Review Article - Interventional Cardiology (2023) Volume 15, Issue 1
Guide extension catheters: Review of technology and future directions
- Corresponding Author:
- Tim A Fischell
Department of Cardiology,
Borgess Heart Institute,
Michigan State University,
Kalamazoo,
Michigan,
USA,
E-mail: tafisc@gmail.com
Received date: 12-Jan-2023, Manuscript No. FMIC-23-87001; Editor assigned: 16-Jan-2023, PreQC No. FMIC-23-87001 (PQ); Reviewed date: 30-Jan-2023, QC No. FMIC-23-87001; Revised date: 06-Feb-2023, Manuscript No. FMIC-23-87001 (R); Published date: 13-Feb-2023, DOI: 10.37532/1755- 5310.2023.15(1).646
Abstract
Although Percutaneous Coronary Intervention (PCI) has revolutionized the management of CAD, the deliverability of devices including balloons, specialty balloons, stents, atherectomy catheters, thrombectomy devices, and intravascular lithotripsy devices has become a common challenge faced by interventional cardiologists. Guide Extension Catheters (GECs) have been developed and are now widely used to create improved backup support to allow the advancement of interventional equipment required for the PCI.
Improved lesion preparation, plaque modification (e.g., with atherectomy), and Guide Extension Catheters (GEC), also called as Mother-Child Technique, has proven critical to procedural success in complex cases. In this review, we discuss the role and limitations of current guide extension devices, with a brief discussion of next-generation GEC.
Keywords
Percutaneous coronary intervention • Coronary artery disease • Guide catheters • Guide extension catheter
Introduction
The treatment for Coronary Artery Disease (CAD) includes lifestyle changes, pharmacological therapy, and revascularization through percutaneous and/or surgical approaches. Percutaneous Coronary Intervention (PCI) is a non-surgical, invasive procedure used to open blocked coronary arteries and increase blood flow to the ischemic myocardium [1].
Although PCI has revolutionized the management of CAD, the deliverability of devices including balloons, specialty balloons, stents, atherectomy catheters, thrombectomy devices, and Intravascular Lithotripsy (IVL) devices has become one of the common challenges faced by interventional cardiologists. Increasing lesion complexity including tortuosity, calcification, length of lesions, and vessel morphology has contributed to the delivery challenges.
Through continuous research, many strategies and techniques have been developed for seamless device delivery despite complex anatomies and lesions, including choosing of an appropriate Guide Catheter (GC) to create excellent backup support (the ability of the guiding catheter to remain in position and provide a stable platform for the advancement of interventional equipment required for the procedure). The size and shape of the GC have an impact on the backup support function. There are two forms of backup support offered by guiding catheters, namely active and passive. The passive support provided by GC is dependent on the physical characteristics of the GC wall (stiffness from manufactured material) and the distal preformed shape of the GC. The preformed shape of the GC influences the ability of the guide to achieve coaxial alignment with the coronary ostium and allows them to rest for support either on the aortic valve or the opposite wall of the aorta. More passive backup support is offered by GC with 7F and 8F sizes than with 5F and 6F sizes. Active support is often achieved by operator-dependent techniques such as manipulation of the guide into a configuration conforming to the aortic root i.e., coaxial alignment, and deep- seating (intubation with deep engagement of the guide into the coronary vessels). The smaller-sized catheters such as 5F and 6F have the advantage of allowing deeper intubation in comparison to larger catheters [2,3].
Other important factors that may improve the success of a procedure include the use of buddy wire, anchor wire, or anchor balloon technique. Improved lesion preparation and plaque modification (e.g., with atherectomy), and Guide Extension Catheters (GECs), also called as Mother-Child Technique are also critical to procedural success in complex cases. In general, the increasing use of radial rather than femoral approach for PCI has made both passive and active GC support, and device delivery more challenging, and has introduced a greater need for guide extension catheters.
