Review Article - Interventional Cardiology (2010) Volume 2, Issue 2
Genetic determinants of adverse outcome (restenosis, malapposition and thrombosis) after stent implantation
- Corresponding Author:
- J Wouter Jukema
Department of Cardiology
C5-P, Leiden University Medical Center
PO Box 9600, 2300 RC Leiden, The Netherlands
Tel: +31 715 266 695
Fax: +31 715 266 885
E-mail: j.w.jukema@lumc.nl
Abstract
Keywords
coronary disease, genes, malapposition, restenosis, stent, thrombosis
The era of percutaneous coronary intervention (PCI) began with the first balloon angioplasty performed by Andreas Gruentzig in 1977 [1]. Although this technique provided impressive immediate results, mid- and long-term follow‑up was characterized by high restenosis rates and the need for repeat revascularization [1,2]. Evolving our techniques, bare-metal prosthetic devices (stents) were designed to act as a barrier against intima growth and recoil, assuring long-time patency of the coronary vessel. In 1986, Sigwart and Puel implanted the first coronary stent in a human patient [3]. However, even though superior to balloon angioplasty alone (32–42% restenosis rate), bare-metal stent (BMS) implantation remains vulnerable to restenosis (22–32% of cases) [4–6] and often requires reintervention. Drug-eluting stents (DES) were conceived as an answer to this problem. For the majority, they consist of a metallic platform covered with a combination of polymer and cellular proliferation inhibitor. The antiproliferative agent is gradually released in the arterial wall at the site of stent deployment preventing restenosis. The first successful DES trials were with sirolimus stents and led to their approval for use in 2002 and 2003 in Europe and the USA, respectively [7,8]. Currently, other DES based upon paclitaxel, everolimus, zotarolimus, biolimus and tacrolimus are available. DES have successfully achieved their task of preventing restenosis, but the experience of the last years has revealed an increased incidence of stent malapposition and stent thrombosis associated with their use [9]. The aim of this article is to briefly present incidence and mechanisms of stent restenosis, stent malapposition and stent thrombosis, and to focus on potential genetic factors related to these complications. The majority of available data is retrieved from candidate gene approach studies, thus limiting the results to specific pretargeted pathophysiologic sequences. Further novel pharmacogenomic approaches, such as genome-wide association studies (GWAS) may be able to identify new genetic factors for a better prediction of outcome after coronary stent deployment.
In-stent restenosis
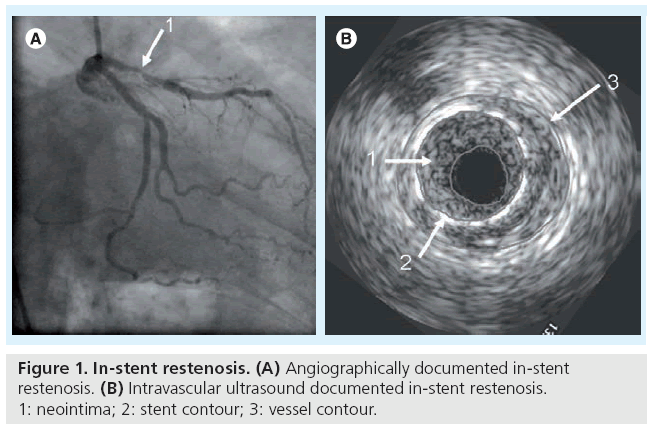
In-stent restenosis (ISR) is defined angiographically when neoformation tissue represents more than 50% of the lumen diameter at the site of the stented vessel (Figure 1). The clinical confirmation of ISR is the recurrence of angina pectoris, which requires further intervention: target lesion revascularization (TLR) or target vessel revascularization (TVR). Although the severity of angiographic stenosis correlates with the need for TLR, half of the patients with angiographically confirmed ISR do not manifest clinical complaints [6,10]. For this reason, authors generally prefer to conduct their research in relation to angiographically documented ISR when an insight into the mechanism of restenosis can be observed, while studies comparing different stents are in relation to clinically driven TLR or TVR.
In-stent restenosis is the result of in-stent cellular proliferation and migration along with extracellular matrix accumulation [11]. Classic predictors of angiographic ISR (both in BMS and DES) include diabetes, renal failure, lesion length, reference vessel diameter and postintervention lumen area [12,13]. Inflammation plays a pivotal role in ISR and it is triggered by the vascular injury during the stent deployment and by the presence of stent struts within the vessel wall [14,15]. Together with inflammation, major contributors are smooth muscle cell migration and proliferation but the process of restenosis involves many different cell-types, including platelets and endothelial cells, and is also characterized by thrombus formation, and to a lesser extent by matrix remodeling.
Genetic factors related to in-stent restenosis
▪ Genetic variations in thrombus formation
In principle, any vascular intervention initiates the formation of a thrombus. Initial studies have shown associations of only a few polymorphisms in the hemostatic system with the risk for adverse events following a PCI. These early reports showed significant associations of the PLA1/A2 polymorphism with acute stent thrombosis and coronary restenosis [16,17]. However, other studies in this field could not confirm these associations [18,19]. From the hypothesis that carriers of the PLA2 allele have a more intense binding of fibrinogen and vitronectin, and thus a higher risk of platelet-rich white thrombus formation, the PLA2 allele can be expected to lead to an increased risk for acute stent thrombosis. However, as platelet inhibition by IIb/IIIa and P2Y12 antagonists can be seen to reduce acute stent thrombosis but not in-stent restenosis rates [20], thrombus formation is probably not a main player in the development of restenosis. This hypothesis is further confirmed by findings demonstrating that especially the strong prothrombotic genetic risk factors for venous thrombosis do not increase the risk for restenosis [21]. Moreover, results from the Genetic Determinants of Restenosis (GENDER) study [21] have shown that the Factor V Leiden polymorphism (a well-known prothrombotic risk factor) was even found to reduce the risk for restenosis after PCI. A total of 3104 consecutive patients with stable angina pectoris or non-ST segment elevation myocardial infarction (non-STEMI), of whom 2309 (74.4%) received stents, were included [21]. The Factor V (1691 G>A or Factor V Leiden) amino acid substitution was associated with a decreased risk of TVR (hazard ratio [HR]: 0.41; 95% CI: 0.19–0.86). The Factor V allele, which is known to lead to increased activation of protein C, might therefore influence restenosis risk by mechanisms not involved in coagulation, but in processes that have a more prominent role in neointimal growth, such as inflammation. Even though in another study of the same patient sample, associations were found between P2Y12 receptor haplotypes and restenosis [22], fewer and smaller effects were present in the stented subgroup. The decrease of the effects in this group could be due to inhibition of this receptor by clopidogrel (although several studies [23–26] failed to demonstrate a functional role of the P2Y12 receptor polymorphism in patients receiving dual antiplatelet therapy). Therefore, the genetic variation in this receptor, and also in many other genes with a role in the hemostatic system, may have been more important at a time in which not every patient was receiving a stent and concomitant platelet inhibition.
