these types of anatomy with low-support guides or from radial approach.
Anomalous variants: For a circumflex from right cusp (a very common variant) consider multipurpose, AL-1/AL 0.75, AR1, or HS. Usually this variant is somewhat downgoing and can be cannulated with counter-clockwise torque and advancement, similar as a right coronary vein graft. In separate circumflex/LAD ostia (no left main) or short left main, torque clockwise for Cx and use longer tipped catheters such as extra-backup shape or Amplatz shapes. Torque counterclockwise for LAD and use a shorter tip such as Judkins or Tig. Counterclock and pushing forward can sometimes redirect a catheter from the Cx into LAD. In the case of RCA from left cusp, consider JL3.5 or AL guides.
Cannulation of coronary bypass grafts
Left-sided vein grafts come off aorta anterior, and can be most easily and quickly cannulated in an RAO view:
• Commonly used shapes Judkins right catheter, Amplatz right, Left Coronary Bypass (LCB), and Amplatz left shapes.
• The tip of the catheter is positioned towards the right of the screen in the RAO view.
• Slight in-and-out movements along whilst simultaneous torquing of the guide will allow it to “catch” the ostium of the graft.
• If the graft cannot cannulated at first, incrementally torquing the guide (such as between 1 and 5 o’clock) and repeating slight in-and-out movements is usually successful. If the tip is not reaching the ostium due to the size of the aorta, AL guides will often be the solution.
• Be aware that the surgeon will usually attach the grafts vertically and/or horizontally adjacent to the others.
• If the graft is arched or upgoing, or disease is complex, AL guides (and even potentially upsizing to a larger French size) are advisable. The technique is to form the AL shape to point upward at the root and withdraw to ostium of graft.
Right-sided grafts typically come off aorta right, and are usually cannulated in the LAO view:
• Judkins right catheters can be used, especially if initial course of the vessel is horizontal.
• If primarily downgoing (which is usually the case), multipurpose guide offers coaxial shape and potential for deep-seating techniques if necessary. Right Coronary Bypass can also be used (RCB).
• The catheter is delivered to the zone above the RCA ostium and rotated with counter-clockwise torque, with small in and out movement, and contrast injection.
• Having a coronary wire within the guide can be helpful to rapidly engage the graft once the guide catches the ostium.
Internal mammary grafts are usually approached with specific IMA guide, although JR4 and LCB can be used. LIMA is cannulated in shallow 10 degrees RAO view. The catheter is delivered over an 0.035” wire distal to the IMA, and slowly pulled back with slight counter-clockwise torque and contrast tests, until it drops into place. Rarely, a coronary wire can be used as a rail to enter LIMA ostium. If the lesion in question is at distal aspect of LAD, a 90 cm guide may be necessary to deliver balloon catheters to lesion.
Guide catheter extension: Simplification of the mother/ daughter technique is achieved using guide catheter extension such as GuideLiner (Vascular Solutions Maple Grove, MN) or Guidezilla (Boston Scientific, Natick, MA) and similar products. These devices can enhance support of a standard guide during the case to deliver devices distally, although are not without some safety concerns. It is good practice to attempt use of at least a buddy wire prior to proceeding with a guide extension given lesser cost and risk. Concerns with the extension catheters include coronary dissection and stent deformation, which occurred in 3.3% and 2.2% of cases, respectively, in one series [10]. In a review of 65 voluntarily reported adverse events to the FDA MAUDE database, issues attributed to GuideLiner included catheter fracture (58%), damage to PCI devices (23%), dissection (14%), perforation (3%), and thrombus (1.5%); with an even higher number of complications overall (408) reported with the Guidezilla [11].
To employ these devices, an appropriately sized extension catheter (from 5F to 8F) is advanced over the previously placed guidewire just to the tip of the guide, followed by a balloon suitable to the target lesion. Injection pressures should be reduced once the catheter is in place to avoid hydrostatic dissection. The balloon is then advanced to the target lesion and inflated. The catheter must be advanced over an inflated balloon to mitigate risk for dissection. If binding proximal to desired position, the extender can be “inch-wormed” over a deflating balloon as it advanced step-wise though the vessel. The extender is gradually withdrawn as the operator works more proximally. Be extremely vigilant on withdrawal of undelivered stents though an extension catheter, as there is substantial risk of dislodging the stent.
