Contrast Agent Evaluation - Imaging in Medicine (2012) Volume 4, Issue 3
Gadoxetic acid-enhanced MRI: a method for the detection of hepatic metastases
Ali Muhi1, Tomoaki Ichikawa2* and Utaroh Motosugi2
1Department of Radiology, Muthanna University, Muthanna, Iraq
2Department of Radiology, University of Yamanashi, Yamanshi, Japan
- *Corresponding Author:
- Ali Muhi
Department of Radiology
Muthanna University, Muthanna, Iraq
Tel.: +81 55 273 1111
Fax: +81 55 273 6744
Email: ichikawa@yamanashi.ac.jp
Abstract
Staging of malignant tumors is increasingly important as new therapeutic strategies develop. The presence of liver metastasis significantly changes the therapeutic approach; therefore, it is important to exclude metastasis with high confidence. Moreover, the proportion of patients with hepatic metastasis considered amenable to resection is increasing with improvements in surgical treatment. With improvements in surgical and medical management of hepatic metastasis comes an increasing need to provide accurate assessment of the exact number, size and location of metastases using imaging. The differentiation of liver metastases from benign lesions, such as hemangioma, focal nodular hyperplasia or adenoma, is very important. Gadoxetic acid is a liver-specific MR contrast agent that has both dynamic and hepatocytespecific properties. Once taken up by functioning hepatocytes, gadoxetic acid enhances hepatic parenchyma, whereas lesions with no hepatocytes or hepatocyte dysfunction, such as metastases, and most hepatocellular carcinomas, remain unenhanced.
Keywords
gadoxetic acid; hepatocyte-specific contrast agents; liver metastases
Liver metastases
Metastases are the most common malignant tumors involving the liver, which is the second organ, after the lymph node, to be affected by metastases. The most common primary malignancies that can metastasize to the liver include: colorectal, gastric, pancreatic, breast and lung cancers. Liver metastases are usually multiple and involve both lobes and vary in size from subcentimeter to several centimeters. Metastases can be hypo- or hyper-vascular. The growth pattern and vascularity of metastasis vary depending on the primary malignancy. Patients with hepatic metastasis are usually asymptomatic and liver function tests are insensitive and not specific. The early detection and accurate diagnosis of metastasis using imaging is crucial so that appropriate treatment can be administered.
Liver-specific contrast agents
Two types of liver-specific contrast agent are available: hepatocyte-and reticuloendothelial cell-specific agents. After intravenous injection, they are accumulated by liver cells (hepatocytes or Kupffer cells) and cause significant changes in the signal intensity of liver leading to increased liver-to-lesion contrast. Functioning hepatocytes take up the hepatocyte-specific agents leading to an increase in the signal intensity of the liver on T1-weighted images (T1WI) giving rise to ‘white’ liver. The available contrast agents of this type include gadoxetic acid (Gd-EOB-DTPA, Primovist®, Bayer Pharma, Berlin, Germany) and gadobenate dimeglumine (Gd-BOPTA, Multihance®, Bracco, Milan, Italy).
A reticuloendothelial cell-specific agent such as Ferucarbotran (Superparamagnetic iron oxide Resovist®, Bayer Pharma, Berlin, Germany) is taken up by Kupffer cells in the liver with the resultant decrease in signal intensity of the liver on T2-weighted images (T2WI). This agent has been removed from the market in the USA and Europe.
Gadoxetic acid is a gadolinium-based, paramagnetic, hydrophilic, highly water-soluble, bolus-injectable MR contrast agent that enables the acquisition of dynamic and hepatobiliary phases [1,2]. Dynamic imaging with gadoxetate acid is feasible with sufficient quality. The functioning hepatocytes selectively take up approximately 50% of the injected dose of the contrast agent, allowing for the acquisition of the hepatobiliary phase images, which is optimal at 20 min postinjection. The recommended dose of gadoxetic acid is fourfold less than the extracellular contrast agents [1–3].
The high specific uptake of the gadoxetic acid into the hepatocyte through the organic anion-transporting polypeptide (OATP1) is mediated by the lipophilic ethoxybenzyl (EOB) moiety, which is linked to the gadolinium complex [4–6]. Following its relatively high uptake in the liver, gadoxetic acid is excreted in equal quantities via bile (50%) and urine (50%). The contrast agent’s excretion via both renal and biliary pathways ensures its elimination in patients with impaired renal or hepatic function if one pathway is impaired.
MR examination using gadoxetic acid is the same as with other gadolinium chelate contrast agents apart from the additional hepatobiliary phase, which is obtained 20 min after contrast injection. For precontrast images, dual phase T1WI, T2WI and diffusion-weighted images (DWI) can be acquired. To reduce the examination time to less than 30 min, T2WI and DWI can be performed after dynamic phase (i.e., after 5 min) and before hepatobiliary phase image (i.e., before 20 min)[7,8]. It has been shown that the signal intensities of the liver parenchyma and focal liver lesions on T2WI and DWI are not significantly modified when these sequences are obtained after contrast injection [7,8]. Alternatively, the examination time can be shortened by obtaining the hepatobiliary phase images at 10 min after contrast injection [9,10].
