Contrast Agent Evaluation - Imaging in Medicine (2010) Volume 2, Issue 4
Gadofosveset injection for magnetic resonance angiography
Amedeo Chiribiri1, Geraint Morton1and Eike Nagel†1
1 King’s College London, BHF Centre of Excellence, Division of Imaging Sciences, NIHR Biomedical Engineering Centre, NIHR Biomedical Engineering Centre, The Rayne Institute, 4th Floor Lambeth Wing, St Thomas’ Hospital, London, UK
- *Corresponding Author:
- Eike Nagel
King’s College London, BHF Centre of Excellence Division of Imaging Sciences
NIHR Biomedical Engineering Centre NIHR Biomedical Engineering Centre
The Rayne Institute 4th Floor Lambeth Wing, St Thomas’ Hospital, London, UK
Tel.: +44 207 188 7242
Fax: +44 207 188 5442
E-mail: eike.nagel@kcl.ac.uk
Abstract
Magnetic resonance angiography has evolved into a robust clinical tool, with improved performance of both hardware and software. However, the poor signal-to-noise ratio remains an important physical limitation. T1-shortening contrast agents help increase the signal-to-noise-ratio but conventional extracellular agents can be injected only in a limited dosage and this is followed by a rapid decrease in blood concentration caused by redistribution in the extracellular space. Gadofosveset trisodium offers a net increase in T1 relaxivity, blood-pool distribution and a longer half-life, promising to significantly improve magnetic resonance angiography image quality once again.
Keywords
cardiovascular system; gadofosveset trisodium; MRA; MS‑325
Recently, magnetic resonance angiography (MRA) has evolved into a robust clinical tool for noninvasive evaluation of vascular structures. Its clinical utility for the evaluation of thoracic, abdominal and peripheral vascular pathology is well established and recent developments in hardware and software have resulted in significant improvement of MRA performances. MRA does not necessarily require administration of contrast agent, but contrast-enhanced MRA has become standard practice owing to its faster speed and flow independence [1]. Recent research in the field of new contrast agents specifically designed for MRA has provided relevant clinical results, overcoming some of the limitations of extracellular gadolinium (Gd) chelates. Human albumin-binding contrast agents promise to significantly improve MRA compared with extracellular contrast agents. Among these compounds, the US FDA recently approved gadofosveset trisodium (MS‑325; Ablavar™, EPIX Pharmaceuticals, Cambridge, MA, USA, and Lantheus Medical Imaging Inc, MA, USA) for use in humans. This article will focus on gadofosveset trisodium’s pharmacological properties and on the evidence of its efficacy available from the literature.
Overview of the market
Several strategies for MRA have been developed using extracellular Gd chelates [2–8]. These agents are designed to reduce the T1 of the blood during first passage, decreasing the signal loss caused by saturation of proton spins in the imaging volume and providing the contrast needed to image the vascular structures. Various studies have demonstrated the superiority of contrast-enhanced MRA in comparison to noncontrast-enhanced techniques, for more accurate depiction of stenotic vessels [8–11]. However, extracellular Gd chelates have relatively low relaxivity and are known to diffuse freely out of the vasculature during first pass (up to a fraction of 50% in certain tissues such as the myocardium), limiting the magnitude and duration of T1 contrast generated. As a consequence, scanner hardware and imaging acquisition protocols have been optimized to offer very fast acquisition times to capture the vascular phase of the contrast during first passage. However, this approach limits the time available for image acquisition, only one body region can be easily imaged at a time, and either very advanced table and coil combinations or repeated injections are needed for more than one station to be imaged with optimal spatial resolution. Furthermore, since the acquisition of MRA images is only possible during first passage, the correct timing of image acquisition is pivotal to achieve the best image quality and, therefore, significant experience and training are required to optimize image acquisition. In addition, firstpass MRA of some vessels, such as the aorta and the pulmonary veins, suffers from image blurring caused by movement of the vessels during the cardiac cycle. This artifact could be avoided using cardiac-triggered sequences and a contrast agent with a longer plasma half-life.
To overcome these limitations, the market demanded a contrast agent capable of generating very high contrast between vascular structures and other tissues (high relaxivity) that also remained in the bloodstream for the duration of imaging and, thus, maintained the contrast between vascular structures and surrounding tissues for longer.
Pharmaceutical research developed three different new classes of blood-pool contrast agents rationally designed to achieve these goals: superparamagnetic contrast agents, such as iron oxide contrast agents (superparamagnetic iron oxide [SPIO] and ultrasmall paramagnetic iron oxide particles [USPIO]) [12]; dendritic Gd-binding compounds (Gadomer 17) [13]; and the class of human albumin-binding contrast agents, such as gadofosveset. Gadofosveset is currently the only blood-pool contrast agent approved by the FDA for MRA.
Introduction to the compound
Gadofosveset trisodium is a blood-pool contrast agent from the family of albumin-binding compounds. It is the first Gd-based blood-pool agent approved for MRA use in humans. Gadofosveset has been designed to overcome the limitations of conventional extracellular contrast agents in MRA. It binds albumin, resulting in three major advantages: restriction of the agent to the bloodpool, net increase of T1-shortening properties and a prolonged half-life.
Chemistry
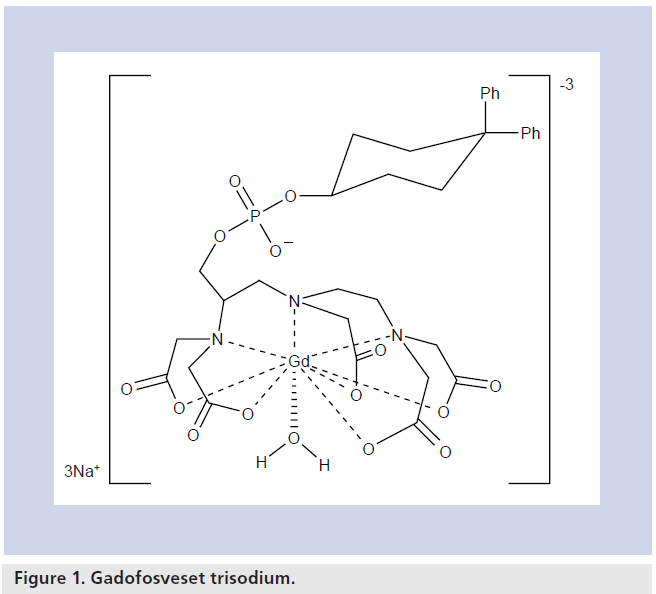
The chemical name for gadofosveset trisodium is trisodium-(2-(R)-((4,4-diphenylcyclohexyl) phosphonooxymethyl)-diethylenetriaminepentaacetato)( aquo)Gd(III). It has a molecular weight of 957 Da. The agent consists of a proteinbinding diphenylcyclohexyl group attached to a Gd chelate by a phosphodiester linkage, resulting in reversible binding to albumin [14]. Figure 1 shows the structural formula of gadofosveset. Its chemical properties contribute to a long plasma half-life and high T1 relaxivity.