Coronary guidewires
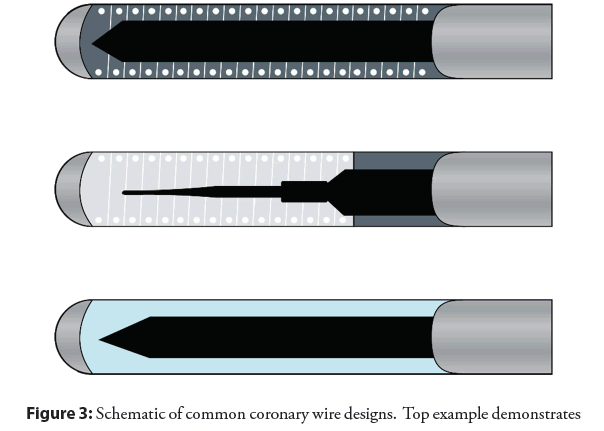
The standard diameter of a coronary wire is 0.014”, the standard length is 190 cm the distal 20-30 mm of which is radiopaque, whilst 300 cm is the “long” version. There are 3 basic components to a coronary guidewire; the core which provides the backbone of the wire, the shapeable tip, and outer coating [12]. The core is variably made of stainless steel which is more rigid and tends to transfer torque, or nitinol alloy which is more flexible and less likely to kink, but reduces transmission of movement. The more distal the core extends (i.e., core-to-tip), and the larger diameter of the core, the more pushable and torque responsive the wire will be; while smaller core diameter and/or use of tapering offers better flexion. The outer coating is commonly hydrophilic, becoming slippery on exposure to blood, improving trackability and “seeking” of channels as well as penetration of thrombus are shown in the Figure 3. Several basic categories exist including workhorse (routine) wires, support wires, and specialty wires (typically CTO). It is not necessary to have exhaustive knowledge of all products available, though familiarity of the basic categories and facility with wires within each type is essential. Wires differ in support, tip rigidity, steerability, trackability, and lubricity. Ultimately the operator will develop his or her preferences.
Figure 3: Schematic of common coronary wire designs. Top example demonstrates core-to-tip design, providing torquability and penetrating power (example Asahi Miracle Bros). Middle example demonstrates a two-component design with tapering of the core which falls short of the tip, with coil shaping ribbon (example Abbott BMW). Bottom example demonstrates polymer-jacketed wire providing lubricity and trackability (example Abbott Whisper).
• Workhorse wires are usually medium body with the core stopping short of the tip, and typically have a radiopaque metal shaping ribbon comprised of a spring coil (steel, platinum, or tungsten) allowing for ease of tracking through the vessel and high level of safety at the distal vessel. These wires are best for straightforward interventions. Workhorse wires may have a silicone coating.
• Specialty CTO wires may lack the distal coil portion, instead employing a hydrophilic jacket (such as PTFE or any number of proprietary polymers) over the distal tapering core with variable tip loads. The hydrophilic coating attracts water on exposure blood creating a lubricious surface for improved trackability in tortuosity and entry of small channels or thrombus. This occurs at the expense of higher risk of perforation.
• Support wires are heavy body wires that tend to have steel cores allowing for extra stiff shafts to enhance balloon and/ or stent deliverability as shown in the Table 1. It is good practice to exchange support wires through delivery catheters or over the wire balloon after wiring with a more forgiving device. Straightening of vessels can lead to “accordion effect” or pseudolesion, which can impede flow and visualization. On the boards (and in practice), withdraw the wire until the floppy distal portion sits within the lesion, administer nitroglycerin, and reassess.
| Wire type | Typical features | Examples |
|---|---|---|
| Workhourse wire | Medium body | Balanced Middle Weight (BMW) (Abbott Vascular) |
| Coil tip | Choice floppy (Boston scientific) | |
| Core stops short of tip | Forte (Boston) | |
| Can have hydrophilic coating | Luge (Boston) | |
| Hydrophilic CTO wire | Variable body | Whisper (Abbott) |
| Often polymer covered tip | Fielder (Asahi Intecc USA) | |
| Tip can be tapered | Pilot series (Abbott) | |
| Aid entry of side branches and small channels | Choice PT floppy (Boston) | |
| Thrombus seeking (STEMI) | Choice PT extra support (Boston) | |
| Penetrating CTO wire | Stiff body | Miracle bros series (Asahi) |
| tapered tip | Confianza (Asahi) | |
| Used for penetrating/drilling Risk of preformation | Gaia series (Asahi) | |
| Support wire | Heavy body | Iron man (Abbott) |
| Coil tip | Mailman (Boston) | |
| Coil can extend to tip | Choice extra support (Boston) | |
| Enhances device delivery | Grand slam (Asahi) | |
| Straighten vessels can be used as "Buddy" wire | All star (Abbott) |
Table 1: Classification and characteristics of commonly used coronary guidewires.