Guide Extension Catheter
One of the most widely used techniques for increasing guide catheter support is the use of guide extension catheters [4]. This technology was first introduced in clinical practice in the United States in 2009. GECs are now considered indispensable equipment for cardiac catheterization laboratories due to their ability to increase backup support of the guide catheter, by providing coaxial alignment and deep intubation to facilitate stent delivery [5]. In addition, they are also used for other complex procedures like thrombectomy, retrieval of dislodged equipment, and selective vessel engagement and opacification [4].
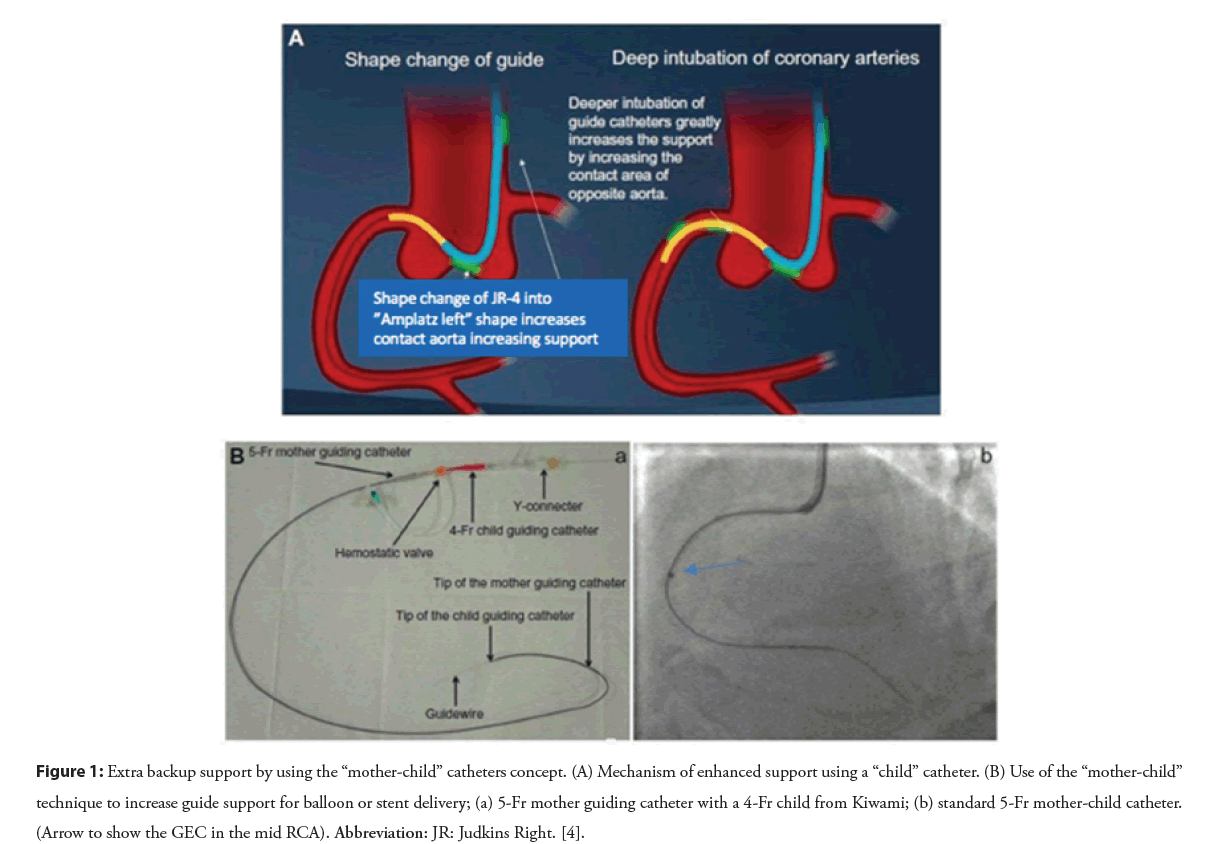
These guide catheter extension devices are basically blunt-ended monorail tubes connected to a rigid pushing element that allows the advancement of the GEC. This is based on a ‘Mother and Child’ Technique’ (Figure 1) [6,7]. In this technique, to improve support for coronary interventions, longer, flexible, and lower-profile (‘child’) guide catheters were developed, which are advanced over the previously inserted guide wires and through the Y hemostatic valve, then inserted into the conventional guide catheters (‘mother’), and extended beyond the distal tip of the guiding catheter to enable deeper vessel intubation, and to achieve improved support and coaxial alignment [8,9]. GEC can be utilized in radial or femoral approaches and may be useful in PCI involving native coronary arteries, grafts, or peripheral interventions.