Pharmacodynamics
Gadofosveset binds albumin with a strong noncovalent interaction, resulting in three major advantages, as follows.
The complex is restricted to the blood-pool, allowing selective enhancement of the vascular structures (see ‘Pharmacokinetics & metabolism’ section). As a direct consequence, the volume of distribution for the agent is reduced and a smaller amount of Gd can be administered.
Albumin binding slows down the molecular tumbling rate of the complex, providing a significant increase in T1-relaxation enhancement compared with gadopentetate dimeglumine (Gd‑DTPA) in plasma [15–18]. Vessels are strongly enhanced on 3D gradient-echo images as a result of a persistent decrease in plasma T1 [3,18]. Gadofosveset has a very high relaxivity in magnetic field strengths in the range used for MRA. It binds albumin selectively and reversibly (see ‘Pharmacokinetics and metabolism’ section) resulting, as a consequence of the molecular weight of albumin, in a slower rate of rotation in solution that is similar to the Larmor frequency of the scanner. The reduction in the molecular tumbling rate of the chelate upon binding optimizes the electron–nuclear interaction between the Gd and the water protons to yield one of the highest relaxivities observed for a Gd chelate. In this condition, a very efficient interaction between Gd and water protons occurs resulting in a higher relaxivity than smaller molecules in free solution in plasma [18]. Furthermore, gadofosveset has the advantage over other Gd-binding macromolecules of a particular interaction between the complex of Gd and its chelant and albumin. The chelant is bound to albumin by multiple noncovalent bindings, ensuring a more efficient immobilization that results in a six- to tenfold higher relaxivity when compared with Gd‑DTPA. Relaxivity is reduced at higher ratios of chelate to albumin, perhaps as a result of a weaker noncovalent binding with less efficient immobilization of the molecule [18].
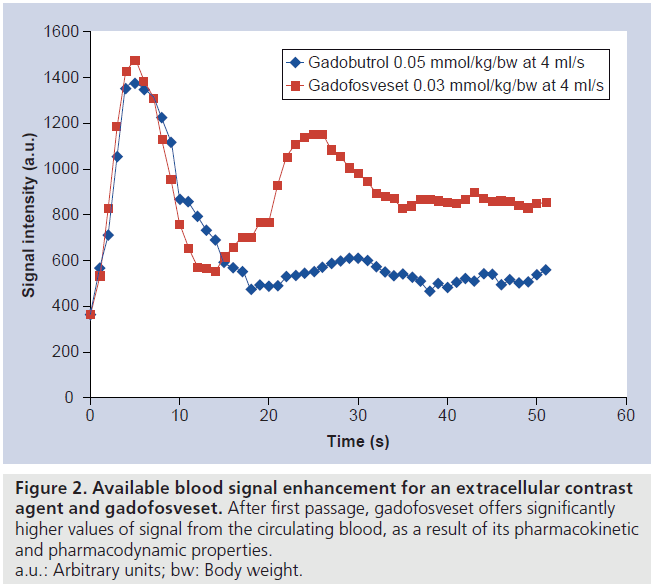
Albumin binding increases the half-life of the agent, which allows prolonged acquisition times of multiple regions of the body [19], employing high resolution sequences that can also be triggered on cardiac activity, and thus reducing artifacts at the origin of the large vessels [15]. Furthermore, as a consequence of the persistent signal-enhancing effect and high relaxivity, longer acquisition times, optimized sequences and high-resolution acquisition can be applied. Figure 2 demonstrates the available blood signal enhancement and its persistence after first passage for extracellular contrast agents and gadofosveset. These characteristics enhance MRA in all regions of the body but are particularly desirable for coronary imaging, where high-resolution and high signal-to-noise ratio are desired [20]. Furthermore, the improved acquisition time window can be used to acquire multiple scans after a single contrast injection.
Figure 2. Available blood signal enhancement for an extracellular contrast agent and gadofosveset. After first passage, gadofosveset offers significantly higher values of signal from the circulating blood, as a result of its pharmacokinetic and pharmacodynamic properties. a.u.: Arbitrary units; bw: Body weight.
The smaller the volume of distribution, the more efficient the T1-lowering effect and a prolonged half-life all significantly contribute to the benefits of MS‑325 over other Gd contrast agents used in clinical practice. The capability of generating the same contrast with the injection of a smaller volume of drug will potentially limit side effects and costs [18].
Albumin-binding contrast agents have several advantages over other types of blood-pool contrast agents. Initially, the development of bloodpool contrast agents focused on macromolecular complexes with Gd, with high relaxivity caused by the slow rotation of the complex in blood [1]. Different types of molecules were evaluated, such as albumin, dextran, polylysin and other polymers [21,22]. Their potential immunogenicity and concerns regarding the excretion of these agents, particularly after repeated injections, limited their development [23].
Apart from albumin-binding contrast agent, USPIO and SPIO are the only other family of blood-pool contrast agents. The usefulness of SPIOs for MRA is limited owing to their predominant T2 shortening effect and very short vascular half-life (less that 10 min) caused by endocytosis in the liver, spleen and other reticolo-endothelial tissues [1]. USPIOs have been evaluated for MRA, exhibiting a similar signalto- noise ratio at 1.5 and 3 T probably owing to confounding T2* shortening effect [1,24].
MS‑325 and intravascular contrast agents in general also present some limitations when compared with diffusible contrast agents. Owing to the restriction of Gd in the blood pool, there is no interstitial enhancement, thus the acquisition of relevant diagnostic information may be impossible and require a second scan with conventional diffusible contrast agents.
Another potential limitation to the use of blood-pool contrast agents for MRA (at steady state) is the venous contamination of the arterial images [25]. Several postprocessing approaches have been proposed to overcome this limitation [26–29].