Coronary angioplasty balloons
Angioplasty balloons in current practice are more commonly used to pre or post-dilate lesions to facilitate stenting as opposed to stand-alone “plain old balloon angioplasty” (POBA). Modern balloons are comprised of a metal hypotube shaft with a channel for the guidewire and a channel for hydrostatic inflation with saline/ contrast. The balloon is advanced over a previously positioned guidewire. If the guidewire channel runs the length of the catheter, the system is considered “Over The Wire” (OTW); and if the wire only runs the distal portion, the system is considered Rapid exchange (RX). Rapid exchange is far more commonly employed. Over the wire balloons can be lower profile, and allow for exchanging of wires at or beyond a lesion, which is often desirable in CTO work. “Nominal” inflation size indicates the diameter achieved when a balloon is inflated to recommended pressure without external forces. Most balloons have two marker dots, one at each end of the inflated portion. Smaller devices ≤ 1.5 mm may have a single marker.
The balloon material itself can be nylon, Polyolefin Copolymer (POC), and Polyethylene Terepthalate (PET) which has increased strength and higher resistance to stretch, making PET useful for noncompliant balloons [13]. Specifically, a compliant (commonly nylon or POC) balloon will have up to 20% size increase from nominal size with supranominal inflation. They are lower profile, flexible, and easy to deliver. Compliant balloons tend to “grow” outside of a resistant zone of lesion in areas of less resistance, which can damage adjacent tissue, cause dissection, or even restenosis in ballooned areas. This is also described as “dog-boning”. Noncompliant balloons (commonly PET) allow for<10% size increase from nominal; are stiffer, thicker, and more difficult to deliver in difficult anatomy. They will hold shape better resulting in less growth outside a lesion (hence theoretically less chance of dissection), providing more force against a resistant lesion. Noncompliant balloons are best for somewhat calcified lesions and for post-dilation of stent. Semi-compliant balloons fall somewhere in the middle; this applies to stent-delivery balloons. The choice of balloon often involves compromise of deliverability, crossability, and compliance. The “rated burst pressure” of a balloon refers to the pressure at which 99% of a given balloon would not burst, while the “mean burst pressure” is the pressure at which 50% will burst, and therefore will be a higher value.
Logistically, when performing POBA, a successful result is considered<50% residual stenosis and presence of TIMI-3 flow, in contradistinction to stenting success which requires<20% residual stenosis [14]. The balloon is commonly sized at 1.0 to 1.2:1 for “POBA-only” result. Undersizing (<1.0:1) compared with oversizing (>1.1:1.0) appears to reduce acute complications including perforation and dissection [15]. POBA is often performed in the range of minutes as opposed to seconds. Vessel spasm can be treated with bolus dosing of intracoronary nitroglycerin at doses of 100-500 mcg. Adherent thrombus can be managed with intracoronary or peripherally administered GPI medications (abciximab, eptifibatide, tirofban), or use of aspiration thrombectomy catheters. Acute vessel recoil is considered a major limitation of POBA, and when severe is treated with stenting (this was the original function for bare metal devices). Dissection is most commonly also treated with stenting although there has historically been a role for the GPI medications to prevent thrombosis [16]. Angiographic reassessment after several minutes may be useful to assess for acute recoil or closure.
The mechanism of Coronary Angioplasty (PTCA) is compression and fracturing of plaque, stretching of adventitia, and intimal tearing which universally occurs but may or may not be visible angiographically. Similar to PCI with stenting, an inflammatory reaction occurs at sites of PTCA which ultimately results in proliferation of smooth muscle and extracellular matrix. Unlike stenting, there is also contraction of the external elastic lamina that occurs that accounts for>60% of the lumenal loss (“negative remodelling”) [17]. The Achilles heel of POBA is restenosis which occurs at a rate of 40%-60% at 6 months [18]; clinical target lesion is revascularization is also quite high at~20% at 1 year which is reduced by stenting and even further with drug eluting stent [19-21].