The 4G/4G genotype of the PAI-1 4G/5G polymorphism determines higher plasminogen activator inhibitor (PAI)-1 levels in plasma [27–29] and tissue [30–32]. The PAI-1 4G allele was associated with an increased risk of restenosis after PCI in the GENDER study [21]. When compared with 5G/5G homozygotes, heterozygous patients were at higher risk for clinically driven TVR (HR: 1.46; 95% CI: 1.05–2.03), whereas patients with the 4G/4G genotype had an even further increased risk (HR: 1.69; 95% CI: 1.19–2.41). Although one smaller study could not confirm this association [33], many reports found a positive correlation between post-PCI PAI-1 levels or activity and restenosis [34,35]. Nevertheless, PAI-1 has a diverse role in several processes involved in restenosis, also in inflammation and proliferation [36]. Even if the 4G allele would increase the risk for restenosis, this could be mediated by a mechansism not related to fibrinolysis inhibition. Taking these findings together, we suggest that coagulation is not a main determinant of the long-term process that leads to restenosis.
▪ Genetic variations in inflammatory factors
Early studies investigating the role of genetics in restenosis demonstrated associations with variants in genes encoding cytokines [37] and selectins [38] – important mediators of inflammation – and suggested a role for inflammation in restenosis. One of these studies was performed by Kastrati et al., and included 1850 consecutive stented patients [37]. They demonstrated a protective effect of allele 2 of a polymorphism in exon 2 of the gene encoding the IL-1 receptor antagonist (IL-1ra), an anti-inflammatory interleukin, on both angiographic and clinical restenosis (OR: 0.78; 95% CI: 0.63–0.97 and OR: 0.73; 95% CI: 0.58–0.92, respectively). Monraats et al. have further established the important role of inflammatory genes in the development of restenosis. In the GENDER study, the rare alleles of the -260 C/T polymorphism in the CD14 gene, the 117 IIe/Thr polymorphism in the colony-stimulating factor 2 gene (also known as granulocyte–macrophage colony-stimulating factor [GM-CSF]) and the -1328 G/A polymorphism in the eotaxin gene, were associated with decreased risk of TVR [39]. Eotaxin is a chemokine that selectively recruits eosinophils and was previously reported to be elevated in plasma of patients with advanced atherosclerosis. After coronary interventions, eotaxin levels increase and remain high for at least 24 h, but no longer than 3 months [40].
Furthermore, the variant alleles of two promoter polymorphisms in the TNF-α gene have been shown to protect against the development of restenosis [41]. Stented patients with the -238A/A genotype (HR: 0.44; 95% CI: 0.23–0.83) and patients with the -1031C/C genotype (HR: 0.72; 95% CI: 0.52–1.00) needed TVR less frequently. Several other inflammatory genes were demonstrated to be involved in the process of restenosis in this cohort, such as IL-10 and caspase-1 (IL-1bconverting enzyme) [42,43]. All these findings support the hypothesis that restenosis is largely (albeit not solely) determined by inflammation.
▪ Genes involved in smooth muscle cell proliferation
Stents specifically aiming to inhibit inflammation (dexamethasone-eluting stents) were not proven to be as effective as stents inhibiting both inflammation and cell proliferation [44]. Despite the fact that restenosis is mainly determined by proliferation and migration of vacular smooth muscle cells (VSMCs), relatively few studies investigated genes involved in proliferation, such as cell-cycle regulatory genes. A recent important finding in this field by Van Tiel et al. was an association between the -838 G/A polymorphism in the cyclin-dependent kinase inhibitor p27(kip1) (a key regulator of smooth muscle cell proliferation) with ISR [45]. Three polymorphisms concerning the p27(kip1) gene (-838C>A; -79C>T; +326G>T) were determined in a cohort of 715 patients undergoing coronary angioplasty and stent placement. Patients with the p27(kip1) -838AA genotype had a decreased risk of ISR (HR: 0.28; 95% CI: 0.10–0.77). This finding was replicated in another cohort study of 2309 patients (HR: 0.61; 95% CI: 0.40–0.93). The -838 A allele corresponded to enhanced promoter activity, which in turn may explain decreased smooth muscle cell proliferation.
▪ Genetic variations in matrix metalloproteinases
Matrix metalloproteinases (MMPs) are Zn2+- requiring proteases capable of degrading a variety of extracellular matrix components. Owing to their significance in vascular remodeling, MMPs are suspected to play an important role in the pathogenesis of atherosclerosis and restenosis [46]. In particular, MMP2, MMP3 and MMP9 are potential players in the process of restenosis after PCI. MMP2 and MMP9 (the gelatinases) are produced by VSMCs and degrade basement membrane components and other matrix proteins to allow migration and proliferation of VSMCs [47]. They are upregulated and activated in VSMCs during intima formation in many different animal models for restenosis involving balloon angioplasty [47]. An increase in MMP2 levels and activity was demonstrated in human coronary sinus blood samples 4 and 24 h after elective coronary angioplasty [48]. This small study, in which only 21 out of 47 patients were stented, also showed an association between MMP2 levels and restenosis. MMP3 (stromelysin-1) expression has been found to be related to plaque instability in pathological studies [49]. MMP3 reduces the matrix content of the vascular wall and is therefore expected to protect against restenosis [49]. Functional studies have shown that the MMP3 -1612 5A/6A promoter polymorphism is associated with altered MMP3 expression. Carriers of the 6A/6A genotype were found to have a reduced MMP3 expression [50–53] and were at increased risk of developing restenosis in a subset of the Regression Growth Evaluation Statin Study (REGRESS) in which stents were not yet frequently used [54], and in two other studies with luminal narrowing after plain balloon angioplasty [55,56]. However, an association between the MMP3 5A/6A polymorphism could not be confirmed in a study that included 217 stented patients. In addition, unpublished results from the GENDER study demonstrated no association between this polymorphism and clinical restenosis in stented patients. Therefore, even though matrix formation is an important process in the development of restenosis, variations in genes involved in matrix remodeling were infrequently investigated or studies yielded negative results and were not published.