Pharmacokinetics & metabolism
Preclinical imaging data, in vitro human plasma binding and signal enhancement characteristics of this agent are reported in an article by Lauffer et al. [18]. Unlike currently available extracellular agents, gadofosveset binds strongly but reversibly to human serum albumin in plasma [14,30]. Protein binding of the chelate reduces its extravasation, yielding primarily intravascular distribution and increased plasma half-life. However, efficient renal excretion is still possible as the reversible binding means that there is always a small amount of unbound agent. Gadofosveset is 80–96% bound to albumin in human plasma [18]. A special chemical group attached to a typical Gd chelate mediates protein binding [31–36]. Gadofosveset is two- to three-times more stable than Gd‑DTPA at pH 7.4 and is ten‑ to 100‑times more kinetically inert [17].
Clinical efficacy
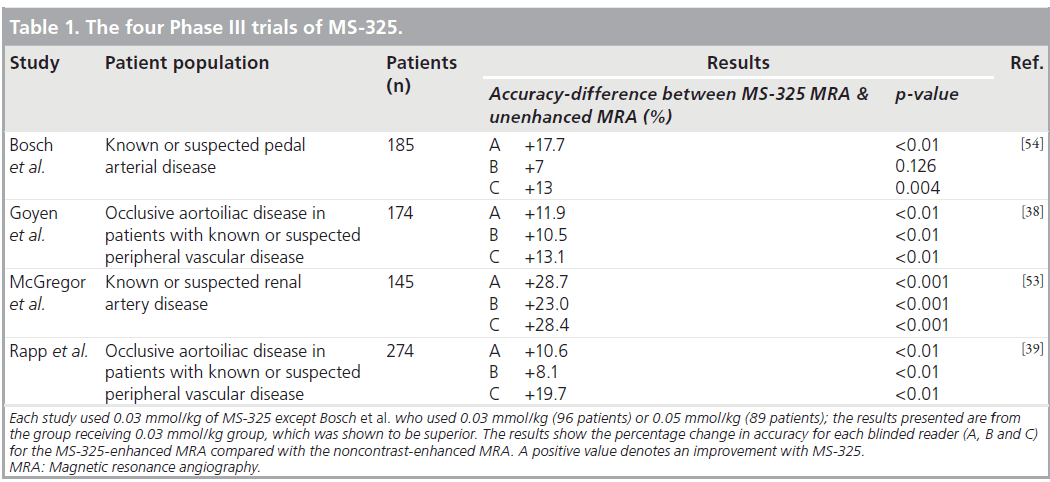
Potential clinical applications for this class of contrast agent include enhancement of vessels in MRA and determination of tissue perfusion, angiogenesis or capillary integrity [19]. Four Phase III studies have demonstrated the clinical efficacy of MS‑325 for different indications. These studies were all well designed using rigorous and state-of-the-art methodology. In all of these studies, unenhanced time-of-flight MRA was compared with MS‑325-enhanced MRA using conventional x‑ray angiography as the reference standard. Images were acquired using standardized, prospectively designed, protocols. In each study the MRA images were interpreted by three different expert, blinded, independent readers. The x‑ray angiography images were interpreted separately by two different expert, blinded, independent readers. A third adjudicator was used if required to provide a consensus. These studies are summarized in Table 1. There have also been many other published clinical studies using MS‑325 for MRA. As a result of these trials there is accumulating evidence for the utility of MS‑325 for a number of different clinical applications and these are discussed later.
Peripheral vascular disease
In a Phase II multicenter dose-finding study of 238 patients with known or suspected peripheral artery disease, MS‑325 administration resulted in a dose-dependent increase in diagnostic accuracy for the detection of occlusive aortoiliac disease. A dose of 0.03 mmol/kg of MS‑325 was found to be safe and accurate in comparison with the invasive reference standard of x‑ray angiography [37].
Two subsequent Phase III studies evaluated MS‑325 for MRA in patients with known or suspected peripheral artery disease using the methods previously described [38,39]. Both of these studies found that MS‑325-enhanced MRA was significantly more accurate than unenhanced MRA for evaluation of occlusive aortoiliac disease (Table 1). Goyen et al. found that sensitivity improved from 49–60 to 71–84%, specificity from 71–78 to 80–90% and accuracy from 68–75 to 80–88% [38]. Another benefit of MS‑325 was a significant reduction in uninterpretable images. Up to 19.5% of the unenhanced images were uninterpretable; while with MS‑325 this was reduced to up to 2.6%. The rate of not interpretable studies was similar with MS‑325- enhanced scans and with x‑ray angiography. The safety profile of MS‑325 was also found to be favorable with minimal and transient side effects reported.
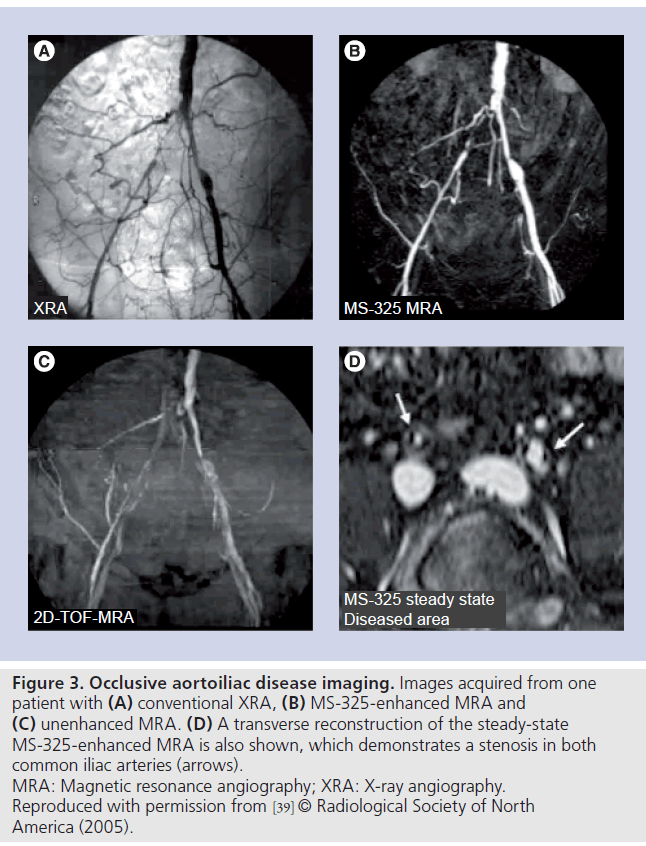
Similarly Rapp et al. found that sensitivity improved from 42–67 to 61–80%, specificity from 75–85 to 85–95% and accuracy from 71–82 to 84–90% (Figure 3) [39]. There was also a significant reduction in the number of studies that, postcontrast, were uninterpretable. This was up to 21.9% in the unenhanced images and only up to 1.2% in the contrast-enhanced MRA images.