When deciding to pre-dilate a lesion before stenting, one considers procedural duration, cost, safety, and efficiency. If attempting to directly pass a stent through difficult vessel anatomy or into a complex lesion, it is inefficient if not outright risky to do so if the stent proves to no-cross. Part and parcel with stent removal is the risk for stent dislodgement within the vessel or at the guide. It is also extremely undesirable to deploy stent at a lesion that does not yield to ballooning, so-called “stent regret”, as options to rectify a suboptimally deployed stent are limited. Lesions favorable for direct stenting include soft plaque, proximal lesions, short lesions, vein grafts, and in ACS cases to exclude adhering thrombus. Lesions likely to benefit from pre-dilatation are visibly calcified lesions (to ensure full expansion and stent delivery), long lesions (to ensure proper stent length), distal lesions, and critical lesions (to allow proper angiography during stent placement). Pre-dilatation should be done with conservative sizing and pressure, with the balloon commonly sized at 0.9:1 to the reference vessel.
Cutting/scoring Balloons (CB) employ atherotome blades or wires affixed to the balloon to improve focal force and reduce slippage of the balloon in restenotic or ostial lesions, and potentially reduce dissection from barotrauma. With the added material, the crossing profile is much higher than a standard balloon and rivals that of a stent-perhaps worse than later generation stents. A good rule of thumb is that if a cutting balloon will cross, so will a stent.
There is no benefit in terms of clinical or angiographic outcomes for stand-alone CB compared with POBA for treatment of atherosclerotic CAD, or for treatment of in-stent restenosis; with a signal to disadvantage for risk of perforation [22-24]. In the case of in-stent restenosis, there may be a slight benefit for CB over POBA before restenting to reduce risk of future ISR [24]. For ostial lesions, there also may be a slight benefit to improve acute gain and lessen subsequent restenosis for ostial lesions based on small trials [25,26]. The PCI guidelines support use of CB for ostial lesions or ISR as a measure to avoid trauma to the vessel with balloon “seeding” (Class IIb) but not for routine use (Class III no benefit) [27]. Cutting balloons may also be logistically beneficial in otherwise undilatable or moderately calcified lesions. The ROTA- CUT trial showed improved lumenal gain with use of CB after rotational atherectomy [28].
Performing a basic coronary intervention
Based on the features of the available equipment as described, and operator experience with each, several decisions are made immediately in a given PCI case as part of a methodical approach: access site, anticoagulation, guide shape and size, wire choice, pre-dilatation, calcium modification, stent type and size, adjunct imaging, post-dilatation, and side branch management. Clever management of these variables will help the case to go smoothly and quickly, and the operator will find that his or her choices will seem to become increasingly clever over time.
Access site is chosen based on previously described considerations, with the femoral approach typically reserved only for more technically involved cases. Anticoagulation must be given before a wire hits the target vessel, which can be tailored to access; such that bivalirudin can be considered in femoral cases to attenuate bleeding concerns [29], and heparin preferable for radial access cases given lack of bleeding benefit from this approach [30]. The guide shape and caliber will be selected based on the degree of difficulty one expects based on angiographic findings, and what devices will likely need to be delivered. Guide fitment and position is continuously monitored based on the pressure waveform, and also visually during insertion and removal of devices, to avoid dissection. The coronary wire in a routine case is selected based on proximity of the lesion, tortuosity and calcification of the vessel, presence/ absence of thrombus. The wire is steered gently into position with free movement of the wire tip never passed with force. If wire debacle is identified, the wire must be withdrawn and redirected, allowed to “find its way”.
Pre-dilatation as a routine measure, while adding small amount of time and cost to the case, can help to size stent and ensure vessel yielding before stent passage. Balloon inflation pressure should be the minimum amount necessary for full expansion. Drug-eluting stenting is considered the gold standard and typically follows pre- dilatation. Stents should be generously sized for length to avoid requirement for another device, as this adds cost and also risk for future restenosis. Stents should be deployed to high pressure at least 16 atm as a starting point. Consideration should be given to a prolonged inflation with consistent maintenance of pressure with the indeflator to allow for full vessel expansion and improved stent apposition [31,32].