Stent malapposition
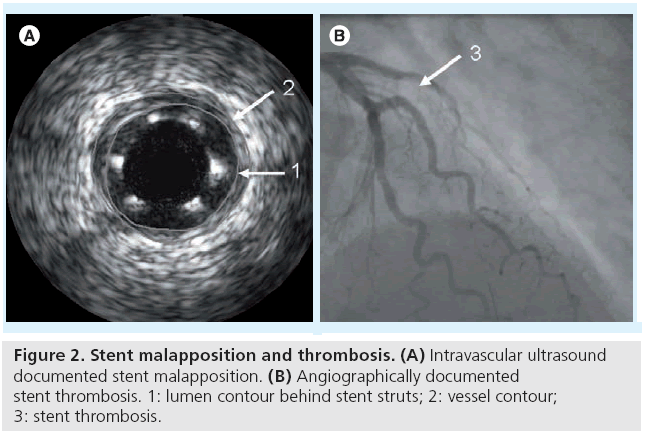
Stent malapposition (SM), commonly detected by intravascular ultrasonography (IVUS), represents a separation of the stent struts from the intimal surface of the arterial wall (in the absence of a side branch) with evidence of blood speckles behind the struts (Figure 2A) [57]. SM may be acute (present immediately after implantation), persistent (present both immediately after implantation and at follow-up) or late-acquired (present only at follow-up). Acute and persistent SM are mainly procedure driven while late-acquired stent malapposition (LASM) is a consequence of positive remodeling of the vessel wall and/or of plaque volume decrease behind the stent (including clot lysis or plaque regression) [58–62]. The main repercussion of late SM (persistent or acquired) is stent thrombosis (ST) [9]. Independent predictors of LASM include lesion length, unstable angina, absence of diabetes and primary stenting in acute myocardial infarction (MI) [63,59]. The risk of LASM in patients with DES is approximately four-times higher compared with those with BMS [9]. This is due to the fact that in BMS, hypersensitivity to the metallic stent is mostly associated with restenosis, whereas in DES, hypersensitivity to the metallic stent, the polymer or to the drug is associated with positive remodeling and excessive inflammation in the vessel wall [64].
Genetic factors related to stent malapposition
We have previously investigated seven polymorphisms involved in inflammatory processes and related to restenosis on the risk of LASM in sirolimus-eluting stent (SES) patients [65]. In total, 104 STEMI patients from the MISSION intervention study [62] were genotyped for the caspase-1 5352 G/A, eotaxin 1382 A/G, CD14 260 A/G, colony-stimulating factor 2 1943 C/T, IL10 -1117 C/T , IL10 4251 C/T and the TNF-α 1211 C/T polymorphisms. LASM occurred in 26 out of 104 (25%) patients. We found a significantly higher risk for LASM in patients carrying the caspase-1 (CASP1) 5352 A allele (RR: 2.32; 95% CI: 1.22–4.42). In addition, mean neointimal growth was significantly lower in patients carrying this LASM risk allele (1.6 vs 4.1%; p = 0.014). The other six polymorphisms related to inflammation were not significantly related to the risk of LASM. Given the limited number of patients included in the study, similar reports are needed to confirm our findings. Moreover, a direct relation between the CASP1 5352 A allele and the risk of ST was not investigated. To our knowledge, no other studies have yet scrutinized the role of genetic variations in LASM.
Stent thrombosis
Stent thrombosis (Figure 2B) is a complication that occurs in 0.8–2% of patients undergoing PCI and is associated with large MI and death [66]. ST is categorized into acute ST (within 24 h from stent implantation), subacute ST (within 1–30 days from stent implantation), late ST (within 30 days – 1 year) and very late ST (>1 year after stent implantation). Subacute and acute ST are classically related to procedure parameters such as stent underdeployment (acute SM) [67,68] or procedure-related complications such as coronary dissections [69,70]. By contrast, (very) late ST appears to be an active phenomenon associated with late SM (persistent or acquired) [9,71], stent type [9], duration of dual antiplatelet therapy [66] and inflammation [58]. Gene variations in the platelet aggregation pathway, responsiveness to clopidogrel or presence of inherited thrombophilic disorders were associated with both acute and late ST.
Genetic factors related to stent thrombosis
▪ Platelet receptor gene polymorphism
Platelet aggregation involves the binding of fibrinogen to the glycoprotein (GP) IIb/IIIa receptor on the platelet surface. One polymorphism of the GP IIIa gene (PLA1/A2 or HPA1a/1b) has been related to the inherited risk of coronary thrombosis [72]. Of importance, the same polymorphism had no influence on the degree of myocardial salvage achieved in 133 acute MI patients undergoing coronary stenting and abciximab administration [73]. The PLA2 polymorphism is a substitution of cytosine for thymidine at position 1565 in exon 2. Walter et al. investigated the association of the PLA2 allele with acute and subacute stent thrombosis in 318 consecutive BMS patients stented for coronary dissection, acute occlusion or high residual restenosis after percutaneous transluminal coronary angioplasty, lesions in bypass grafts and restenotic lesions [74]. They found that patients with the PLA2 allele had an increased risk of stent thrombosis compared with patients homozygous for PLA1 (OR: 5.26; 95% CI: 1.55–17.85). Kastrati et al. partially confirmed these findings in their prospective study including 1759 patients with stable and unstable angina pectoris [75]. No difference was observed at 30 days after stent placement in terms of ST or a composite end point of death, MI or urgent revascularization between PLA1/A1 and PLA1/A2 carriers. However, the incidence of ST and the composite end point were higher in the PLA2 homozygotes versus PLA1 homozygotes (8.7 vs 1.7%; p = 0.002 and 13.0 vs 5.4%; p = 0.06, respectively).