Figure 3. Occlusive aortoiliac disease imaging. Images acquired from one patient with (A) conventional XRA, (B) MS-325-enhanced MRA and (C) unenhanced MRA. (D) A transverse reconstruction of the steady-state MS-325-enhanced MRA is also shown, which demonstrates a stenosis in both common iliac arteries (arrows). MRA: Magnetic resonance angiography; XRA: X-ray angiography. Reproduced with permission from [39] © Radiological Society of North America (2005).
Further work in patients with peripheral vascular disease suggested improved accuracy of peripheral arterial MS‑325-enhanced MRA, with digital subtraction angiography (DSA) as the reference standard, using high-spatialresolution steady-state MRA compared with standard-resolution arterial-phase first-pass MRA [40]. This was a small study with only 27 patients and it is worth noting that 100% accuracy was achieved with steady-state MRA. Another small study of 20 patients also demonstrated that MS‑325-enhanced MRA of the lower legs at 3 T is also very accurate and comparable with that of invasive selective DSA [41].
In a study of 40 patients MS‑325 performed well compared with a double dose of gadopentetate dimeglumine for first-pass whole-body MRA [42]. Quantitative assessment revealed relative contrast values that were significantly higher with MS‑325 in the vascular segments in the supraaortic/thoracic and the abdominal regions. However, the relative contrast values were lower with MS‑325 in the lower limb arteries. On qualitative analysis, MS‑325 also performed well and appeared to be at least as good as gadopentetate dimeglumine. Klessen et al. also felt that further optimization of the injection protocol may improve the results with MS‑325 as the flow rates used were based on experience with extracellular contrast media [42].
Another study of 30 patients demonstrated very high agreement between steady-state MRA with MS‑325 and DSA. Interobserver agreement was excellent for aortoiliac and femoropopliteal disease (Cohen’s k-values of 0.93 and 0.86, respectively) and was less strong but remained good for infrapopliteal disease (k-value of 0.78) [43]. The correlation between whole-body DSA and MS‑325 was not as good in another recent study (k-values of 0.44 and 0.63 for two observers). However, this was a small study of 11 patients and used first-pass rather than steady-state imaging [44].
Cardiac imaging Coronary arteries
For free-breathing, high-resolution, 3D coronary MRA, the use of intravascular contrast agents may be helpful for contrast enhancement between coronary blood and myocardium. In six patients, 0.1 mmol/kg of the intravascular contrast agent MS‑325/AngioMARK® was given intravenously followed by double-oblique, freebreathing, 3D inversion-recovery coronary MRA with real-time navigator gating and motion correction [19]. Contrast-enhanced, 3D coronary MRA images were compared with images obtained with a T2 prepulse without exogenous contrast. The contrast-enhanced images demonstrated a 69% improvement in the contrast-tonoise ratio (6.6 ± 1.1 vs 11.1 ± 2.5; p < 0.01) compared with the T2 prepulse approach. By using the intravascular agent, extensive portions (>80 mm) of the native left and right coronary system could be displayed consistently with submillimeter in-plane resolution. The intravascular contrast agent MS‑325/AngioMARK leads to a considerable enhancement of the blood/muscle contrast for coronary MRA compared with T2 preparation techniques.
Coronary angiography with MS‑325 has been developed further by Prompona et al. [45]. This study compared contrast-enhanced whole-heart coronary MRA at 3 T using a 3D flash sequence with inversion-recovery prepulse after MS‑325 injection with coronary MRA at 1.5 T using a 3D steady-state free-precession sequence with T2 preperation techniques in a group of 20 volunteers. Quantitative analysis demonstrated a higher overall contrast-to-noise ratio between coronary blood and myocardium with MS‑325- enhanced coronary MRA at 3 T. Qualitative analysis also demonstrated an improved image quality of the distal coronary segments compared with noncontrast-enhanced steady-state freeprecession coronary MRA at 1.5 T in the same group of volunteers. Similar findings were also reported by Kelle et al. who found that MS‑325 resulted in significant improvements in contrastto- noise, blood–myocardial contrast, image quality, visible vessel length and vessel sharpness over noncontrast imaging at 1.5 T in 16 volunteers [46].