When balloons are being removed, remember that the counterforce acts to “suck” the guide catheter into the ostium, which has to be balanced by consistent back pressure on the guide with the left hand, especially when withdrawing balloons from freshly deployed long stents or rigid vessels. This is a very common cause of vessel dissection. The balloon should be allowed to fully deflate before removal. It is advantageous to keep the guide catheter tip in view while the balloon is being freed from the lesion using a staccato tugging maneuver. One can also employ a forward push first, akin to freeing one’s fingers from a finger trap.
When deploying a stent, it is worthwhile to be aware that under deployed stents become restenotic stents, and severely under deployed or malapposed stents can become thrombosed stents down the line. The goal minimal stent diameter (MSD) is typically>5.0 mm2 for a DES and>6.5 mm2 for BMS to ensure future vessel patency, and therefore IVUS can be considered to optimize stent deployment as well [33]. If a vessel is calcified angiographically, or by IVUS (which is widely considered more accurate in determination of vessel calcium), calcium management should be employed before stenting. This may include noncompliant ballooning, cutting balloon (up to moderate calcium), and lithotripsy or atherectomy (severe calcium). If a stent is not expanding fully due to vessel rigidity, options can include inflation of post balloon until burst (grenadoplasty), ultra-high-pressure ballooning such as with Chocolate balloon (Medtronic Minneapolis, MN), and lithotripsy with Shockwave (Shockwave Medical Santa Clara, CA). Use of laser with contrast or blood medium is described [34], but in the author’s experience is not particularly effective.
Post-dilation of stents should be done as a general rule, with a non-compliant balloon~0.25 mm larger than the stent and shorter than the stent length [35]. This is especially true for longer lesions, overlapping stents, and smaller vessels. The realities of stent underexpansion with the stent balloon are somewhat sobering– based on the POST-IT IVUS based study,>70% of stents were suboptimally deployed with the stent balloon alone in that the MSD was at least 10% less than the averaged vessel area. Post- dilatation essentially doubled optimum stent deployment and significantly increased MSD [36]. In the STOP study, only 21% of stents achieved optimized deployment at 16 atm (i.e. MSD achieved was>60% distal reference vessel), which increased to 54% with going to 20 atm [37]. In a large registry of>90,000 patients, low pressure<15 atm or very high pressure>20 atm stent deployment were both associated with ISR and stent thrombosis [38]. In smaller vessels, or longer lesions with tapering length, the stent should be sized to the distal reference and deployed to moderate pressure (10 atm-14 atm) to avoid distal vessel injury, followed by higher pressure post-dilation on the proximal aspect. Always reassess with at least 2 orthogonal views, and with the wire withdrawn, to assess whether any proximal or distal complication has occurred. Liberalized use of nitroglycerin can help discern spasm vs. true lesion, and will save placement of further devices.
Discussion
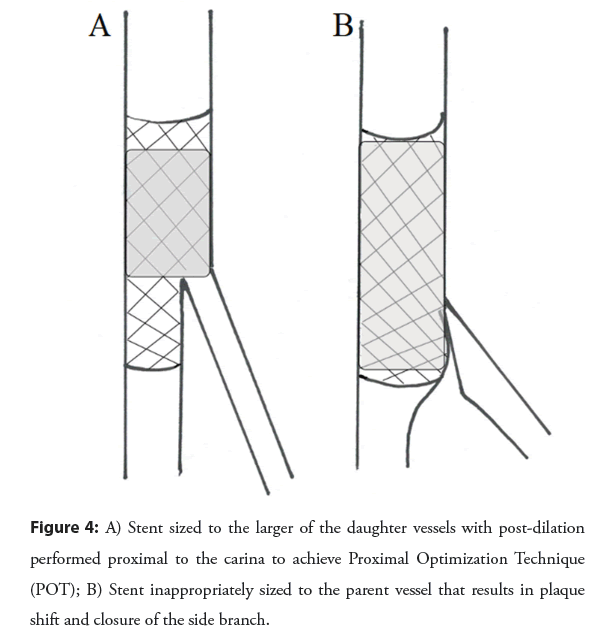
Bifurcation lesions should be approached as possible with a single stent technique, given failure of multiple trials to demonstrate any clinically important advantage to up front multi-stent techniques [39]. It is good practice to at least place a wire in the Side Branch (SB) to allow for a measure of protection against plaque shift and mark the position of the branch as well [40]. After main vessel stenting, provisional ballooning of the SB, followed by provisional T-stenting only as necessary, is practical and efficient. Proximal Optimization Technique (POT) should be done to ensure proximal apposition and facilitate SB re-wiring, and repeated if any ballooning has been done through the stent struts to reappose struts (POT/ SB/ Re-POT) as shown in the Figure 4. POT/SB/Re- POT achieves better vessel circularity than kissing balloon [41].