More recently, Sucker et al. assessed the relevance of prothrombotic platelet-receptor polymorphisms for the onset of coronary stent thrombosis in 316 patients [76]. They compared the prevalence of GP Ibα, GP IIb, GP IIIa (including PLA1/A2) and GP Ia prothrombotic polymorphisms in patients with coronary stent thrombosis occurring in the first 6 month after stent implantation and healthy control subjects. Carriers of the aforementioned prothrombotic versions did not appear to be at any increased risk for stent thrombosis. Selection of patients (differences in number of elective and acute stent implantations) and the treatment of more complex coronary lesions in the latter study or the limited power might explain these discrepancies [76]. Angiolillo et al. have investigated the differential platelet sensitivity between PLA1 homozygotes and PLA2 carriers in 38 patients undergoing coronary stent implantation and receiving a clopidogrel 300 mg loading dose [77]. They demonstrated that PLA2 carriers have a lower inhibition of platelet reactivity following the standard clopidogrel loading dose, which might finally lead to stent thrombosis.
▪ Genetic variations in response to clopidogrel
In current practice, patients undergoing PCI and stent deployment are prescribed clopidogrel 300–600 mg as a loading dose followed by 1 year dual antiplatelet therapy (aspirin 80–325 mg and clopidogrel 75 mg daily) and continued with life-long aspirin intake. A good responsiveness to clopidogrel is therefore crucial in order to prevent thrombotic events following stent deployment.
Clopidogrel is an inactive prodrug that requires two-step oxidation by the hepatic cytocrome P450 (CYP) enzymes to transform into an active metabolite that further inhibits the ADP P2Y12 receptor producing the antiaggregation effect. The genes encoding the CYP enzymes are polymorphic and several variants were related to a decreased catalytic activity and subsequent attenuated effect of the drug.
The CYP3A5 gene has a functional polymorphism that includes the expressor (*1) and nonexpressor (*3) alleles [78,79]. Suh et al. compared clinical outcome in 348 patients (with stable angina, unstable angina or non-STEMI) who had PCI with BMS implantation [79]. Antiplatelet therapy consisted of aspirin (100– 300 mg daily, prescribed indefinitely) and clopidogrel (75 mg daily after 300 mg loading dose) administered for at least 4 weeks after the procedure. Atherothrombotic events (a composite of cardiac death, MI and nonhemorrhagic stroke) occurred more frequently within 6 months after stent implantation among the patients with the nonexpressor genotype than among those with the expressor genotype (14/193 vs 3/155; p = 0.023). Moreover, the CYP3A5 polymorphism was a predictor of athrothrombotic events in clopidogrel users.
These findings are especially interesting since a number of studies (which were not aimed at clinical end points) found no association between the CYP3A5 variants and clopidogrel response and/or residual platelet aggregation (RPA) [80–82] nor did a number of studies with clinical end points [83,84].
Trenk et al. investigated whether the CYP2C19 681G>A *2 polymorphism was associated with high (>14%) RPA on clopidogrel and whether high on-clopidogrel RPA affects clinical outcome after elective coronary stent placement [85]. RPA was assessed in 797 consecutive patients after a 600-mg loading dose and after the first 75 mg maintenance dose of clopidogrel before discharge. Patients were followed-up for 1 year. Between the *2 carriers and *1/*1 carriers (wildtype) the authors found significant (p < 0.001) differences in the proportion of patients with RPA>14%, both after loading (62.4 vs 43.4%, respectively) and at predischarge (41.3 vs 22.5%, respectively). RPA over 14% at discharge was associated with a threefold increase (95% CI: 1.4–6.8; p = 0.004) in the 1‑year incidence of death and MI. However, the authors could not demonstrate a direct relation between the CYP2C19*2 allele and clinical outcome.
This relationship was demonstrated by Giusti et al. in a subanalysis of the The Low Responsiveness to Clopidogrel and Sirolimus- or Paclitaxel-Eluting Stent Thrombosis (RECLOSE) trial [86]. The role of the CYP2C19*2 polymorphism in the occurrence of DES ST (definite or probable) or the composite end point of ST (definite or probable) and cardiac mortality within 6‑month follow-up was assessed in 772 patients undergoing PCI and receiving either sirolimus or paclitaxel DES. Patients with acute coronary syndrome (ACS) and STEMI were included as well as patients with left main disease, chronic total occlusions, bifurcation lesions or diffuse disease. All patients received aspirin (325 mg) and a loading dose of clopidogrel 600 mg before the procedure, followed by a maintenance dose of clopidogrel 75 mg and aspirin 325 mg daily. Patients with ST or ST and cardiac mortality end point had a higher prevalence of the *2 allele (54.1 vs 31.3%; p = 0.025 and 51.7 vs 31.2%; p = 0.020, respectively). At multivariate logistic regression analysis, the CYP2C19*2 allele was an independent risk factor for ST (OR: 3.43; 95% CI: 1.01– 12.78; p = 0.047) and ST and cardiac mortality (OR: 2.7; 95% CI: 1.00–8.42; p = 0.049).
Mega et al. reconfirmed these findings on long-term assessment of patients from TRITON-TIMI 38 study [83]. Of 1389 patients treated with clopidogrel who underwent PCI and stenting, a number were followed-up for 15 months. Patients were initially admitted with non-STEMI (71%) and STEMI (29%). They received a clopidogrel 300 mg loading dose, followed by 75 mg daily maintenance dose for up to 15 months. For CYP2C19, the presence of at least one copy of the *2 allele was associated with a higher rate of composite death from cardiovascular causes, non-fatal MI, non-fatal stroke (HR: 1.42; 95% CI: 0.98– 2.05) and of definite/probable ST (HR: 3.33; 95% CI: 1.28–8.62) than did non-carriers.
Sibbing et al. assessed the role of the mutant *2 allele of the CYP2C19 polymorphism on the 30‑day incidence of definite ST in 2485 consecutive patients undergoing coronary stent placement [87]. There are a number of differences with regard to the previous study [83]: STEMI patients were excluded; the end point was acute and subacute definite ST; and, patients received clopidogrel 600 mg loading dose.
Drug-eluting stents were used in 25% and BMS in 75% of the patients. Of the patients studied, 73% were CYP2C19 wild-type homozygotes (*1/*1) and 27% carried at least one of the *2 allele. The cumulative 30‑day incidence of ST was significantly higher in CYP2C19*2 allele carriers versus wild-type homozygotes (1.5 vs 0.4%; HR: 3.81; 95% CI: 1.45–10.02; p = 0.006). The risk of ST was highest (2.1%) in patients carrying the CYP2C19 *2/*2 genotype (p = 0.002).