Regarding the safety of gadoxetic acid, it is safe and well tolerated with no major side effects [1–3]. Gadoxetic acid was classified by the Committee for Medicinal Products for Human Use (CHMP) in Europe as a medium-risk agent for the development of nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate <30 ml/min/1.73 m2) [101].
Gadoxetic acid for detection of liver metastasis
Detection of liver metastases
Liver metastases are classified according to their enhancement in the arterial-dominant phase of dynamic contrast-enhanced MRI into hyper- and hypo-vascular.
Hypervascular metastases derive from hypervascular primary tumors such as islet cell tumor, carcinoid, thyroid carcinoma, renal carcinoma, pheochromocytoma, melanoma and breast carcinoma. Metastases from colon, pancreatic and breast carcinomas may also be hypervascular. Hypervascular metastases show enhancement in the arterial-dominant phase of dynamic gadoxetic acid-enhanced MRI and wash-out in the portal/late venous phases that results in an iso- or hypo-intense signal (Figure 1).
Hypovascular metastases are the most frequent malignancies in the liver and colon cancer is the most common primary site. On hepatic arterial phase images, hypovascular metastases can show a ring-like enhancement and wash-out in portal/late phase resulting in an iso- or hypointense signal (Figure 2). Similar to the hypervascular metastases, hypovascular metastases also show contrast enhancement but to a lesser degree than the surrounding hepatic parenchyma. Enhancement of liver metastases depends on presences of tumor cellularity, fibrous tissue, necrosis and size.
Portal venous phase is the best phase for imaging hypovascular metastases during dynamic contrast-enhanced MRI, whereas hypervascular metastases are best imaged during the arterial-dominant phase. Arterialdominant phase is useful for lesion detection as well as lesion characterization by identifying hypervascularity. Therefore, correct timing of the arterial-dominant phase is indispensable for characterization of focal liver lesions. Gadoxetic acid has a T1 relaxivity higher than gadopentetate dimeglumine (Magnevist®, Bayer HealthCare) [11], but the recommended dose of gadoxetic acid is only one-quarter that of extracellular contrast media such as gadopentetate dimeglumine, which result in weaker T1-shortening particularly for the arterial phase during dynamic imaging. Some radiologists use a double dose of gadoxetic acid to improve vascular enhancement in the dynamic imaging, however, this approach is not favored because it increases the cost. Motosugi et al. demonstrated that a double dose significantly increases the arterial-phase lesion–liver contrast ratio [12]. In our experience, the standard dose of gadoxetic acid is equivalent to double dose for lesion detection and characterization in most cases during the arterial phase and hepatocyte phase images. However, the evaluation of wash-out or wash-in of contrast media during the portal venous phase (65–70 s) or equilibrium phase (3–5 min) may be challenging since the hepatic enhancement during those phases is caused by presence of gadoxetic acid in the vascular/extracellular space (similar to the distribution of an extracellular contrast agent such as gadopentetate dimeglumine), as well as in the intracellular space because the hepatocyte uptake begins with the first pass and is certainly perceivable within the first 90 s [11,13].
In the hepatobiliary phase, using gadoxetic acid-enhanced MRI, both hyper- and hypovascular metastases are hypointense. In this phase, metastases will not show uptake of the contrast agent compared with the surrounding normal liver parenchyma, which shows specific uptake of the contrast agent leading to improve lesion conspicuity by increasing lesion–liver contrast. This leads to improved detection of liver metastasis with high confidence, particularly for small lesions (<1 cm in diameter) [14–16].
Unenhanced conventional MRI, including T1WI and T2WI, is complimentary to gadoxetic acid-enhanced MRI and can improve lesion characterization. On T1WI, the signal intensity of metastases is typically low and on T2WI it is moderately hyperintense. Lesions smaller than 1 cm are difficult to detect with conventional MRI.
Differentiating metastasis from hemangioma
Cavernous hemangioma is the most common benign hepatic tumor with a reported incidence of 20% in the general population [17]. It is a common incidental finding during the evaluation of other diseases. Hemangioma is mostly asymptomatic. Cavernous hemangioma appears hypointense on T1WI and hyperintense on T2WI, with increasing hyperintensity by increasing echo time (heavy T2WI). It usually demonstrates characteristic peripheral nodular enhancement with centripetal filling on dynamic imaging (Figure 3). Large lesions may have s nonenhancing central scar. Hemangiomas appear hypointense on hepatobiliary phase images.