Cardiac venous system
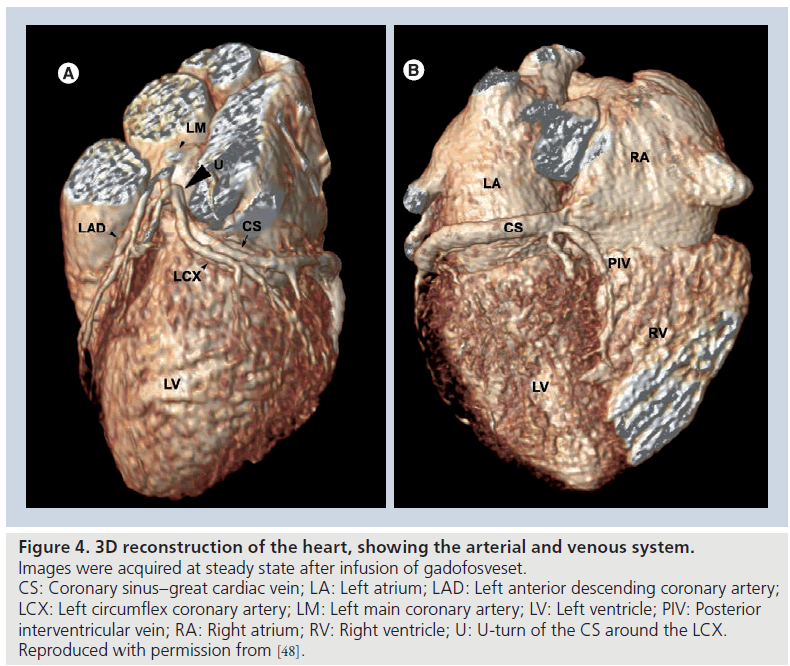
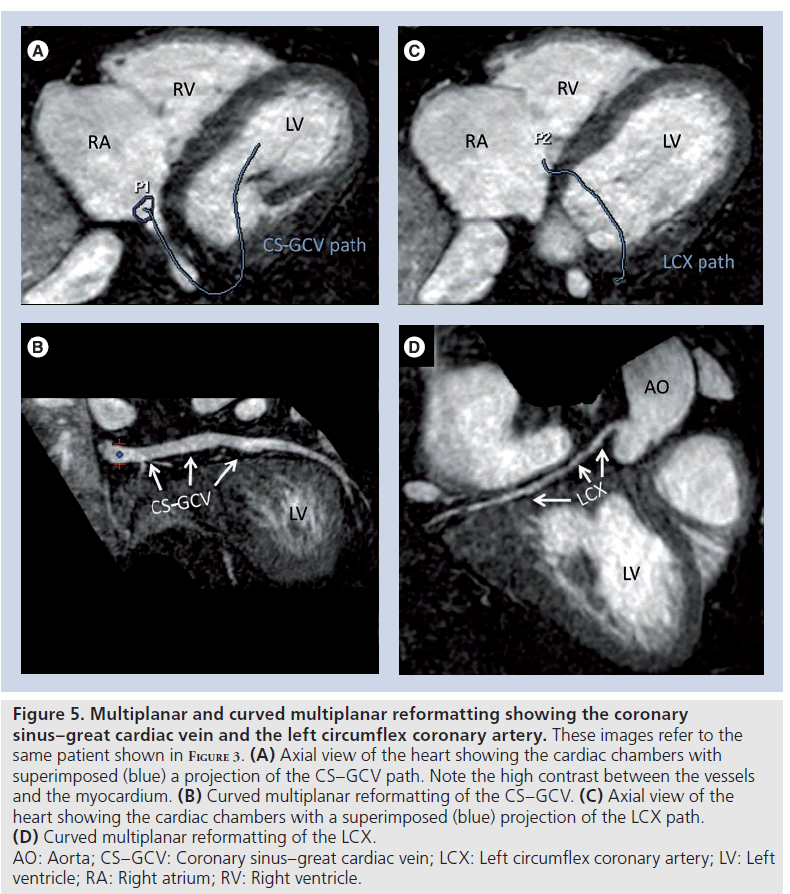
Gadofosveset has been successfully used to visualize the cardiac venous system [47,48]. For this particular application, the use of T1 shortening contrast agents is one of the possible technical solutions, since T2 preparation techniques for contrast generation are less effective on deoxygenated venous blood and a relatively long acquisition time is needed for high-resolution scans (Figures 4 & 5).
Figure 4. 3D reconstruction of the heart, showing the arterial and venous system. Images were acquired at steady state after infusion of gadofosveset. CS: Coronary sinus–great cardiac vein; LA: Left atrium; LAD: Left anterior descending coronary artery; LCX: Left circumflex coronary artery; LM: Left main coronary artery; LV: Left ventricle; PIV: Posterior interventricular vein; RA: Right atrium; RV: Right ventricle; U: U-turn of the CS around the LCX. Reproduced with permission from [48].
Figure 5. Multiplanar and curved multiplanar reformatting showing the coronary sinus–great cardiac vein and the left circumflex coronary artery. These images refer to the same patient shown in Figure 3. (A) Axial view of the heart showing the cardiac chambers with superimposed (blue) a projection of the CS–GCV path. Note the high contrast between the vessels and the myocardium. (B) Curved multiplanar reformatting of the CS–GCV. (C) Axial view of the heart showing the cardiac chambers with a superimposed (blue) projection of the LCX path. (D) Curved multiplanar reformatting of the LCX. AO: Aorta; CS–GCV: Coronary sinus–great cardiac vein; LCX: Left circumflex coronary artery; LV: Left ventricle; RA: Right atrium; RV: Right ventricle.
Left atrial imaging
MS‑325-enhanced inversion-recovery steadystate free-precession MRA of the left atrium resulted in improved image quality on qualitative assessment compared with a noncontrast T2 prepared MRA [49]. However, in this study, five of the 15 patients enrolled were excluded as a result of navigator efficiency of less than 35%.
Carotid arteries
In a Phase II study, Bluemke et al. described good sensitivity (100 and 63%) and specificity (100 and 100%) using 0.01 and 0.03 mmol/kg MS‑325, respectively, with 3D spoiled-gradient recalled-echo MRA compared with conventional angiography for the detection of over 70% carotid artery stenosis [50]. MRA was performed during steady-state conditions of circulating contrast agent approximately 5 min after injection. There was a trend toward increased accuracy at lower doses but also more uninterpretable studies at the lower doses (although these were not significant as it was a Phase II study with only 26 patients).
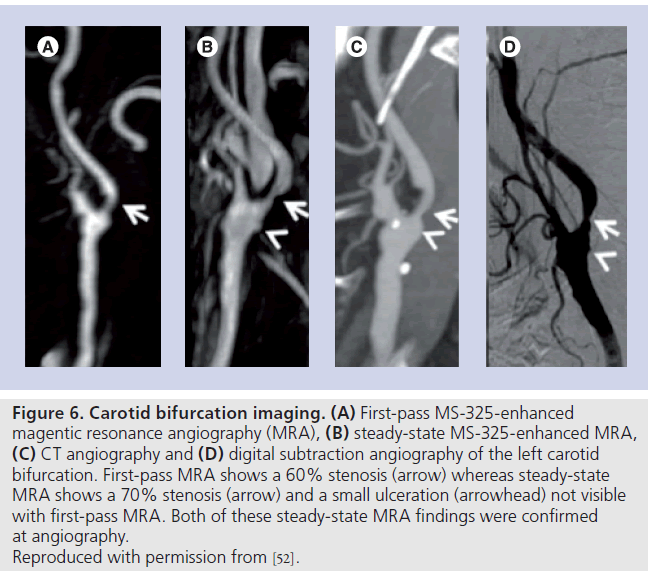
A subsequent study of 84 patients demonstrated very high accuracy for grading stenosis severity using MS‑325 MRA compared with digital subtraction angiography [51]. Steady-state image reading was superior to first pass (Figure 6) but combining both resulted in sensitivity, specificity, positive and negative predictor values of 98, 100, 100 and 97%, respectively. Similarly high accuracy was demonstrated in another smaller study of 20 patients using steady-state MS‑325 enhanced acquisitions [52].
Figure 6. Carotid bifurcation imaging. (A) First-pass MS-325-enhanced magentic resonance angiography (MRA), (B) steady-state MS-325-enhanced MRA, (C) CT angiography and (D) digital subtraction angiography of the left carotid bifurcation. First-pass MRA shows a 60% stenosis (arrow) whereas steady-state MRA shows a 70% stenosis (arrow) and a small ulceration (arrowhead) not visible with first-pass MRA. Both of these steady-state MRA findings were confirmed at angiography. Reproduced with permission from [52].