Recently, Collet et al. demonstrated the role of the CYP2C19*2 allele in 259 young patients (aged <45 years) who survived a first MI and received clopidogrel treatment for at least 1 month [88]. The primary end point was a composite of death, MI and urgent coronary revascularization occurring during exposure to clopidogrel. The secondary end point was angiography- documented ST. Median clopidogrel treatment duration was approximately 1 year. The primary end point occurred more frequently in carriers than in noncarriers (15 vs 11 events; HR: 3.69; 95% CI: 1.69–8.05; p = 0.0005), as did ST (eight vs four events; HR: 6.02; 95% CI: 1.81–20.04; p = 0.0009). The effect of the CYP2C19*2 genetic variant persisted from 6 months after clopidogrel initiation up to the end of follow-up (HR: 3.00; 95% CI: 1.27–7.10; p = 0.009). The CYP2C19*2 genetic variant appeared to be the only independent predictor of cardiovascular events (HR: 4.04; 95% CI: 1.81–9.02; p = 0.0006).
In a study of 2208 patients presenting with acute MI (among which 1535 underwent PCI), patients carrying any two CYP2C19 loss-offunction alleles (*2, *3, *4 or *5), had a higher rate of death from any cause, nonfatal stroke or MI during 1 year of follow-up than patients with none (21.5 vs 13.3%; adjusted HR: 1.98; 95% CI: 1.10–3.58) [84]. Among the patients who underwent PCI during hospitalization, the rate of cardiovascular events among carriers of CYP2C19 loss-of-function alleles was 3.58-times higher (95% CI: 1.71–7.51) than among those with none.
In order to develop a risk score with better prediction of RPA, Geisler et al. analyzed the CYP2C19*2 genotype and previously identified nongenetic risk factors (age >65 years, Type 2 diabetes mellitus, decreased left ventricular function, renal failure and ACS) [81]. They demonstrated a significant correlation of the nongenetic factors (c2 = 5.32; p = 0.021) and CYP2C19*2 (c2= 21.31; p < 0.0001) with high RPA, and the highest association for the combination of both (c2= 25.85; p < 0.0001). This was the first study to demonstrate that prediction of clopidogrel responsiveness may be substantially improved by adding the CYP2C19*2 genotype to nongenetic risk factors. The important influence of the CYP2C19*2 genotype over platelet function and cardiovascular outcomes was recently confirmed by Shuldiner et al. in the first GWAS paper identifying CYP2C19 as a candidate gene. In the Pharmacogenomics of Antiplatelet Intervention (PAPI) Study, clopidogrel was administered for 7 days to 429 healthy individuals and the response was measured by ex vivo platelet aggregometry [89]. A GWAS was performed followed by genotyping the loss-offunction cytochrome CYP2C19*2 variant. The relation between CYP2C19*2 genotype and platelet aggregation was replicated in 227 clopidogrel- treated patients undergoing PCI (p = 0.02). Patients with the CYP2C19*2 variant were more likely (20.9 vs 10.0%) to have a cardiovascular ischemic event or death during 1 year of follow-up (HR: 2.42; 95% CI: 1.18–4.99; p = 0.02).
▪ Factor V Leiden mutation
Factor V Leiden is the most common inherited thrombophilic disorder, resulting from a single mutation (1691 G>A) in the Factor V gene. Individuals heterozygous for this mutation are at increased risk for venous thrombosis, and in homozygous individuals the risk becomes extremely high. Although conceivable, there is only one case report to document a possible relation between a Factor V Leiden heterozygous patient and stent thrombosis (simultaneous occlusion of two stents, one in the left anterior descending artery and one in the right coronary artery at 4 days after implantation in a patient receiving standard dual antiplatelet therapy) [90]. Further larger studies are therefore needed before Factor V Leiden may be linked to ST.
Limitations
Many studies have managed to identify genes and polymorphisms involved in the post-stenting outcome after scrutinizing various plausible pathophysiologic mechanisms. However, to predict an accurate scale of adverse effects, an interaction assessment between genetic, nongenetic (traditional risk factors) as well as epigenetic factors is of extreme importance. This information remains momentarily scarce.
Also of importance, findings from certain studies sometimes cannot be confirmed by other studies. This is largely explained by variation in study settings and therefore the replication of findings in independent studies needs to be further emphasized.
The candidate gene approach used to date in the majority of investigations narrows the results to specific areas of interest.
Conclusion
In-stent restenosis and stent thrombosis remain important limitations of the current PCI practice. Besides the procedure-related risk factors and medication, solid evidence demonstrated that a patient’s own response to stent implantation influences the outcome. Individual genetic responses involve inflammation, cellular proliferation, platelet receptors and drug metabolism pathways. A better understanding of stent pathology has led to the identification of new important genes and genetic polymorphisms. These discoveries may help us better identify the vulnerable patients who need extraordinary therapeutic measures. Conversely, genetic–epidemiologic studies have identified genes that have subsequently revealed important pathophysiologic mechanisms.
Future perspective
The speed by which new genes are being related to stent pathology is matched by the speed of new developments in stent technology and medication. Novel pharmacogenomic approaches (e.g., GWAS and the 1000 genome project) may help to identify unknown genetic factors for a better prediction of outcome after stent implantation [91].
It is, however, difficult to predict whether screening for established polymorphisms will prove, in the future, a cost-effective method for improved stent-type selection or medication in the daily routine.
The classic stents appear to be rapidly being replaced by new and complex body–polymer– drug constructs that address most, if not all, of the current problems. The new generation of stents may appear capable of modulating local inflammation to permit a good re-endothelization, to prevent stent thrombosis, to reduce the duration of antiplatelet medication and, if necessary, even to degrade after local healing is achieved.
New drugs such as prasugrel, ticagrelor and cangrelor seem to effectively inhibit platelet aggregation with little or no interindividual response variability. The combination of lessons learned from genetic and pathophysiologic studies, the newly available resources (e.g., stents, antiplatelet drugs and imaging) and refined implantation techniques will definitely improve PCI performances and extend its use.
Financial & competing interests disclosure
Professor Dr J Wouter Jukema is an established clinical investigator of the Netherlands Heart Foundation (2001D032) and project leader of the HP 7 – Pharmacogenetic EU project HEALTH-F2–2007 223004 PHASE. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Genetic variants associated with an increased or decreased risk of in-stent restenosis
▪ Genetic variations in thrombus formation.
-- Associated with increased risk – 4G allele of the PAI-1 4G/5G polymorphism.
-- Associated with decreased risk – Factor V 1691G>A (Factor V Leiden) amino acid substitution.