Figure 1: Extra backup support by using the “mother-child” catheters concept. (A) Mechanism of enhanced support using a “child” catheter. (B) Use of the “mother-child” technique to increase guide support for balloon or stent delivery; (a) 5-Fr mother guiding catheter with a 4-Fr child from Kiwami; (b) standard 5-Fr mother-child catheter. (Arrow to show the GEC in the mid RCA). Abbreviation: JR: Judkins Right. [4].
Guide extension catheters have become a mainstay for coronary intervention, because of their ability to facilitate device delivery [6]. It is estimated that guide extension catheters are now used in ~18% of all PCI. The large majority of these are used as a “bailout” technique after stent delivery has failed using conventional guiding catheter support techniques. The need for guide extension catheters has become more important as operators move to increased use of radial interventions, with less guiding catheter backup support, and more complex PCI [11-15]. In most cases the guide extension is used to get additional backup by advancing the tip of the guide extension system into the proximal or mid portion of a coronary artery, but usually not distally.
Clinical uses of guide extension catheters
• To facilitate smooth equipment delivery. The design aids in allowing device delivery in complex and tortuous lesions.
• To improve the coaxial alignment and backup support of the guide catheter. The deeper the intubation length, the greater the support. Deep intubation may be challenging, especially through diseased and calcified vessels, but can be facilitated using a balloon for distal anchoring (balloon-surfing technique) or “inch worming” using balloon-assisted tracking technique.
• Allow selective contrast injection. It can improve vessel visualization and minimize overlap with other vessels that can complicate image interpretation. This also helps to limit contrast usage.
• GEC can also help to improve Optical Coherence Tomography (OCT) imaging quality by ensuring adequate blood clearance [16].
• Can help in delivering drugs distally, especially in no-reflow situations where you need the drug to be delivered to the distal coronary bed.
• In Bifurcation cases where you need to use rotablation and maintain two wires GEC can be helpful.
• Thrombectomy. Due to their large lumen size, guide extension catheters may allow the removal of large thrombi. Stys, et al. reported the use of guide catheter extensions for thrombectomy after a dedicated aspiration catheter failed [17].
• Retrieval of lost devices. A novel guide extension catheter trapping technique by trapping the lost stent from within, with an NC balloon into a guide extension catheter was used to retrieve a lost stent reported in a clinical case report [18].
• Facilitating Chronic Total Occlusion (CTO) interventions. This is another area of application. Kovacic et al. reported the use of the GuideLiner catheter in “balloon-uncrossable” CTOs. Moreover, the use of a guide extension catheter can facilitate entry of the retrograde guidewire into the ante-grade catheter when employing the reverse controlled ante grade and retrograde tracking and dissection (Reverse CART) technique [19].
• To engage aberrant-origin coronary arteries or bypass grafts. In situations, where an aberrant-origin coronary or graft cannot be engaged with any GC, GEC devices can be used to reach the target vessel, to complete the procedure [20].
• Use in the presence of a previous Transcatheter Valve. In situations, where crossing a transcatheter aortic valve by a guiding catheter is not possible due to inadequate backup support, the use of GECs, wherein, a guiding wire is first delivered through the valve cage followed by advancing the guide extension catheter, can create direct coronary engagement [20].
• Based on reported studies guide extension catheter was mainly used to facilitate stent delivery (59%), to improve the alignment of the guide catheter (29%), and for selective contrast injections (13%) [20].