This simple technique was also associated with reduction in target lesion failure (4% vs. 6%) and stent thrombosis (0.4% vs. 1.3%) when compared with no POT in a large bifurcation registry [42].
Failure to pass a stent
This is encountered routinely and is “bread-and-butter” interventional cardiology. It is important to anticipate this issue and pro-actively employ strategies to avoid it. It is also important to have a plan of attack if this is encountered during the case. Reasons that a stent may fail to cross include rigidity of the vessel, tightness of the lesion, tortuosity of the vessel, redundancy of the vessel (as in the case of a “sigmoid” RCA), poor guide support, poor wire support, and stent biasing against the vessel wall or previous stent. Calcium is a major challenge in these situations and may need to be specifically dealt with. Reflex approaches when a calcified and/or tortuous vessel is being treated include upsizing to 7F and/or coming from the groin, use of supportive guides, further lesion prep with pre-dilatation, and use of buddy wires. Guide extension catheters have also become quite commonplace.
Techniques to employ when unable to deliver a stent to the target lesion
Tactics to deliver a stent:
• Anticipate based on lesion characteristics.
• Use groin approach and/or 7F if calcified or tortuous.
• Use guide catheters with passive support.
• Deep seat guide or have assistant apply forward pressure.
• Use long sheath to support the guide.
• Deep breath to straighten segment of vessel.
• Buddy wire with a delivery wire.
• Try both wires before next maneuver.
• Predicate with noncompliant ballon.
• Predicate zone proximal to lesion if necessary.
• Steady forward pressure.
• Rapid bursts of forward pressure.
• Guide extension catheter over inflated ballon.
• Shorten the stent to reduce biasing.
• Change to lower profile or more flexible stent.
• Apply rotaglide to stent to improve tracking.
• Buddy ballon to trap wire distal to lesion, or inflated in a proximal side branch.
• Buddy wire “in jail” by trapping buddy with a stent more proximally.
• Wiggle wire to reduce biasing.
• Rotational or orbital atherectomy to smooth vessel course.
• Accept PTCA result (recall~20% clinical failure).
Failure to pass a balloon: This is encountered much less frequently than failure to pass a stent and is a challenging situation indeed, most often in severely calcified lesions, CTOs, or through stent struts. Many of the same tactics used to beef up delivery equipment also apply [43]. One is uniquely challenged in that the lesion has already been crossed with a wire that presumably cannot be exchanged, unless a balloon or microcatheter can be passed, which is begging the question. A very low-profile balloon such as Sapphire balloon (Teleflex, NC) should be tried, which has sizes down to 1 mm. If a buddy wire can be passed, inflation of a balloon over one of the wires at the proximal aspect of the lesion may exert pressure on the second wire to “pry” into the cap. A balloon can be advanced as far as possible and inflated until rupture to allow for hydrostatic modification of the lesion (grenadoplasty). Conversely, a balloon sized to the vessel proximal to the lesion can be inflated to crack the cap. A CTO microcatheter can be tried to cross the lesion to allow for exchange of a wire with more body. Finally, if suitable wire can be delivered, laser or rotational atherectomy can be employed.