Renal arteries
MS‑325 has also been used to improve renal artery MRA in a Phase III multicenter trial of 145 patients with known or suspected renal arterial disease (see previously and Table 1) [53]. MS‑325-enhanced MRA significantly improved the sensitivity, specificity and accuracy compared with nonenhanced MRA for the three readers. The sensitivity, specificity and accuracy with contrast were reasonably good (57–66, 77–83 and 73–79%, respectively) in this study. In addition, the number of uninterpretable scans decreased from 30% without contrast to less than 2% following administration of MS‑325.
Pedal artery disease
Another Phase III study of 185 patients with known or suspected pedal artery disease reported that the accuracy of MS‑325-enhanced MRA (73–81%) was significantly better than that of unenhanced MRA (60–67%) (Table 1). The sensitivity of MRA was good without contrast (77–87%) and did not significantly improve with the use of MS‑325. It is worth noting that in this population the specificity of unenhanced MRA for the detection of over 50% stenosis was poor (28–39%) and MS‑325 MRA moderate (60–66%) [54]. A decrease in the number of uninterpretable studies was also noted with 0.03 mmol/kg of MS‑325. This study also found a dose of 0.03 mmol/kg of MS‑325 to be safe as well as reasonably effective.
Other vasculature
There are also some limited retrospective data from 25 patients, which suggest that motioncompensated high-resolution steady-state MRA of the thoracic vasculature using MS‑325 offers superior image quality compared with standard first-pass MRA [55]. MS‑325 may also improve first-pass MRA of the hand; however, to date, there are only data from a very small number of volunteers and no patients [56]. Finally, Alonso‑Burgos et al. have reported some pilot data that suggest that MS‑325 MRA might be useful for mapping the location and size of perforator vessels in superior gluteal artery perforator and deep inferior epigastric artery perforator flaps when planning reconstructive breast surgery [57].
Therefore, clinical efficacy of MS‑325 has been demonstrated for a number of vascular beds and early data relating to other regions of the body suggest that the number of clinical applications will continue to expand.
Other potential clinical applications
Blood-pool contrast agents could offer significant advantages when imaging smaller vessels and vessels with slow or complex flow [1]. For this reason, blood-pool contrast agents have been proposed for perfusion imaging [58], detection of gastrointestinal bleeding [59] or tumor imaging to demonstrate angiogenesis [60,61].
Safety & tolerability
The FDA has requested that a boxed warning be added to the labeling of all Gd-based MRI contrast agents marketed in the USA. This applies also to gadofosveset. The boxed warning describes the risk for nephrogenic systemic fibrosis in patients receiving these agents who suffer renal dysfunction that meets one of the following two conditions:
• Acute or chronic severe renal insufficiency (i.e., where the glomerular filtration rate is <30 ml/min/1.73 m2);
• Acute renal dysfunction caused by the hepato-renal syndrome or in the perioperative liver transplantation period of any severity
In patients with renal insufficiency, acute renal failure has occurred with the use of other Gd agents. The risk of renal failure may increase with increasing doses of Gd contrast. Patients with these conditions should not be exposed to gadofosveset except as recommended by a physician.
In clinical trials, gadofosveset caused anaphylactoid and/or anaphylactic reactions in two of 1676 subjects.
In clinical trials, a QT interval corrected for heart rate change of 30–60 ms from baseline was observed in 6% of patients at 45 min postinjection. This prolongation was not associated with arrhythmias or other symptoms [101].
Regulatory affairs
Gadofosveset is currently approved for MRA in the USA and the EU.
To the best of our knowledge, no comparison is available between gadofosveset and conventional extracellular agents in terms of cost and cost:efficacy ratio. As previously explained, the use of blood-pool contrast agents can have some limitations, particularly in patients requiring an interstitial phase in Gd pharmacokinetics. In these cases, the choice of the contrast agent must be individualized based on the clinical indication and, if an injection of intravascular contrast agent is needed, a second scan with an interstitial contrast agent might be needed after a few days.
Conclusion & future perspective
Gadofosveset, the first Gd-based blood-pool contrast agent approved for MRA, has the potential to open new horizons in the diagnosis of vascular diseases, increase the robustness of MRA and allow easier imaging of multiple vascular beds. First approved in 2005, it has shown to be useful for a broad and rapidly evolving spectrum of clinical applications and should allow us to address a number of new clinical questions in cardiovascular medicine.
Financial & competing interests disclosure
Eike Nagel received research grants from Philips Medical Systems and Bayer Schering Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material s discussed in the manuscript apart f rom those disclosed.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as: * of interest * of considerable interest
References
- Bremerich J, Bilecen D, Reimer P: MR angiography with blood pool contrast agents. Eur. Radiol. 17, 3017–3024 (2007).
- Adamis MK, Li W, Wielopolski PA et al.: Dynamic contrast-enhanced subtraction MR angiography of the lower extremities: initial evaluation with a multisection two-dimensional time-of-flight sequence. Radiology 196, 689–695 (1995).
- Grist TM, Korosec FR, Peters DC et al.: Steady-state and dynamic MR angiography with MS-325: initial experience in humans. Radiology 207, 539–544 (1998).
- Korosec FR, Frayne R, Grist TM, Mistretta CA: Time-resolved contrast-enhanced 3D MR angiography. Magnetic resonance in medicine. Magn. Reson. Med. Sci. 36, 345–351 (1996).
- Krinsky GA, Rofsky NM, DeCorato DR et al.: Thoracic aorta: comparison of gadolinium-enhanced three-dimensional MR angiography with conventional MR imaging. Radiology 202, 183–193 (1997).
- Levy RA, Prince MR: Arterial-phase three-dimensional contrast-enhanced MR angiography of the carotid arteries. AJR Am. J. Roentgenol. 167, 211–215 (1996).
- Prince MR, Narasimham DL, Jacoby WT et al.: Three-dimensional gadoliniumenhanced MR angiography of the thoracic aorta. AJR Am. J. Roentgenol. 166, 1387–1397 (1996).
- Prince MR, Yucel EK, Kaufman JA, Harrison DC, Geller SC: Dynamic gadolinium-enhanced three-dimensional abdominal MR arteriography. J. Magn. Reson. Imaging 3, 877–881 (1993).
- Petersen MJ, Cambria RP, Kaufman JA et al.: Magnetic resonance angiography in the preoperative evaluation of abdominal aortic aneurysms. J. Vasc. Surg. 21, 891–898; discussion 899 (1995).