Commonly Used Guide Extension CathetersA,B,C,D,E
The most used rapid exchange guide extension catheters are:
1. GuideLiner Catheter (Teleflex, Morrisville, NC, USA)-First GEC approved by FDA in 2009. The GuideLiner V3 Guide extension catheter is the latest version
2. GuideZilla (Boston Specific, Natick, MS, USA) FDA approved in 2013, and the most recent GuideZilla II Guide Extension Catheter
3. TrapLiner-(Teleflex, Morrisville, NC, USA)-USFDA approved in 2017.
4. Telescope (Medtronic, Santa Rosa, CA USA)-FDA approved 2019/2020
5. Guidion (Interventional Medical Device Solutions) is available in Europe, Guideplus Catheter (Nipro Corp, Japan), Heartrail II Guiding Catheter (Terumo, Japan) and will not be discussed in this paper.
(A) GuideLiner V3 catheterA
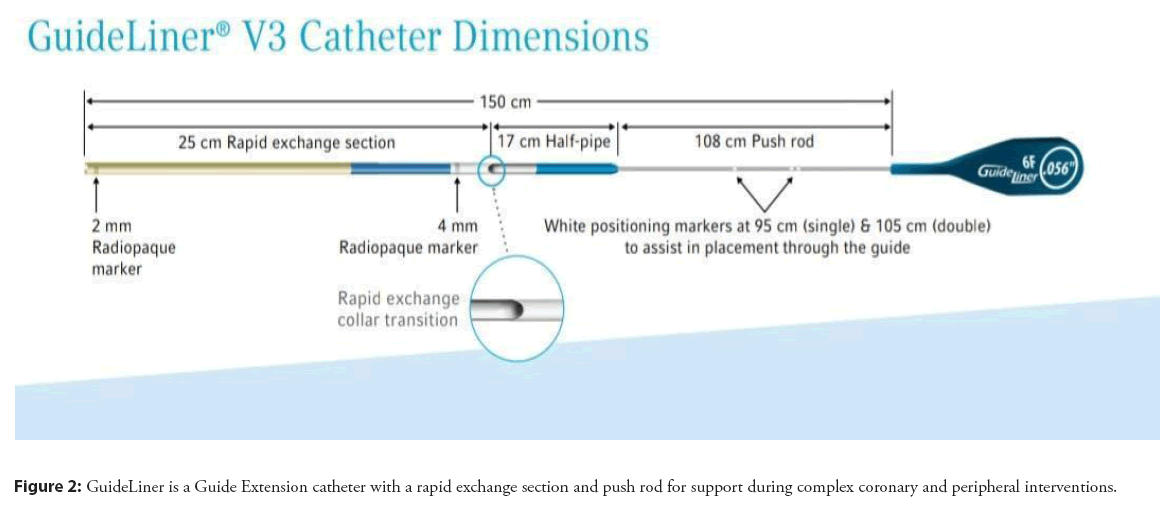
GuideLiner V3 Catheter, a rapid exchange guide extension catheter, is the latest version of GuideLiner catheters. They are used along with guide catheters and guidewires to reach distal parts of the coronary and/or peripheral vasculature, to facilitate the placement of interventional devices. Features are mentioned below:
• Highly Flexible, Coil-Reinforced Guide Extension-The GuideLiner V3 Catheter’s coil-reinforced guide extension provides excellent flexibility and kink resistance allowing dependable deep- seating delivery through tortuous vessels to reach distal locations.
• Half-pipe technology-The half-pipe channel is designed to minimize device/collar interaction by directing and aligning devices through the collar transition, facilitating smooth device entry and seamless delivery. This technology minimizes the opportunity for device separation by creating a 17 cm polymer bond between the extension and push rod (Figure 2).
• Added Backup Support-With as little as 5 mm of extension, bench testing has shown that the GuideLiner V3 Catheter significantly increases the backup support of a 6 Fr. guide catheter.
• Available in five sizes: 5, 5.5, 6, 7 and 8 Fr.
(B) TrapLiner catheter (2-in-1 Device-Guide Extension catheter with trapping capabilities)B
The TrapLiner Catheter is a 2-in-1 rapid exchange guide extension catheter that combines the benefits of guide extension with the ability to trap an 0.014” guidewire against the inner wall of a guide catheter, by means of a balloon. This functionality helps prevent the loss of guidewire position or unintentional guidewire advancement during catheter exchange and eliminates the need for alternative catheter exchange techniques. This is typically used with the 7 or 8 French versions and is typically used only for CTO intervention (Figure 3).