▪ Genetic variations in inflammatory factors.
-- Associated with increased risk – A/A genotype of the IL-10 -2849 G/A polymorphism; A/A genotype of the IL-10 -1082 G/A polymorphism; G/G genotype of the IL-10 +4259 A/G polymorphism; A/A genotype of the caspase-1 5352 G/A polymorphism.
-- Associated with decreased risk – *2 allele of the IL-1RA gene; T/T genoype of the CD14–260 C/T polymorphism; Thr allele of the CSF2–117 Ile/Thr polymorphism; A allele of the CCL 11 (eotaxin) 1328 G/A polymorphism; A/A genotype of the TNF -238 G/A polymorphism; C/C genotype of the TNF -1031 T/C polymorphism.
▪ Genes involved in smooth muscle cell proliferation.
-- Associated with decreased risk – A/A genotype of the p27(kip1)-838G/A polymorphism.
Genetic variants associated with an increased risk of stent thrombosis
▪ Platelet receptor gene polymorphism.
-- PLA2 allele of the GPIIIa PLA1/A2.
▪ Genetic variations in response to clopidogrel.
-- *3 allele of the CYP3A5 gene (encodes hepatic cytocrome P450 CYP enzymes); *2 allele of the CYP2C19 gene (encodes hepatic cytocrome P450 CYP enzymes).
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Gruntzig AR, Senning A, Siegenthaler WE: Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 301(2), 61–68 (1979).
- Holmes DR Jr, Vlietstra RE, Smith HC et al.: Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am. J. Cardiol. 53(12), 77C–81C (1984).
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L: Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 316(12), 701–706 (1987).
- Fischman DL, Leon MB, Baim DS et al.: A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 331(8), 496–501 (1994).
- Serruys PW, de Jaegere P, Kiemeneij F et al.: A comparison of balloon-expandablestent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331(8), 489–495 (1994).
- Cutlip DE, Chhabra AG, Baim DS et al.: Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation 110(10), 1226–1230 (2004).
- Moses JW, Leon MB, Popma JJ et al.: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Serruys PW, Kutryk MJ, Ong AT: Coronaryartery stents. N. Engl. J. Med. 354(5), 483–495 (2006).
- Hassan AK, Bergheanu SC, Stijnen T et al.: Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. Eur. Heart J. doi : 10.1093/ eurheartj/ehn553 (2010) (Epub ahead of print).
- Scott NA: Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Deliv. Rev. 58(3), 358–376 (2006).
- Hoffmann R, Mintz GS, Dussaillant GR et al.: Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation 94(6), 1247–1254 (1996).
- Rathore S, Terashima M, Katoh O et al.: Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: contemporary practice in real world patients. EuroIntervention 5(3), 349–354 (2009).
- Bhargava B, Karthikeyan G, Abizaid AS, Mehran R: New approaches to preventing restenosis. BMJ 327(7409), 274–279 (2003).
- Farb A, Sangiorgi G, Carter AJ et al.: Pathology of acute and chronic coronary stenting in humans. Circulation 99(1), 44–52 (1999).
- Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R: Morphological predictors of restenosis after coronary stenting in humans. Circulation 105(25), 2974–2980 (2002).
- Kastrati A, Schomig A, Seyfarth M et al.: PlA polymorphism of platelet glycoprotein IIIa and risk of restenosis after coronary stent placement. Circulation 99(8), 1005–1010 (1999).
- Wheeler GL, Braden GA, Bray PF, Marciniak SJ, Mascelli MA, Sane DC: Reduced inhibition by abciximab in platelets with the PlA2 polymorphism. Am. Heart J. 143(1), 76–82 (2002).
- Mamotte CD, van Bockxmeer FM, Taylor RR: PIa1/a2 polymorphism of glycoprotein IIIa and risk of coronary artery disease and restenosis following coronary angioplasty. Am. J. Cardiol. 82(1), 13–16 (1998).
- Volzke H, Grimm R, Robinson DM et al.: Candidate genetic markers and the risk of restenosis after coronary angioplasty. Clin. Sci. (Lond.) 106(1), 35–42 (2004).
- Acute platelet inhibition with abciximab does not reduce in-stent restenosis (ERASER study). The ERASER Investigators. Circulation 100(8), 799–806 (1999).
- Pons D, Monraats PS, de Maat MP et al.: The influence of established genetic variation in the haemostatic system on clinical restenosis after percutaneous coronary interventions. Thromb. Haemost. 98(6), 1323–1328 (2007).
- Rudez G, Pons D, Leebeek F et al.: Platelet receptor P2RY12 haplotypes predict restenosis after percutaneous coronary interventions. Hum. Mutat. 29(3), 375–380 (2008).
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E et al.: Lack of association between the P2Y12 receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb. Res. 116(6), 491–497 (2005).
- Giusti B, Gori AM, Marcucci R et al.: Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet.Genomics 17(12), 1057–1064 (2007).
- Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS: Genetic polymorphisms of the platelet receptors P2Y(12), P2Y(1) and GP IIIa and response to aspirin and clopidogrel. Thromb. Res. 119(3), 355–360 (2007).
- von Beckerath N, von Beckerath O, Koch W, Eichinger M, Schomig A, Kastrati A: P2Y12 gene H2 haplotype is not associated with increased adenosine diphosphate-induced platelet aggregation after initiation of clopidogrel therapy with a high loading dose. Blood Coagul. Fibrinolysis 16(3), 199–204 (2005).
- Kathiresan S, Gabriel SB, Yang Q et al.: Comprehensive survey of common genetic variation at the plasminogen activator inhibitor-1 locus and relations to circulating plasminogen activator inhibitor-1 levels. Circulation 112(12), 1728–1735 (2005).
- Diamanti-Kandarakis E, Palioniko G, Alexandraki K, Bergiele A, Koutsouba T, Bartzis M: The prevalence of 4G5G polymorphism of plasminogen activator inhibitor-1 (PAI-1) gene in polycystic ovarian syndrome and its association with plasma PAI-1 levels. Eur. J.Endocrinol. 150(6), 793–798 (2004).
- Asselbergs FW, Williams SM, Hebert PR et al.: The gender-specific role of polymorphisms from the fibrinolytic, renin-angiotensin, and bradykinin systems in determining plasma t-PA and PAI-1 levels. Thromb. Haemost. 96(4), 471–477 (2006).