The hepatobiliary phase is not helpful in the differentiation between the two entities because neither a metastasis nor hemangioma exhibit specific uptake of the gadoxetic acid, therefore both lesions will appear as hypointense against the surrounding bright liver in the hepatobiliary phase. The differentiation between metastasis and hemangioma primarily depends on signal intensity on T2WI and enhancement in the dynamic phase. The standard dose of gadoxetic acid is one-quarter that of gadopentetate dimeglumine and Gd-BOPTA, which may result in a relatively weaker enhancement particularly for the arterial phase. Typical hemangioma will show high signal intensity (bright) on T2WI with peripheral nodular enhancement on arterial phase with centripetal filling on late phase, whereas metastases show intermediate signal intensity on T2WI and ring-like enhancement on arterial phase [18,19]. Atypical small hemangiomas that show intermediate signal intensity in the T2WI, no enhancement during the arterial phase and minimal enhancement during the late dynamic phase, may be difficult to differentiate from metastases.
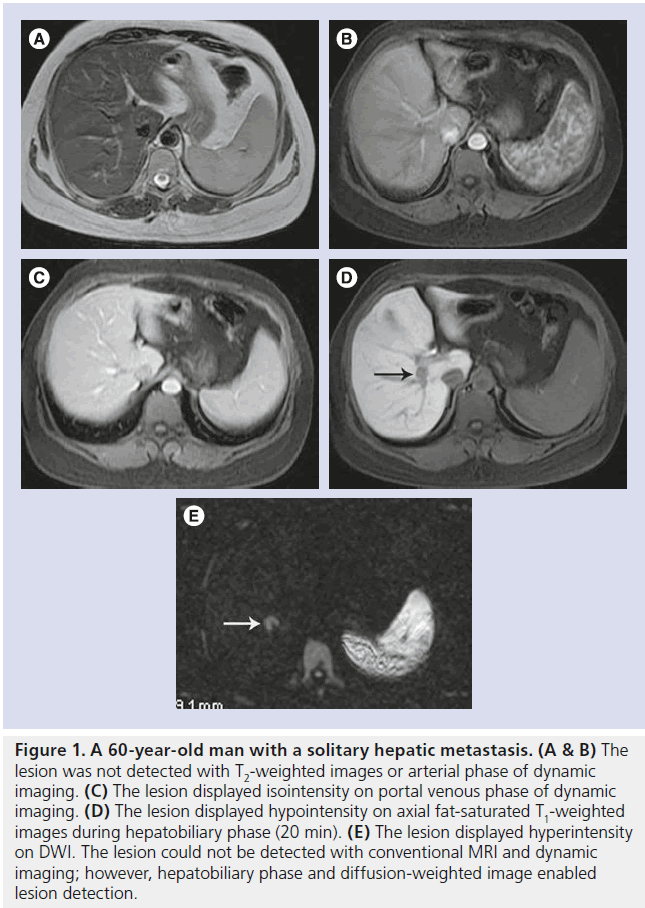
Figure 1. A 60-year-old man with a solitary hepatic metastasis. (A & B) The lesion was not detected with T2-weighted images or arterial phase of dynamic imaging. (C) The lesion displayed isointensity on portal venous phase of dynamic imaging. (D) The lesion displayed hypointensity on axial fat-saturated T1-weighted images during hepatobiliary phase (20 min). (E) The lesion displayed hyperintensity on DWI. The lesion could not be detected with conventional MRI and dynamic imaging; however, hepatobiliary phase and diffusion-weighted image enabled lesion detection.
Differentiating metastasis from focal nodular hyperplasia
Focal nodular hyperplasia (FNH) is the second most common benign tumor of the liver and is more frequently seen in females. It is usually asymptomatic. Its origin is unclear, but vascular injury or malformation was suggested as a causative factor [17]. It often has a spoke wheellike central scar. FNH displays as Iso- to hypointense on T1WI and Iso- to hyper-intense on T2WI. FNH displays hypervascular on the arterial phase of dynamic imaging with no washout in the portal venous phase. It enhances, becoming iso- or hyperintense to normal liver on hepatobiliary phase images (Figure 4). The central scar, if present, usually appears hyperintense on T2WI; it shows poor enhancement on the arterial phase of dynamic imaging. The central scar sometimes displays as hypointense on hepatobiliary phase images [20]. It has been reported that FNH can be diagnosed with accuracy as high as 88% using gadoxetic acid-enhanced MRI [20–22]. FNH can be distinguished from metastases with high confidence using gadoxetic acid-enhanced MRI.