GuideZilla II Guide Extension Catheter provides additional backup support and facilitates easy delivery of ancillary devices.
It creates a smooth pathway for a balloon and/or stent delivery by providing greater flexibility and a smooth surface. This is important with complex lesions, calcium, tortuous vessels, and distal lesions (Figure 4). Features are mentioned below:
• 1 × 1 Braid provides extra backup support without over- straightening the vessel.
• Z-Glide Hydrophilic coating on the outer diameter reduces friction and enhances deliverability through complex, tortuous anatomy.
• Stiff-yet-flexible stainless steel hypo tube shaft (push rod) provides exceptional pushability and kink resistance when advancing the extension catheter.
• Hypo tube push rod for enhanced pushability
• Short Hypo tube Transition (6 mm) for minimal device interaction and smooth device entry
• Platinum Iridium Helical Collar for optimal Visibility and Smooth Device Interaction
• Distal radiopaque marker bands (wo) facilitate accurate placement and positioning.
• Soft, flexible, and atraumatic tip designed to minimize the risk of complications to the vessel.
• Available in four sizes: 6, 7, 8, and 6 Fr Long
• The inner surface of the catheter has a Polytetrafluoroethylene coating (PTFE) that reduces friction when devices are passed through it.
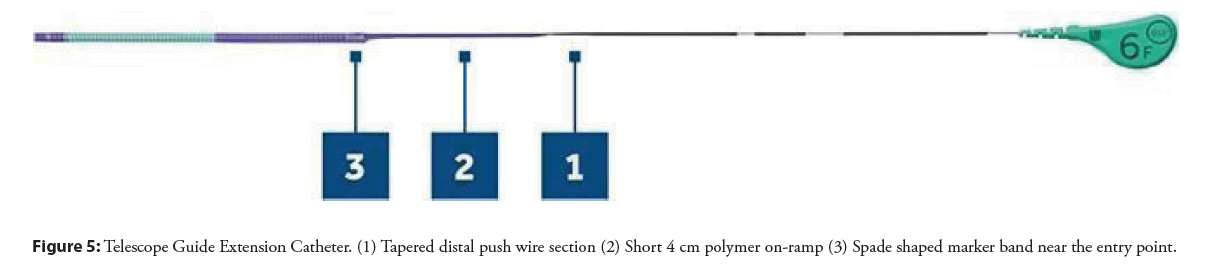
• It has a 2 mm TruFlex soft polymer tip which responsively deflects and provides flexibility. Pushwire. It has a solid, round pushwire that confers greater force and pushability, minimizing the risk of kinking. The pushwire also tapers from proximal to distal where it connects with the catheter.
• A short 4 cm ramp with a polymer coating, which reduces potential difficulty with passing and delivering stents.
• Platinum Iridium marker bands-3 mm long spade-shaped marker band near the entry port; and 1 mm long marker band, 2 mm from distal tip, confirms orientation of catheter on fluoroscopy.