- Castello R, Espana F, Vazquez C et al.: Plasminogen activator inhibitor-1 4G/5G polymorphism in breast cancer patients and its association with tissue PAI-1 levels and tumor severity. Thromb. Res. 117(5), 487–492 (2006).
- Burzotta F, Iacoviello L, Di Castelnuovo A et al.: 4G/5G PAI-1 promoter polymorphism and acute-phase levels of PAI-1 following coronary bypass surgery: a prospective study. J. Thromb.Thrombolysis. 16(3), 149–154 (2003).
- Festa A, D’Agostino R Jr, Rich SS, Jenny NS, Tracy RP, Haffner SM: Promoter (4G/5G) plasminogen activator inhibitor-1 genotype and plasminogen activator inhibitor-1 levels in Blacks, Hispanics, and non-Hispanic Whites: the Insulin Resistance Atherosclerosis Study. Circulation 107(19), 2422–2427 (2003).
- Bottiger C, Koch W, Lahn C et al.: 4G/5G polymorphism of the plasminogen activator inhibitor-1 gene and risk of restenosis after coronary artery stenting. Am. Heart J. 146(5), 855–861 (2003).
- Prisco D, Fedi S, Antonucci E et al.: Postprocedural PAI-1 activity is a risk marker of subsequent clinical restenosis in patients both with and without stent implantation after elective balloon PTCA. Thromb. Res. 104(3), 181–186 (2001).
- Ishiwata S, Tukada T, Nakanishi S, Nishiyama S, Seki A: Postangioplasty restenosis: platelet activation and the coagulation-fibrinolysis system as possible factors in the pathogenesis of restenosis. Am. Heart J. 133(4), 387–392 (1997).
- Hoekstra T, Geleijnse JM, Schouten EG, Kluft C: Plasminogen activator inhibitor-type 1: its plasma determinants and relation with cardiovascular risk. Thromb. Haemost. 91(5), 861–872 (2004).
- Kastrati A, Koch W, Berger PB et al.: Protective role against restenosis from an interleukin-1 receptor antagonist gene polymorphism in patients treated with coronary stenting. J. Am. Coll. Cardiol. 36(7), 2168–2173 (2000).
- Rauchhaus M, Gross M, Schulz S et al.: The E-selectin SER128ARG gene polymorphism and restenosis after successful coronary angioplasty. Int. J.Cardiol. 83(3), 249–257 (2002).
- Monraats PS, Pires NM, Agema WR et al.: Genetic inflammatory factors predict restenosis after percutaneous coronary interventions. Circulation 112(16), 2417–2425 (2005).
- Economou E, Tousoulis D, Katinioti A et al.: Chemokines in patients with ischaemic heart disease and the effect of coronary angioplasty. Int. J.Cardiol. 80(1), 55–60 (2001).
- Monraats PS, Pires NM, Schepers A et al.: Tumor necrosis factor-a plays an important role in restenosis development. FASEB J. 19(14), 1998–2004 (2005).
- Monraats PS, Kurreeman FA, Pons D et al.: Interleukin 10: a new risk marker for the development of restenosis after percutaneous coronary intervention. Genes Immun. 8(1), 44–50 (2007).
- Monraats PS, de Vries F, de Jong LW et al.: Inflammation and apoptosis genes and the risk of restenosis after percutaneous coronary intervention. Pharmacogenet. Genomics 16(10), 747–754 (2006).
- Pesarini G, Ferrero V, Tomai F et al.: Steroid-eluting stents in patients with acute coronary syndromes. Angiographic results of DESIRE: Dexamethasone-Eluting Stent Italian Registry. J. Invasive.Cardiol. 21(3), 86–91 (2009).
- van Tiel CM, Bonta PI, Rittersma SZ et al.: p27kip1–838C>A single nucleotide polymorphism is associated with restenosis risk after coronary stenting and modulates p27kip1 promoter activity. Circulation 120(8), 669–676 (2009).
- Agema WR, Jukema JW, Pimstone SN, Kastelein JJ: Genetic aspects of restenosis after percutaneous coronary interventions: towards more tailored therapy. Eur. Heart J. 22(22), 2058–2074 (2001).
- Newby AC: Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 69(3), 614–624 (2006).
- Hojo Y, Ikeda U, Katsuki T, Mizuno O, Fujikawa H, Shimada K: Matrix metalloproteinase expression in the coronary circulation induced by coronary angioplasty. Atherosclerosis 161(1), 185–192 (2002).
- Ye S: Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc. Res. 69(3), 636–645 (2006).
- Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM: Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J. Biol.Chem. 271(22), 13055–13060 (1996).
- Beyzade S, Zhang S, Wong YK, Day IN, Eriksson P, Ye S: Influences of matrix metalloproteinase-3 gene variation on extent of coronary atherosclerosis and risk of myocardial infarction. J. Am. Coll. Cardiol. 41(12), 2130–2137 (2003).
- Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ: Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ. Res. 92(11), 1254–1261 (2003).
- Lichtinghagen R, Bahr MJ, Wehmeier M et al.: Expression and coordinated regulation of matrix metalloproteinases in chronic hepatitis C and hepatitis C virus-induced liver cirrhosis. Clin. Sci. (Lond.) 105(3), 373–382 (2003).
- De Maat MP, Jukema JW, Ye S et al.: Effect of the stromelysin-1 promoter on efficacy of pravastatin in coronary atherosclerosis and restenosis. Am. J. Cardiol. 83(6), 852–856 (1999).
- Humphries S, Bauters C, Meirhaeghe A, Luong L, Bertrand M, Amouyel P: The 5A6A polymorphism in the promoter of the stromelysin-1 (MMP3) gene as a risk factor for restenosis. Eur. Heart J. 23(9), 721–725 (2002).
- Hoppmann P, Koch W, Schomig A, Kastrati A: The 5A/6A polymorphism of the stromelysin-1 gene and restenosis after percutaneous coronary interventions. Eur. Heart J. 25(4), 335–341 (2004).
- Mintz GS, Nissen SE, Anderson WD et al.: American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 37(5), 1478–1492 (2001).
- Cook S, Wenaweser P, Togni M et al.: Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 115(18), 2426–2434 (2007).
- Hong MK, Mintz GS, Lee CW et al.: Incidence, mechanism, predictors, and long-term prognosis of late stent malapposition after bare-metal stent implantation. Circulation 109(7), 881–886 (2004).