Conclusion
In summary, working knowledge of day-to-day coronary equipment is important for the interventional cardiologist to improve procedural efficiency and outcomes. Exhaustive knowledge of all available equipment is neither necessary nor practical, as the operator becomes familiar and proficient with a smaller array of guides, wires, balloons, etc. that work best in his or her hands. A standard approach for PCI can be employed in the majority of routine cases, and hopefully can serve as a starting point for cases that prove to be more complex. In all cases, PCI must be approached methodically. One learns to control the variables that can be controlled such as access, anticoagulation, the nature of the equipment, the use calcium modification and/or adjunct imaging, and simple measures such as post-dilatation. As experience grows, the operator will find that his or her choices will seem to become increasingly clever over time. Importantly, there will also be variables that cannot be controlled. Therefore, the interventionist must always anticipate possible challenges and complications, and mindfully employ tools techniques and tools from the outset to improve procedural and clinical outcomes.
References
- Ali R, Greenbaum AB, Kugelmass AD, et al. A review of available angioplasty guiding catheters, wires, and balloons making the right choice. Interv Cardiol. 7(2):100-103 (2012).
- Di Mario C, Ramasami N. Techniques to enhance guide catheter support. Catheter Cardiovasc Interv. 72(4):505-512 (2008).
- Bruyne De, Stockbroeckx J, Demoor D, et al. Role of side holes in guide catheters: Observations on coronary pressure and flow. Catheter Cardiovasc Diagn. 33(2):145-152 (1994).
- Grossman M, Gurum HS, Richard N, et al. Percutaneous coronary intervention complications and guide catheter size: Bigger is not better. JACC: Interventions. 2(7):636-644 (2009).
- Agostoni P, Zoccai G, Benedicits L, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 44(2):349-356 (2004).
- Ando G, Capodanno D. Radial versus femoral access in invasively managed patients with acute coronary syndrome: A systematic review and meta-analysis. Ann Intern Med. 163(12):932-940 (2015).
- Ikari Y, Nagaoka M, Kim JY, et al. The physics of guiding catheters for the left coronary artery in transfemoral and transradial interventions. J Invasive Cardiol.17(12):636-641(2005).
- Nguyen T, Douglas J, Hermiller J, et al. Guides and wires. J Interv Cardiol. 14(1):113-133 (2001).
- Grossman DE, Tuzcu EM, Simpfendorfer C, et al. Percutaneous transluminal angioplasty for shepherd’s crook right coronary stenosis. Catheter Cardiovasc Diagn.15(3):189-191 (1988).
- Waterbury TM, Sorajja P, Bell MR, et al. Experience and complications associated with the use of guide extension catheters in percutaneous coronary intervention. Catheter Cardiovasc Interv. 88(7):1057-1065 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Chen Y, Shah AA, Shlofmitz E, et al. Adverse events associated with the use of guide extension catheters during percutaneous coronary intervention: Reports from the Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 2019; 20(5):409-412.
[CrossRef] [Google Scholar] [PubMed]
- Erglis A. Coronary guidewires. EuroIntervention. 6(1):168-169 (2010).
- Fornel D. Overview of angioplasty balloon technology advances. Diagn Interv Imaging. (2021).
- Smith SS, Dove JT, Jacobs AK, et al. ACC/AHA guidelines for percutaneous coronary intervention. Circulation. 103:3019-3041(2001).
- Roubin GS, Douglas JS, King SB, et al. Influence of balloon size on initial success, acute complications, and restenosis after percutaneous transluminal coronary angioplasty. A prospective randomized study. Circulation. 78(3):557-565 (1988).
- Bergelson BA, Fishman RF, Tommaso CL, et al. Abrupt vessel closure: Changing importance, management, and consequences. Am Heart J. 134(3):362-381 (1997).
- Mints GS, Popma A, Pichard AD, et al. Arterial remodelling after coronary angioplasty: A serial intravascular ultrasound study. Circulation. 94(1):35-43 (1996).
- Thornton MA, Gruentzig AR, Hollman J, et al. Coumadin and aspirin in prevention of recurrence after transluminal coronary angioplasty: A randomized study. Circulation. 69(4):721-727 (1984).
- George CJ, Baim DS, Brinker JA, et al. One-year follow-up of the stent restenosis (STRESS I) study. Am J Cardiol. 81(7):860-865 (1998).
- Macaya C, Serruys PW, Ruygrok P, et al. Continued benefit of coronary stenting versus balloon angioplasty: One-year clinical follow-up of Benestent trial. J Am Coll Cardiol. 27(2):255-261 (1996).
- Brophy JM, Belisle P, Joseph L, et al. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med. 138(10):777-786 (2003).