- Shetty AN, Shirkhoda A, Bis KG, Alcantara A: Contrast-enhanced three-dimensional MR angiography in a single breath-hold: a novel technique. AJR Am. J. Roentgenol. 165, 1290–1292 (1995).
- Snidow JJ, Johnson MS, Harris VJ et al.: Three-dimensional gadolinium-enhanced MR angiography for aortoiliac inflow assessment plus renal artery screening in a single breath hold. Radiology 198, 725–732 (1996).
- Neuwelt EA, Hamilton BE, Varallyay CG et al.: Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 75, 465–474 (2009).
- Dong Q, Hurst DR, Weinmann HJ, Chenevert TL, Londy FJ, Prince MR: Magnetic resonance angiography with gadomer-17. An animal study original investigation. Invest. Radiol. 33, 699–708 (1998).
- Parmelee DJ, Walovitch RC, Ouellet HS, Lauffer RB: Preclinical evaluation of the pharmacokinetics, biodistribution, and elimination of MS-325, a blood pool agent for magnetic resonance imaging. Invest. Radiol. 32, 741–747 (1997). & Describes pharmacokinetics and biodistribution of gadofosveset in detail.
- Caravan P: Protein-targeted gadoliniumbased magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Acc. Chem. Res. 42, 851–862 (2009). nn Describes the pharmacodynamics of gadovofsveset in detail.
- Caravan P, Cloutier NJ, Greenfield MT et al.: The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. J. Am. Chem. Soc. 124, 3152–3162 (2002).
- Caravan P, Comuzzi C, Crooks W, McMurry TJ, Choppin GR, Woulfe SR: Thermodynamic stability and kinetic inertness of MS-325, a new blood pool agent for magnetic resonance imaging. Inorg. Chem. 40, 2170–2176 (2001).
- Lauffer RB, Parmelee DJ, Dunham SU et al.: MS-325: albumin-targeted contrast agent for MR angiography. Radiology 207, 529–538 (1998).
- Stuber M, Botnar RM, Danias PG et al.: Contrast agent-enhanced, free-breathing, three-dimensional coronary magnetic resonance angiography. J. Magn. Reson. Imaging 10, 790–799 (1999). & Describes the usefulness of gadofosveset in coronary magnetic resonance angiography.
- Nassenstein K, Waltering K-U, Kelle S et al.: Magnetic resonance coronary angiography with Vasovist: in vivo T1 estimation to improve image quality of navigator and breath-hold techniques. Eur. Radiol. 18, 103–109 (2008).
- Burtea C, Laurent S, Colet J-M, Vander Elst L, Muller RN: Development of new glucosylated derivatives of gadolinium diethylenetriaminepentaacetic for magnetic resonance angiography. Invest. Radiol. 38, 320–333 (2003).
- Kobayashi H, Kawamoto S, Jo S-K, Bryant HL, Brechbiel MW, Star RA: Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjug. Chem. 14, 388–394 (2003).
- Baxter AB, Melnikoff S, Stites DP, Brasch RC: AUR Memorial Award 1991. Immunogenicity of gadolinium-based contrast agents for magnetic resonance imaging. Induction and characterization of antibodies in animals. Invest. Radiol. 26, 1035–1040 (1991).
- Allkemper T, Heindel W, Kooijman H, Ebert W, Tombach B: Effect of field strengths on magnetic resonance angiography: comparison of an ultrasmall superparamagnetic iron oxide blood-pool contrast agent and gadopentetate dimeglumine in rabbits at 1.5 and 3.0 Tesla. Invest. Radiol. 41, 97–104 (2006).
- Engelbrecht MR, Saeed M, Wendland MF, Canet E, Oksendal AN, Higgins CB: Contrast-enhanced 3D-TOF MRA of peripheral vessels: intravascular versus extracellular MR contrast media. J. Magn. Reson. Imaging 8, 616–621 (1998).26 Lei T, Udupa JK, Saha PK, Odhner D: Artery-vein separation via MRA – an image processing approach. IEEE Trans. Med. Imaging 20, 689–703 (2001).
- Lei T, Udupa JK, Saha PK et al.: 3D MRA visualization and artery-vein separation using blood-pool contrast agent MS-325. Acad. Radiol. 9(Suppl. 1), S127–S133 (2002).
- Santini F, Patil S, Meckel S, Scheffler K, Wetzel SG: Double-reference crosscorrelation algorithm for separation of the arteries and veins from 3D MRA time series. J. Magn. Reson. Imaging 28, 646–654 (2008).
- van Bemmel CM, Spreeuwers LJ, Viergever MA, Niessen WJ: Level-set-based artery-vein separation in blood pool agent CE-MR angiograms. IEEE Trans. Med. Imaging 22, 1224–1234 (2003).
- Lauffer RB, Parmelee DJ, Ouellet HS et al.: MS-325: a small-molecule vascular imaging agent for magnetic resonance imaging. Acad. Radiol. 3(Suppl. 2), S356–S358 (1996).
- Adam G, Neuerburg J, Spüntrup E, Mühler A, Scherer K, Günther RW: Dynamic contrast-enhanced MR imaging of the upper abdomen: enhancement properties of gadobutrol, gadolinium-DTPA-polylysine, and gadolinium-DTPA-cascade-polymer. Magn. Reson. Med. 32, 622–628 (1994).
- Bogdanov AA, Weissleder R, Frank HW et al.: A new macromolecule as a contrast agent for MR angiography: preparation, properties, and animal studies. Radiology 187, 701–706 (1993).
- Desser TS, Rubin DL, Fan Q et al.: Quantitation of saturation effects versus dose in three-dimensional time-of-flight magnetic resonance angiography with blood-pool contrast agents. Invest. Radiol. 29(Suppl. 2), S65–S68 (1994).
- Meyer D, Schaefer M, Bouillot A, Beauté S, Chambon C: Paramagnetic dextrans as magnetic resonance contrast agents. Invest. Radiol. 26(Suppl. 1), S50–S52; discussion S60–S64 (1991).
- Schmiedl U, Ogan MD, Moseley ME, Brasch RC: Comparison of the contrastenhancing properties of albumin-(Gd-DTPA) and Gd-DTPA at 2.0 T: and experimental study in rats. AJR Am. J. Roentgenol. 147, 1263–1270 (1986).