• Available in sizes 6 and 7 Fr
(E) Comparison of various currently used GECs
The Comparison of guide extension catheter are shown in Table 1
| S No. | Feature | GuideLiner V3 Catheter | GuideZilla II Guide Extension Catheter | TrapLiner | Telescope |
|---|---|---|---|---|---|
| 1 | Manufacturer | Teleflex | Boston Specific | Teleflex | Medtronic |
| 2 | Year of approval by FDA, for the initial version | 2009 | 2013 | 2017 | 2019 |
| 3 | Size (Fr) | 5, 5.5, 6, 7 and 8 | 6, 7, 8, and 6 Long (XL) | 6 ,7 and 8 F | 6F and 7 |
| 4 | Total length | 150 cm | 150 cm | 150 cm | 150 cm |
| 5 | Proximal Shaft | 108 cm Stainless Steel ribbon pushrod | Stainless Steel Hypotube | 0.020" Stainless steel Hypotube push rod | 125 cm |
| 6 | Guide Segment/Rapid Exchange Section | 25 cm | 25 cm on 6F, 7F, 8F (40 cm on 6F Long) |
13 cm | 25 cm |
| 7 | Collar | All Polymer Collar | Stainless steel collar embedded in polymer | All Polymer Collar | All Polymer Collar |
| 8 | Collar Transition | 17 cm Half pipe | 6 mm Hypotube transition | 3 cm halfpipe | short 4 cm polymer on ramp |
| 9 | Coating | Silicon Wipe Hydrophilic coating | Z-Glide hydrophilic coating | Silicon Wipe Hydrophilic coating | Hydrophilic coating |
| 10 | Coil reinforced Guide extension | Braided Guide Extension | Coil reinforced Guide extension | Coil reinforced Guide extension | |
| 11 | Radiopaque Marker Bands | (a) Distal Marker Band (2 mm from distal tip) (b) Proximal Marker band (4 mm from Collar) | (a) Distal Marker Band (b) Radiopaque band near Collar | Platinum Iridium marker bands (a) 4 mm from Collar (b) 2 mm from distal tip | Platinum Iridium bands (a) 1 mm long, 2 mm from distal tip (b) 3 mm long, spade-shaped at entry port |
| 12 | Additional Features | - | - | Trapping Balloon | - |
Table 1: Comparison of various guide extension catheters.
(F) Inner and outer diameter comparison of various GECs
Inner and outer diameter comparisons are clearly indicated in Table 2
| Size | GuideLiner V3 Catheter | GuideZilla II Guide Extension Catheter | TrapLiner Catheter | Telescope Catheter | ||||
|---|---|---|---|---|---|---|---|---|
| ID | OD | ID | OD | ID | OD | ID | OD | |
| 6F | 0.056” (1.42 mm) | 0.067 (1.70 mm) | 0.057” (1.45 mm) | 0.067” (1.70 mm) | 0.056” (1.42 mm) | 0.056” (1.42 mm) | 0.067 (1.70 mm) | |
| 6FL | -- | -- | 0.057” (1.45 mm) | 0.067” (1.70mm) | ----- | ------ | --- | ----- |
| 7F | 0.062” (1.57 mm) | 0.075” (1.90 mm) | 0.063” (1.60 mm) | 0.073’ (1.86 mm) | 0.062” (1.57 mm) | - | 0.062” (1.57 mm) | 0.075” (1.90 mm) |
| 8F | 0.071” (1.80 mm) | 0.085” (2.16 mm) | 0.072” (1.83 mm) | 0.083” (2.11 mm) | 0.071” (1.80 mm) | - | ----- | ------- |
Note: F-French Size (1F=0.33 mm); ID: Inner Diameter (Inches); OD: Outer Diameter (Inches)
Table 2: Inner and outer diameter comparison of various guide extension catheters.
Complications Associated with the Use of Guide Extension Catheters
Although guide extension catheters are extremely useful, there are potential complications associated with their use. It is very important that they are used properly, and in a safe manner. Some of the common complications associated with the use of GECs in PCI include:
Coronary dissection
Despite having a relatively atraumatic tip for most GEC catheters, some pressure damping, or wedging may occur during the advancement of GEC. The blunt-ended tip of GEC can get under the plaque and create minor or severe flow-limiting dissection in ~3% of cases [21,22]. Forceful advancement or injection may increase the risk of coronary dissection, especially if the tip is embedded at an edge of the plaque. In case of suspected complications, techniques such as balloon-assisted delivery of the extension may be considered. Life-threatening, severe dissections have occurred, but can often be bailed out with stenting.
Wire and pushrod wrapping: Difficulty in stent advancement
During the procedure, it is important to maintain the separation of the pushrod and the guidewire. If these cross or wrap with each other, the devices, particularly stents, cannot pass through the collar of the tubular monorail section of the extension, leading to deformation or dislodgement of stents. Large stents (≥ 4 mm and covered stents) might not pass-through smaller GECs. Even without wrapping, the advancement of larger stents through smaller GECs can lead to stent disruption or stripping of the stent from the stent delivery system [23,24]. A variety of techniques can be employed to remove the stripped stent [18,23].