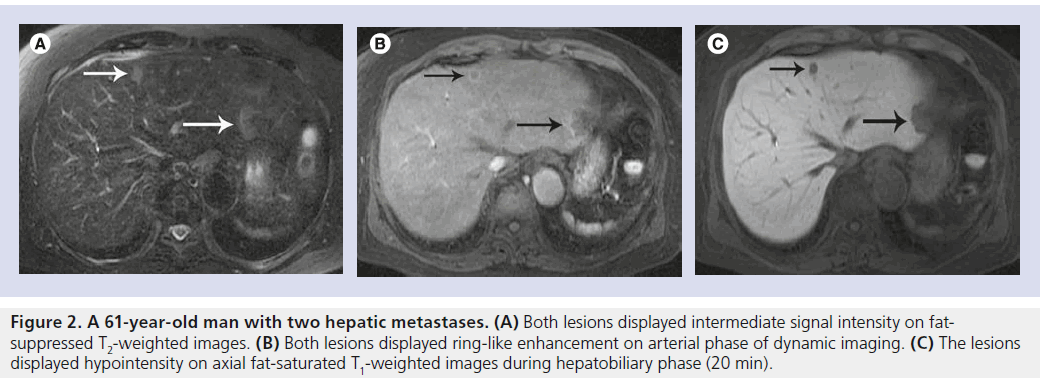
Figure 2. A 61-year-old man with two hepatic metastases. (A) Both lesions displayed intermediate signal intensity on fatsuppressed T2-weighted images. (B) Both lesions displayed ring-like enhancement on arterial phase of dynamic imaging. (C) The lesions displayed hypointensity on axial fat-saturated T1-weighted images during hepatobiliary phase (20 min).
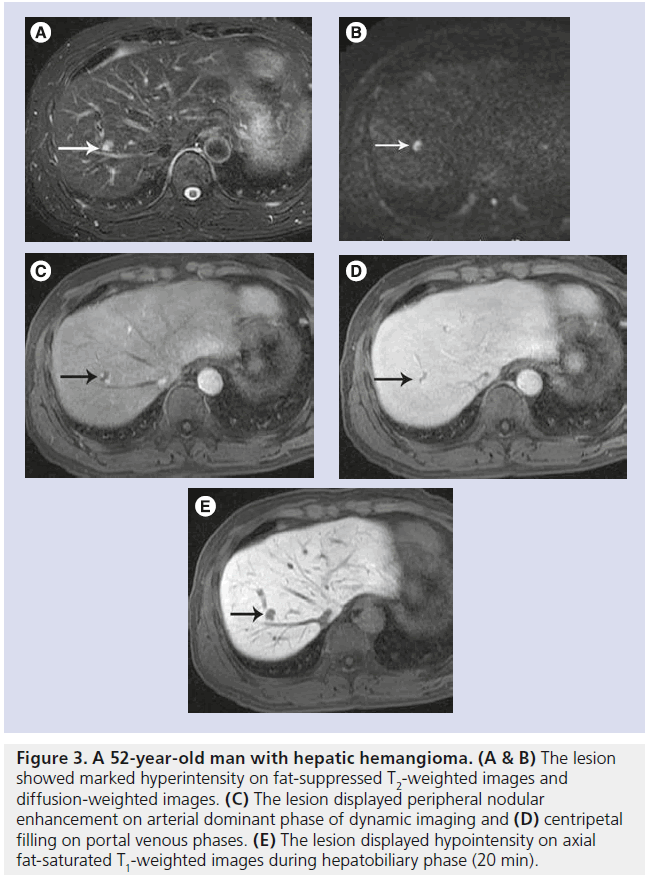
Figure 3. A 52-year-old man with hepatic hemangioma. (A & B) The lesion showed marked hyperintensity on fat-suppressed T2-weighted images and diffusion-weighted images. (C) The lesion displayed peripheral nodular enhancement on arterial dominant phase of dynamic imaging and (D) centripetal filling on portal venous phases. (E) The lesion displayed hypointensity on axial fat-saturated T1-weighted images during hepatobiliary phase (20 min).