- Wang SC, Wikström MG, White DL et al.: Evaluation of Gd-DTPA-labeled dextran as an intravascular MR contrast agent: imaging characteristics in normal rat tissues. Radiology 175, 483–488 (1990).
- Perreault P, Edelman MA, Baum RA et al.: MR angiography with gadofosveset trisodium for peripheral vascular disease: Phase II trial. Radiology. 229(3), 811–820. (2003).
- Goyen M, Edelman M, Perreault P et al.: MR angiography of aortoiliac occlusive disease: a Phase III study of the safety and effectiveness of the blood-pool contrast agent MS-325. Radiology 236, 825–833 (2005).
- Rapp JH, Wolff SD, Quinn SF et al.: Aortoiliac occlusive disease in patients with known or suspected peripheral vascular disease: safety and efficacy of gadofosvesetenhanced MR angiography – multicenter comparative Phase III study. Radiology 236, 71–78 (2005).
- Hadizadeh DR, Gieseke J, Lohmaier SH et al.: Peripheral MR angiography with blood pool contrast agent: prospective intraindividual comparative study of high-spatial-resolution steady-state MR angiography versus standard-resolution first-pass MR angiography and DSA. Radiology 249, 701–711 (2008).
- 1 Bonel HM, Saar B, Hoppe H et al.: MR angiography of infrapopliteal arteries in patients with peripheral arterial occlusive disease by using gadofosveset at 3.0 T: diagnostic accuracy compared with selective DSA. adiology 253, 879–890 (2009).
- Klessen C, Hein PA, Huppertz A et al.: First-pass whole-body magnetic resonance angiography (MRA) using the blood-pool contrast medium gadofosveset trisodium: comparison to gadopentetate dimeglumine. Invest. Radiol. 42, 659–664 (2007).
- Grijalba FU, Esandi MC: Comparison of gadofosveset-enhanced three-dimensional magnetic resonance angiography with digital subtraction angiography for lower-extremity peripheral arterial occlusive disease. Acta Radiol. 51(3), 284–289 (2010).
- Nielsen YW, Eiberg JP, Løgager VB, Hansen MA, Schroeder TV, Thomsen HS: Whole-body MR angiography with body coil acquisition at 3 T in patients with peripheral arterial disease using the contrast agent gadofosveset trisodium. Acad. Radiol. 16, 654–661 (2009).
- Prompona M, Cyran C, Nikolaou K, Bauner K, Reiser M, Huber A: Contrastenhanced whole-heart MR coronary angiography at 3.0 T using the intravascular contrast agent gadofosveset. Invest. Radiol. 44, 369–374 (2009).
- Kelle S, Thouet T, Tangcharoen T et al.: Whole-heart coronary magnetic resonance angiography with MS-325 (Gadofosveset). Med. Sci. Monit. 13, CR469–CR474 (2007).
- Chiribiri A, Kelle S, Götze S et al.: Visualization of the cardiac venous system using cardiac magnetic resonance. Am. J. Cardiol. 101, 407–412 (2008).
- Chiribiri A, Kelle S, Köhler U et al.: Magnetic resonance cardiac vein imaging: relation to mitral valve annulus and left circumflex coronary artery. JACC Cardiovasc. Imaging 1, 729–738 (2008).
- Wagner M, Rief M, Asbach P et al.: Gadofosveset trisodium-enhanced magnetic resonance angiography of the left atrium-A feasibility study. Eur. J. Radiol. 51(3), 284–289 (2009).
- Bluemke DA, Stillman AE, Bis KG et al.: Carotid MR angiography: Phase II study of safety and efficacy for MS-325. Radiology 219, 114–122 (2001).
- Anzidei M, Napoli A, Marincola BC et al.: Gadofosveset-enhanced MR angiography of carotid arteries: does steady-state imaging improve accuracy of first-pass imaging? Comparison with selective digital subtraction angiography. Radiology 251, 457–466 (2009).
- Anzidei M, Napoli A, Geiger D et al.: Preliminary experience with MRA in evaluating the degree of carotid stenosis and plaque morphology using high-resolution sequences after gadofosveset trisodium (Vasovist) administration: comparison with CTA and DSA. Radiol. Med. 115(4), 634–647 (2010).
- McGregor R, Vymazal J, Martinez-Lopez M et al.: A multi-center, comparative, Phase 3 study to determine the efficacy of gadofosveset-enhanced magnetic resonance angiography for evaluation of renal artery disease. Eur. J. Radiol. 65, 316–325 (2008).
- Bosch E, Kreitner K-F, Peirano MF et al.: Safety and efficacy of gadofosveset-enhanced MR angiography for evaluation of pedal arterial disease: multicenter comparative Phase 3 study. AJR Am. J. Roentgenol. 190, 179–186 (2008).
- Naehle CP, Müller A, Willinek WA et al.: First-pass and steady-state magnetic resonance angiography of the thoracic vasculature using gadofosveset trisodium. J. Magn. Reson. Imaging 30, 809–816 (2009).
- Reisinger C, Gluecker T, Jacob AL, Bongartz G, Bilecen D: Dynamic magnetic resonance angiography of the arteries of the hand. A comparison between an extracellular and an intravascular contrast agent. Eur. Radiol. 19, 495–502 (2009).
- Alonso-Burgos A, García-Tutor E, Bastarrika G, Benito A, Domínguez PD, Zubieta JL: Preoperative planning of DIEP and SGAP flaps: preliminary experience with magnetic resonance angiography using 3-tesla equipment and blood-pool contrast medium. J. Plast. Reconstr. Aesthet. Surg. 63, 298–304 (2010).
- Jerosch-Herold M, Wilke N, Wang Y et al.: Direct comparison of an intravascular and an extracellular contrast agent for quantification of myocardial perfusion. Cardiac MRI Group. Int. J. Card. Imaging 15(6), 453–464 (1999).
- Gupta H, Weissleder R, Bogdanov AA, Brady TJ: Experimental gastrointestinal hemorrhage: detection with contrast-enhanced MR imaging and scintigraphy. Radiology 196, 239–244 (1995).
- Turetschek K, Floyd E, Helbich T et al.: MRI assessment of microvascular characteristics in experimental breast tumors using a new blood pool contrast agent (MS-325) with correlations to histopathology. J. Magn. Reson. Imaging 14, 237–242 (2001).
- van Dijke CF, Brasch RC, Roberts TP et al.: Mammary carcinoma model: correlation of macromolecular contrast-enhanced MR imaging characterizations of tumor microvasculature and histologic capillary density. Radiology 198, 813–818 (1996).