Air embolism
Because of pressure damping, there may be insufficient backflow for adequate venting of the guiding catheter with a GEC in place. Patience is required to ensure complete de-airing of the system to avoid air embolization [10].
Ischemia
The obstruction caused by the deeply intubated extension may cause luminal obstruction or pseudo-obstruction in more tortuous arteries, causing myocardial ischemia. This is rapidly treatable by proximal, disengagement of the GEC from the distal vessel segment [20].
MAUDE Database for Present GECs
Based on the study of FDA’s Manufacturer and User Facility Device Experience (MAUDE) database for reports associated with both the GuideLiner (65 cases: 2010-2018) and the GuideZilla (408 cases: 2016-2018), the most common adverse events are catheter fracture (58%; 40%), inability to pass devices or damaged PCI devices (23%; 13%), unable to pass catheter to target lesion (nil; 29%), coronary artery dissection (14%; 2.5%), and catheter kinking (nil; 14%) in those reported cases, during PCI interventions involving GuideLiner and GuideZilla respectively [25-27].
Techniques to Deliver a Guide Extension Catheter
• Balloon surfing technique: It requires the use of a balloon when we deliver a GEC to the “rail” and center the device away from the vessel wall and any potential vessel damage. The technique involves positioning a small balloon at the distal tip of the GEC, with half sticking out of the catheter. The balloon is used as a bumper to get past the lesion or to the lesion and minimizes the razor effect of the GEC tip. A dedicated dilator such as a Navigation catheter (Vascular Solutions, Minneapolis, MN), or a microcatheter can be utilized instead of a balloon catheter [25,28,29].
• Balloon anchor technique: The technique involves inflating a balloon proximal to, or within the target lesion to assist in GEC advancement. The balloon acts as an anchor that is used to pull the guide extension down the vessel. It entails inflating the balloon, whether a compliant or noncompliant balloon, at the distal vessel where the lesion is located, and while the balloon is inflated, tracking the guide catheter extension system to the lesion, to the balloon.
• Balloon-assisted tracking- “Inchworm” technique: This technique involves positioning an inflated balloon halfway at the distal tip of the catheter during delivery and advancing the GEC immediately as the balloon is deflated, thus advancing the GEC distally over the deflated balloon.
Future Advances
The first generation of guide catheter extension devices (Gen-1 GE) are monorail delivered, tubular structures, and include the GuideLiner, the GuideZilla, and the Telescope. Delivering these first-generation guide extension devices distally often requires continued manipulation, with balloon inflations to anchor [28], or balloon inflations at the distal end of the guide extension to “inchworm” slowly, mm by mm down the coronary artery [30]. Although additional balloon manipulation techniques are often successful, they require additional time, radiation dosing, catheter exchanges, and contrast, and may cause balloon barotrauma in proximal non-target segments of the coronary artery, with potential risks of dissection, or restenosis.
Although most operators appreciate the power of deep intubation of guide extension in complex cases, performing deep intubation with the current first-generation guide extension systems can be technically challenging, not possible, and potentially dangerous.
Even when one delivers the guide extension over the shaft of a balloon catheter or stent delivery system, the blunt-edged tubular structure can catch on fibrous lesions, calcium, or stent struts, lift up plaque, embolize material or cause coronary dissection. The inability to deliver the guide extension catheter distally enough results in failure of guide extension in ~20% of cases [21]. Coronary dissection has been reported in ~3% of cases, even without deep intubation [21,25,31]. Newer generation guide catheter extension devices such as the CrossLinerTM have been developed to overcome these problems.