Differentiating metastasis from liver adenoma
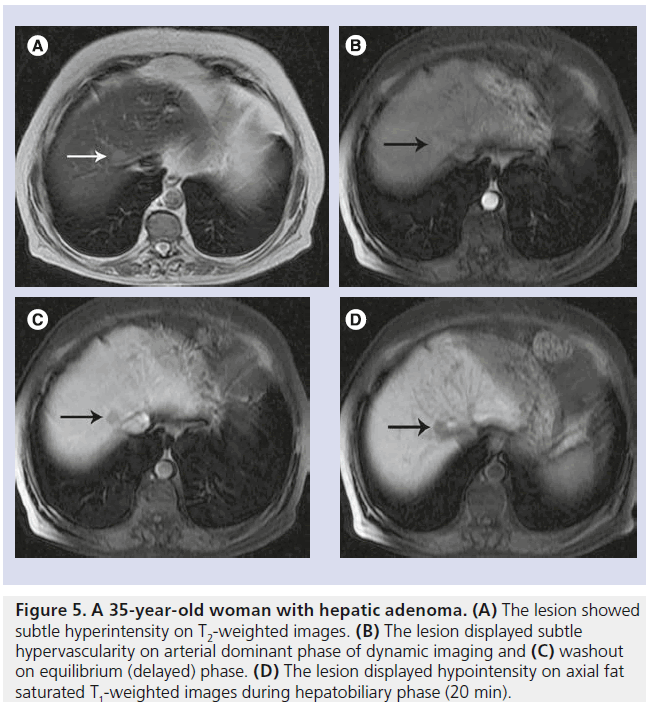
Hepatic adenoma is a rare, benign liver tumor that is derived from hepatocytes and predominantly affects women who take oral contraceptive pills. The tumor is round, well demarcated and often solitary. Typical features of adenomas on histopathology include fatty contents, intracellular glycogen and a thin pseudocapsule. Hepatic adenoma usually appears heterogeneous with areas of hyperintensity (caused by the presence of lipid or hemorrhage) on T1WI. On T2WI, it usually demonstrates intermediate hyperintensity and it displays hypervascularity on arterial phase of dynamic imaging (Figure 5). On hepatobiliary phase, the majority of adenomas display a weak-to-strong hypointensity, whereas metastases almost always show strong hypointensity on hepatobiliary phase [23,24].
Gadoxetic acid for detection of metastases in comparison with other types of contrast agents or imaging techniques
Several studies have demonstrated that gadoxetic acid-enhanced MRI has greater accuracy and sensitivity than dynamic contrast-enhanced (multidetector CT [MDCT]) and contrastenhanced ultrasound, particularly for lesions ≤1 cm [14–16]. The recently reported sensitivity of dynamic MDCT for detecting hepatic metastases ranges from 48 to 82%, with most studies reporting sensitivity between 60 and 80% [25–31]. The positive predictive value of dynamic MDCT and contrast-enhanced ultrasound in the detection of metastasis was equivalent to that of gadoxetic acid-enhanced MRI even for lesions smaller than 10 mm [14]. MDCT has the disadvantage of radiation exposure delivered to the patients during the initial examination and each subsequent follow-up.
Since gadoxetic acid is a recently introduced contrast medium, the specific properties of this agent, including enhancement during the dynamic phase and the hepatobiliary phase, need to be well understood by radiologists to enable lesion characterization. The conventional modalities, such as ultrasound and dynamic contrast-enhanced CT, may be needed in some instances for small lesions such as cysts (anechoic with ultrasound with posterior acoustic enhancement) or hemangiomas (peripheral nodular enhancement with centripetal filling with dynamic CT). However, in a multicenter study that evaluated the health–economic value of three imaging strategies, including gadoxetic acid, contrast-enhanced MRI with extracellular contrast media, and dynamic contrast-enhanced CT in patients with suspected colorectal liver metastases, indicated that gadoxetic acid-enhanced MRI can lead to cost savings by improving preoperative planning and decreasing intraoperative changes. The higher cost of imaging with gadoxetic acid-enhanced MRI is compensated by decreasing the need for additional imaging and less intraoperative modifications [32].
Superparamagnetic iron oxide has proven to be effective in the detection of metastases, with a sensitivity range of 78–97% [14,27–30,33,34]. Superparamagnetic iron oxide-enhanced MRI showed comparable diagnostic accuracy to Gd-EOB-MRI [14,34].
Both gadoxetic acid and gadobenate dimeglumine can achieve similar enhancement in normal liver parenchyma and the diagnostic performance of the two agents for the detection of liver metastases is assumed to be equivalent.
Combining DWI with gadoxetic acid-enhanced MRI
Gadoxetic acid-enhanced MRI has higher accuracy than DWI for the detection of liver metastases. The addition of DWI to gadoxetic acid-enhanced MRI has the potential to increase sensitivity for the detection of liver metastases, particularly for small metastases.
Combined interpretation of DWI and gadoxetic acid-enhanced MRI also improves characterization of focal liver lesions [35–38].