- Mauri L, Bonan R, Weiner B, et al. Cutting balloon angioplasty for the prevention of restenosis: Results of the cutting balloon global randomized trial. Am J Cardiol. 90(10):1079-1083 (2002).
- Albiero R, Silber S, Mario C, et al. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenenosis: Results of the Restenosis Cutting Balloon Evaluation Trial (RESCUT). J Am Coll Cardiol. 43(6):943-949 (2004).
- Albiero R. Cutting balloon versus conventional balloon angioplasty for the treatment of coronary artery disease. Eur Cardiol. 1(1):48-52 (2005).
- Muramatsu T, Tsukahara R, Ho M, et al. Efficacy of cutting balloon angioplasty for lesions at the ostium of the coronary arteries. J Invasive Cardiol. 11(4):201-206 (1999).
- Kurbaan AS, Kelly PA, Sigwart U, et al. Cutting balloon angioplasty and stenting for aorto-ostial lesions. Heart. 77(4):350-352 (1997).
- Levine GN, Bates ER, James C, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American college of cardiology foundation/ American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 124(23):e574-e651 (2011).
- Furuichi S, Tobaru T, Asano R, et al. Rotational atheterectomy followed by cutting-balloon plaque modification for drug-eluting stent implantation in calcified coronary lesions. J Invasive Cardiol. 24(5):191-195 (2012).
- Lincoff MA, Bittl J, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIa/IIIb blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention. JAMA. 289(7):853-973 (2003).
- Shazad A, Keep I, Mars C, et al. Unfractionated Heparina versus Bivalirudin in Primary Percutaneous Coronary Intervention (HEAT-PPCI): An open-label, single centre, randomised controlled trial. Lancet. 384(9957):1849-1858 (2014).
- Cook J, Mhatre A, Wang W, et al. Prolonged high-pressure is required for optimal stent deployment as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 83(4):521-527 (2014).
- Saad M, Bavineni M, Uretsky B, et al. Improved stent expansion with prolonged compared with short balloon inflation: A meta-analysis. Catheter Cardiovasc Interv. 92:873-880 (2018).
- Sonoda S, Morino Y, Ako J, et al. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: Serial intravascular ultrasound analysis from the SIRIUS trial. JACC. 43(11):1959-1963 (2004).
- Egred M. A novel approach for under-expanded stent: Excimer laser in contrast medium. J Invasive Cardiol. 24(9):e161-e163 (2012).
- Lui HK. The perfect fit: Getting the most out of your coronary stent. (2005).
- Brodie BR, Cooper C, Jones M, et al. Is adjunctive balloon postdilation necessary after coronary stent deployment? Final results from the POSTIT trial. Catheter Cardiovasc Interv. 59(2):184-192 (2003).
- Rana O, Shah NC, Wilson S, et al. The impact of routine and intravascular ultrasound-guided high-pressure post-dilatation after drug-eluting stent deployment: The Stent Optimization (STOP) study. J Invasive Cardiol. 26(12):640-646 (2014).
- Frobert O, Sarno G, James SK, et al. Effect of stent inflation pressure and post-dilatation on the outcome of coronary artery intervention. A report of more than 90,000 stent implantations. PLoS ONE. 8(2):e56348 (2013).
- Zimarino M, Corazzini A, Ricci F, et al. Late thrombosis after double versus single drug-eluting stent in the treatment of coronary bifurcation: A meta-analysis of randomized and observational studies. JACC Cardiovasc Interv. 6(7):687-695 (2013).
- Brunel P, Lefevre T, Darremont O, et al. Provisional T-stenting and kissing balloon in the treatment of coronary bifurcation lesions: Results of the French multicentre TULIPE study. Catheter Cardiovasc Interv. 68(1):67-83 (2006).
- Foin N, Juan LG, Nakatani S, et al. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: Implications for the management of incomplete stent apposition. Circ Cardiovasc Interv. 7(2):180-189 (2014).
- Chevalier B, Mamas MA, Hovasse T, et al. Clinical outcomes of the Proximal Optimization Technique (POT) in bifurcation stenting. EuroIntervention. 17(11):e910-e918 (2021).
- Dash D. Interventional management of balloon-uncrossable coronary total occlusion: Is there any way uut? Korean Circ J. 48(4):277-286 (2018).