- Mintz GS, Shah VM, Weissman NJ: Regional remodeling as the cause of late stent malapposition. Circulation 107(21), 2660–2663 (2003).
- Shah VM, Mintz GS, Apple S, Weissman NJ: Background incidence of late malapposition after bare-metal stent implantation. Circulation 106(14), 1753–1755 (2002).
- van der Hoeven BL, Liem SS, Dijkstra J et al.: Stent malapposition after sirolimus-eluting and bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: acute and 9-month intravascular ultrasound results of the MISSION! intervention study. JACC. Cardiovasc. Interv. 1(2), 192–201 (2008).
- Tanabe K, Serruys PW, Degertekin M et al.: Incomplete stent apposition after implantation of paclitaxel-eluting stents or bare metal stents: insights from the randomized TAXUS II trial. Circulation 111(7), 900–905 (2005).
- Virmani R, Farb A, Guagliumi G, Kolodgie FD: Drug-eluting stents: caution and concerns for long-term outcome. Coron. Artery Dis. 15(6), 313–318 (2004).
- Bergheanu SC, Pons D, van der Hoeven B et al.: Inflammatory polymorphisms and the risk of late-acquired stent malapposition. J. Am. Coll. Cardiol. 53(10), A392–A392 (2009).
- Schulz S, Schuster T, Mehilli J et al.: Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur. Heart J. 30(22), 2714–2721 (2009).
- Cutlip DE, Leon MB, Ho KK et al.: Acute and nine-month clinical outcomes after ‘suboptimal’ coronary stenting: results from the Stent Anti-thrombotic Regimen Study (STARS) registry. J. Am. Coll. Cardiol. 34(3), 698–706 (1999).
- Uren NG, Schwarzacher SP, Metz JA et al.: Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur. Heart J. 23(2), 124–132 (2002).
- Huber MS, Mooney JF, Madison J, Mooney MR: Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am. J. Cardiol. 68(5), 467–471 (1991).
- van Werkum JW, Heestermans AA, Zomer AC et al.: Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 53(16), 1399–1409 (2009).
- Bergheanu SC, van der Hoeven BL, Hassan AKM et al.: Post-intervention IVUS is not predictive for very late in-stent thrombosis in drug-eluting stents. Acta Cardiologica 64(5), 611–616 (2009).
- Weiss EJ, Bray PF, Tayback M et al.: A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N. Engl. J. Med. 334(17), 1090–1094 (1996).
- Gorchakova O, Koch W, Mehilli J et al.: PlA polymorphism of the glycoprotein IIIa and efficacy of reperfusion therapy in patients with acute myocardial infarction. Thromb. Haemost. 91(1), 141–145 (2004).
- Walter DH, Schachinger V, Elsner M, Dimmeler S, Zeiher AM: Platelet glycoprotein IIIa polymorphisms and risk of coronary stent thrombosis. Lancet 350(9086), 1217–1219 (1997).
- Kastrati A, Koch W, Gawaz M et al.: PlA polymorphism of glycoprotein IIIa and risk of adverse events after coronary stent placement. J. Am. Coll. Cardiol. 36(1), 84–89 (2000).
- Sucker C, Scheffold N, Cyran J, Ghodsizad A, Scharf RE, Zotz RB: No evidence for involvement of prothrombotic platelet receptor polymorphisms in acute coronary stent thrombosis. Int. J. Cardiol. 123(3), 355–357 (2008).
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E et al.: PlA polymorphism and platelet reactivity following clopidogrel loading dose in patients undergoing coronary stent implantation. Blood Coagul. Fibrinolysis 15(1), 89–93 (2004).
- Kuehl P, Zhang J, Lin Y et al.: Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 27(4), 383–391 (2001).
- Suh JW, Koo BK, Zhang SY et al.: Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ 174(12), 1715–1722 (2006).
- Brandt JT, Close SL, Iturria SJ et al.: Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 5(12), 2429–2436 (2007).
- Geisler T, Schaeffeler E, Dippon J et al.: CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics 9(9), 1251–1259 (2008).
- Smith SM, Judge HM, Peters G et al.: Common sequence variations in the P2Y12 and CYP3A5 genes do not explain the variability in the inhibitory effects of clopidogrel therapy. Platelets 17(4), 250–258 (2006).
- Mega JL, Close SL, Wiviott SD et al.: Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360(4), 354–362 (2009).
- Simon T, Verstuyft C, Mary-Krause M et al.: Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360(4), 363–375 (2009).
- Trenk D, Hochholzer W, Fromm MF et al.: Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J. Am. Coll. Cardiol. 51(20), 1925–1934 (2008).
- Giusti B, Gori AM, Marcucci R et al.: Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am. J. Cardiol. 103(6), 806–811 (2009).
- Sibbing D, Stegherr J, Latz W et al.: Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur. Heart J. 30(8), 916–922 (2009).
- Collet JP, Hulot JS, Pena A et al.: Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 373(9660), 309–317 (2009).
- Shuldiner AR, O’Connell JR, Bliden KP et al.: Association of cytochrome P4502C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302(8), 849–857 (2009).
- Eshtehardi P, Eslami M, Moayed DA: Simultaneous subacute coronary drug-eluting stent thrombosis in two different vessels of a patient with factor V Leiden mutation. J. Cardiovasc. Med. (Hagerstown.) 9(4), 410–413 (2008).
- Hayden EC: International genome project launched. Nature 451(7177), 378–379 (2008).
▪ Early report showing significant association of PLA1/A2 polymorphism with in-stent restenosis.
▪▪ Extensive study to examine the relationship between polymorphisms that are known to play a role in the hemostatic system and the risk of clinical restenosis.
▪▪ First large prospective study to demonstrate the association between four inflammatory polymorphisms with target vessel revascularization after percutaneous coronary intervention.
▪ Study identifying the role of a smooth muscle cell key regulator polymorphism of in-stent restenosis.
▪ First study to associate a platelet receptor polymorphism with stent thrombosis.
▪▪ Study investigating all the known CYP2C19 reduced-function alleles and the risk for a worse clinical outcome, including stent thrombosis.
▪ The variation of cytochrome P450 enzymatic function in clopidogrel resistance and 1-year clinical outcome after bare-metal stent implantation.
▪ Demonstrates that the CYP2C19*2 allele is associated with definite stent thrombosis during the first 30 days after stent implantation.
▪ First paper on genome-wide association studies identifying CYP2C19 as a candidate gene for clopidogrel therapy modulation.