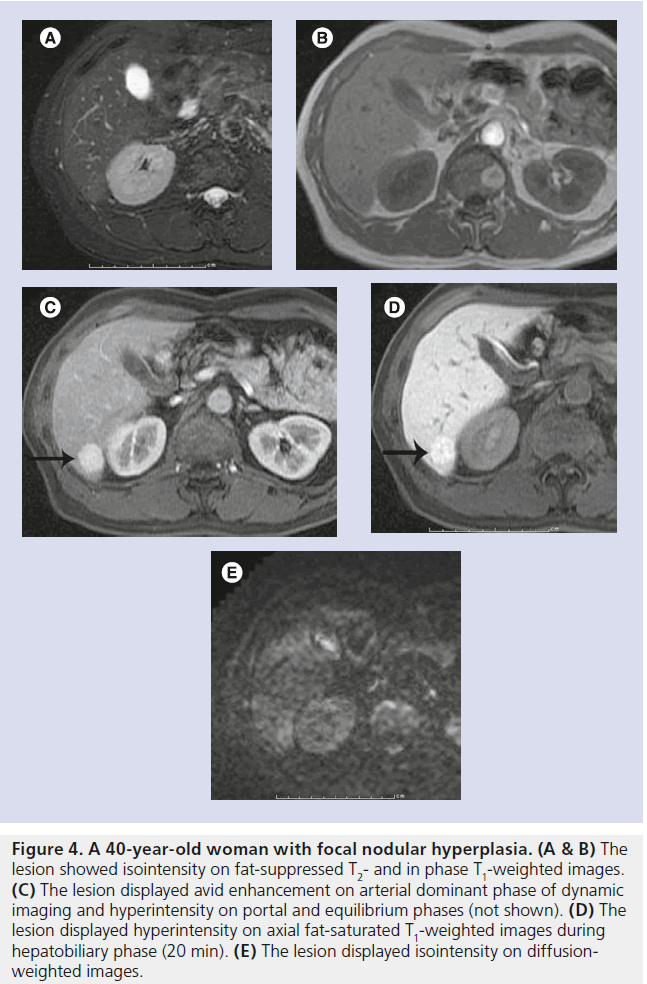
Figure 4. A 40-year-old woman with focal nodular hyperplasia. (A & B) The lesion showed isointensity on fat-suppressed T2- and in phase T1-weighted images. (C) The lesion displayed avid enhancement on arterial dominant phase of dynamic imaging and hyperintensity on portal and equilibrium phases (not shown). (D) The lesion displayed hyperintensity on axial fat-saturated T1-weighted images during hepatobiliary phase (20 min). (E) The lesion displayed isointensity on diffusionweighted images
Gadoxetic acid-enhanced MRI for assessment of extrahepatic primary tumors
Initial encouraging reports showed that gadoxetic acid-enhanced MRI was equivalent to dynamic contrast-enhanced MDCT in depicting pancreatic carcinoma and had better sensitivity for depicting liver metastases from pancreatic cancer [39]. Gadoxetic acid-enhanced MRI was also used for the staging of rectal carcinoma, including staging of extrahepatic disease [40]. However, dynamic contrast-enhanced MDCT is superior for the local staging of the extrahepatic tumors and for the detection of lung metastases because of its higher spatial resolution.
Figure 5. A 35-year-old woman with hepatic adenoma. (A) The lesion showed subtle hyperintensity on T2-weighted images. (B) The lesion displayed subtle hypervascularity on arterial dominant phase of dynamic imaging and (C) washout on equilibrium (delayed) phase. (D) The lesion displayed hypointensity on axial fat saturated T1-weighted images during hepatobiliary phase (20 min).
Conclusion
Gadoxetic acid-enhanced MRI allows both dynamic and hepatobiliary phase imaging with a single MR contrast agent. The evaluation of vascularity on dynamic phase and hepatocytespecific uptake on hepatobiliary phase enables accurate detection and characterization of focal liver lesions. Based on our experience and the published literature, gadoxetic acid-enhanced MRI is superior to dynamic contrast-enhanced MDCT, contrast-enhanced ultrasound and contrast-enhanced MRI with gadopentetate dimeglumine, especially for detection of small (<1 cm) metastases and for characterization of hypervascular lesions. The diagnostic performance of gadoxetic acid-enhanced MRI is equivalent to that of other liver-specific contrast agents, such as Gd-BOPTA, however, gadoxetic acid is preferred owing to shorter examination time. Thus, gadoxetic acid-enhanced MR is the imaging modality of choice for the detection of liver metastases.
In the preoperative staging, the aim is to identify patients who are candidates for curative resection and to prevent unnecessary surgeries. The number, size and location of tumors will determine whether the patient will benefit from surgery, therefore a precise preoperative imaging modality is required. The currently available imaging modalities, such as US, CT and conventional MRI, are still limited for detecting small metastases less than 10 mm and gadoxetic acidenhanced MRI proved superior to those modalities for detecting small metastasis. Furthermore, gadoxetic acid-enhanced MRI is also useful for anatomical and functional assessment for preoperative planning.
In the postoperative period, gadoxetic acidenhanced MRI is useful for evaluation of postoperative complications including vascular and biliary complications. It is also important for the detection of recurrence in the follow-up period where lesion characterization is not so important. Although hepatobiliary-specific agents are more expensive than nonspecific extracellular fluid agents, this expense is often offset by the additional information obtained, which may negate the need for other imaging studies, follow-up, biopsy or even surgery.
Future perspective
Most metastases arise in normal liver. Most metastases appear hypointense relative to the surrounding hepatic parenchyma on the hepatobiliary phase of gadoxetic acid-enhanced MRI. However, challenges remain in three areas:
▪ Differentiation of small benign lesions smaller than 1 cm that do not show typical signal intensity on precontrast conventional MRI or typical enhancement on dynamic phase images, from metastases;
▪ Detection and staging of the extrahepatic primary tumors;
▪ Reducing the long examination time compared with dynamic MDCT.
Further studies are also required to confirm the role of gadoxetic acid-enhanced MRI in the evaluation of response of liver metastases to chemotherapy or locoregional treatment.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
▪ of interest
- Hamm B, Staks T, Muhler A et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 195(3), 785–792 (1995).
- Reimer P, Rummeny EJ, Shamsi K et al. Phase II clinical evaluation of Gd-EOBDTPA: dose, safety aspects, and pulse sequence. Radiology 199(1), 177–183 (1996).
- Weinmann HJ, Schuhmann-Giampieri G, Schmitt-Willich H, Vogler H, Frenzel T, Gries H. A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn. Reson. Med. 22(2), 233–237 (1991).
- van Montfoort JE, Stieger B, Meijer DK, Weinmann HJ, Meier PJ, Fattinger KE. Hepatic uptake of the magnetic resonance imaging contrast agent gadoxetate by the organic anion transporting polypeptide Oatp1. J. Pharmacol. Exp. Ther. 290(1), 153–157 (1999).
- Pascolo L, Cupelli F, Anelli PL et al. Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem. Biophys. Res. Commun. 257(3), 746–752 (1999).
- Libra A, Fernetti C, Lorusso V et al. Molecular determinants in the transport of a bile acid-derived diagnostic agent in tumoral and nontumoral cell lines of human liver. J. Pharmacol. Exp. Ther. 319(2), 809–817 (2006).
- Choi JS, Kim M-J, Choi J-Y, Park M-S, Lim JS, Kim KW. Diffusion-weighted MR imaging of liver on 3.0-Tesla system: effect of intravenous administration of gadoxetic acid disodium. Eur. Radiol. 20, 1052–1060 (2010).
- Kim YK, Kwak HS, Kim CS, Han YM. Detection and characterization of focal hepatic tumors: a comparison of T2-weighted MR images before and after the administration of gadoxectic acid. J. Magn. Reson. Imaging 30, 437–443 (2009).
- Sofue K, Tsurusaki M, Tokue H, Arai Y, Sugimura K. Gd-EOB-DTPA-enhanced 3.0 T MR imaging: quantitative and qualitative comparison of hepatocyte-phase images obtained 10 min and 20 min after injection for the detection of liver metastases from colorectal carcinoma. Eur. Radiol. 21, 2336–2343 (2011).
- Motosugi U, Ichikawa T, Tominaga L et al. Delay before the hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging: is it possible to shorten the examination time? Eur. Radiol. 19, 2623–2629 (2009).
- Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. Am. J. Roentgenol. 195(1), 13–28 (2010).
- Motosugi U, Ichikawa T, Sano K et al. Double-dose gadoxetic acid-enhanced magnetic resonance imaging in patients with chronic liver disease. Invest. Radiol. 46(2), 141–145 (2011).
- Vogl TJ, Kummel S, Hammerstingl R et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd- DTPA. Radiology 200, 59 –67 (1996).
- Muhi A, Ichikawa T, Motosugi U et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J. Magn. Reson. Imaging 4(2), 326–335 (2011). & Comparative study of the diagnostic performance of various imaging techniques for the detection of hepatic metastasis from colorectal cancer.
- Huppertz A, Balzer T, Blakeborough A et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230, 266–275 (2004).
- Bluemke DA, Sahani D, Amendola M et al. Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter Phase III study. Radiology 237, 89–98 (2005).
- Woodward PJ, Kennedy A, Sohaey R. Diagnostic Imaging: Obstetrics: Published by Amirsys®. Lippincott Williams & Wilkins, NY, USA (2011).
- Motosugi U, Ichikawa T, Onohara K et al. Distinguishing hepatic metastasis from hemangioma using gadoxetic acid-enhanced magnetic resonance imaging. Invest. Radiol. 46(6), 359–365 (2011). & Summarizes the imaging features that distinguish hepatic metastasis from hemangioma using gadoxetic acidenhanced MRI.
- Goshima S, Kanematsu M, Watanabe H et al. Hepatic hemangioma and metastasis: differentiation with gadoxetate disodiumenhanced 3-T MRI. Am. J. Roentgenol. 195(4), 941–946 (2010).
- Grazioli L, Bondioni MP, Haradome H et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acidenhanced MR imaging in differential diagnosis. Radiology 262(2), 520–529 (2012).
- Zech CJ, Grazioli L, Breuer J, Reiser MF, Schoenberg SO. Diagnostic performance and description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPAenhanced liver magnetic resonance imaging: results of a multicenter trial. Invest. Radiol. 43(7), 504–511 (2008).
- Huppertz A, Balzer T, Blakeborough A et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230(1), 266–275 (2004).
- Lee JM, Zech CJ, Bolondi L et al. Consensus report of the 4th International Forum for Gadolinium-Ethoxybenzyl- Diethylenetriamine Pentaacetic Acid Magnetic Resonance Imaging. Korean J. Radiol. 12(4), 403–415 (2011).
- Giovanoli O, Heim M, Terracciano L, Bongartz G, Ledermann HP. MRI of hepatic adenomatosis: initial observations with gadoxetic acid contrast agent in three patients. Am. J. Roentgenol. 190(5), W290–W293 (2008).
- Huppertz A, Haralda S, Kraus A et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT-initial Observations. Radiology 234(2), 468–478 (2005).
- Schwartz L, Brody L, Brown K et al. Prospective, blinded comparison of helical CT and CT arterial portography in the assessment of hepatic metastasis from colorectal carcinoma. World J. Surg. 30, 1892–1899 (2006).
- Motosugi U, Ichikawa T, Nakajima H et al. Imaging of small hepatic metastases of colorectal carcinoma: how to use superparamagnetic iron oxide-enhanced magnetic resonance imaging in the multidetector-row computed tomography age? J. Comput. Assist. Tomogr. 33(2), 266–272 (2009).
- Onishi H, Murakami T, Kim T et al. Hepatic metastases: detection with multi-detector row CT, SPIO-enhanced MR imaging, and both techniques combined. Radiology 239(1), 131–138 (2006).
- Muhi A, Ichikawa T, Motosugi U et al. Diagnosis of colorectal hepatic metastases: contrast-enhanced ultrasonography versus contrast-enhanced computed tomography versus superparamagnetic iron oxideenhanced magnetic resonance imaging with diffusion-weighted imaging. J. Magn. Reson. Imaging 32(5), 1132–1140 (2010).
- Kim YK, Ko SW, Hwang SB, Kim CS, Yu HC. Detection and characterization of liver metastases: 16-slice multidetector computed tomography versus superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur. Radiol. 16(6), 1337–1345 (2006).
- Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257(3), 674–684 (2010).
- Zech CJ, Grazioli L, Jonas E et al. Healtheconomic evaluation of three imaging strategies in patients with suspected colorectal liver metastases: Gd-EOB-DTPA-enhanced MRI vs. extracellular contrast mediaenhanced MRI and 3-phase MDCT in Germany, Italy and Sweden. Eur. Radiol. 19(Suppl. 3), S753–S763 (2009).
- Del Frate C, Bazzocchi M, Mortele KJ et al. Detection of liver metastases: comparison of gadobenate dimeglumine-enhanced and ferumoxides-enhanced MR imaging examinations. Radiology. 225(3), 766–772 (2002).
- Kim YK, Lee YH, Kwak HS, Kim CS, Han YM. Detection of liver metastases: Gadoxetic acid-enhanced three-dimensional MR imaging versus ferucarbotran-enhanced MR imaging. Eur. J. Radiol. 73(1), 131–136 (2010).
- Koh DM, Collins DJ, Wallace T, Chau I, Riddell AM. Combining diffusionweighted MRI with Gd-EOB-DTPAenhanced MRI improves the detection of colorectal liver metastases. Br. J. Radiol. doi:10.1259/bjr/91771639 (2011) (Epub ahead of print).
- Kim YK, Kim CS, Han YM, Lee YH. Detection of liver malignancy with gadoxetic acid-enhanced MRI: is addition of diffusionweighted MRI beneficial? Clin. Radiol. 66(6), 489–496 (2011).
- Löwenthal D, Zeile M, Lim WY et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd- EOB-DTPA enhanced MR imaging. Eur. Radiol. 21(4), 832–840 (2011).
- Shimada K, Isoda H, Hirokawa Y, Arizono S, Shibata T, Togashi K. Comparison of gadolinium-EOB-DTPA-enhanced and diffusion-weighted liver MRI for detection of small hepatic metastases. Eur. Radiol. 20(11), 2690–2698 (2010).
- Motosugi U, Ichikawa T, Morisaka H et al. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology 260(2), 446–453 (2011). & Comparative study of the diagnostic performance of the gadoxetic acid-enhanced MRI for the detection of pancreatic cancer and associated liver metastases.
- Huppertz A, Franiel T, Wagner M et al. Whole-body MRI with assessment of hepatic and extraabdominal enhancement after administration of gadoxetic acid for staging of rectal carcinoma. Acta Radiol. 51(8), 842–850 (2010).