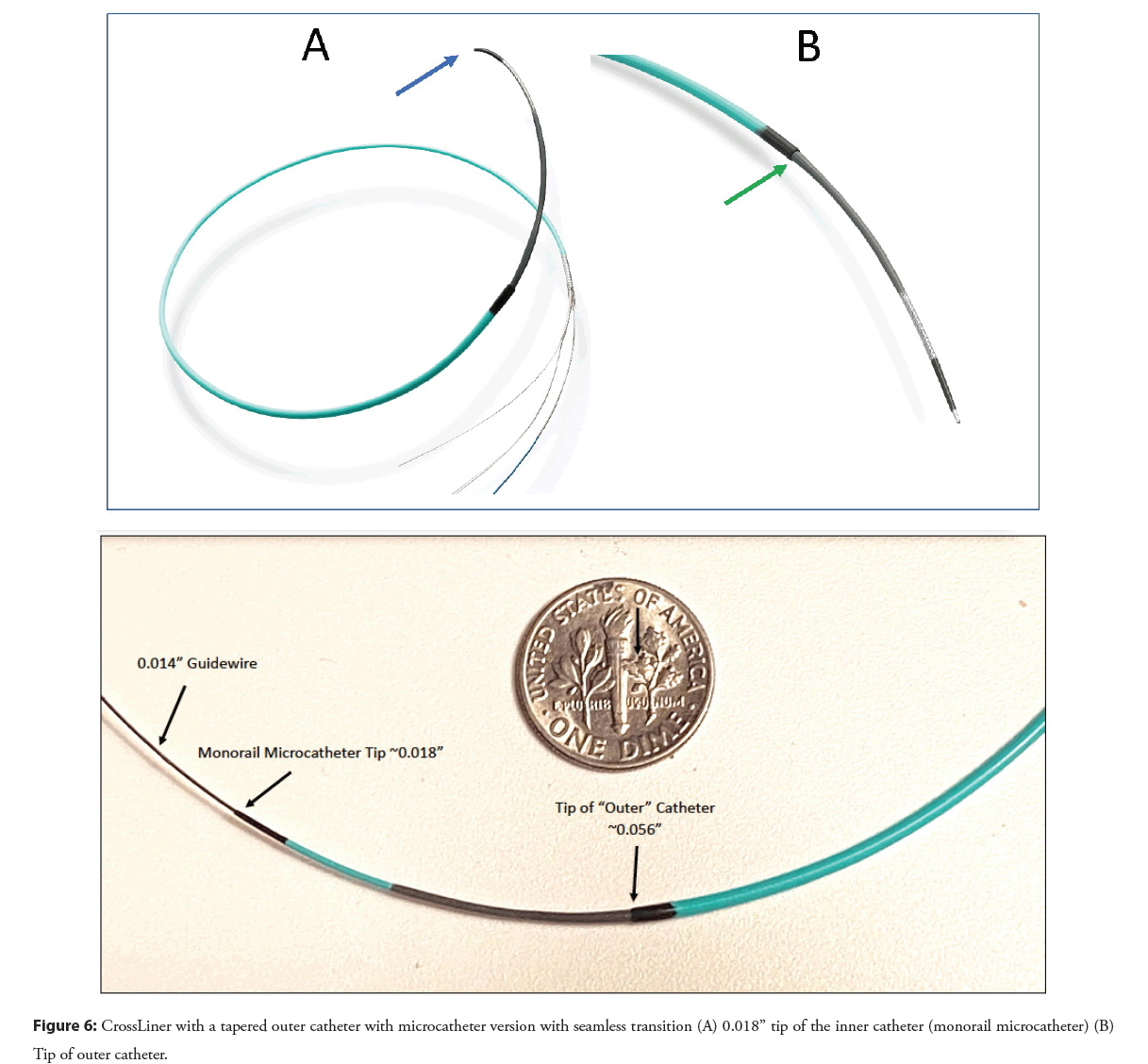
The CrossLiner is a next-generation guide extension system that may offer advantages over first-generation devices. The CrossLiner has a tapered inner monorail catheter with a microcatheter tip that is connected inside the outer catheter which is the guide extension tube (Figure 6). By creating a seamless edge at the junction of the inner and outer catheters the CrossLiner can pass relatively atraumatically down a diseased coronary with deliverability that appears like a balloon angioplasty catheter. The CrossLiner was tested in a head-to-head study with the GuideLiner and GuideZilla in a porcine coronary model, with stenting, and proved to be much more deliverable in this model [29]. Further clinical evaluation will be required to assess the role of this new device in PCI once it is FDA cleared for use.
Guide catheter extension devices are extremely helpful in complex and difficult PCI procedures, but they need to be used with appropriate “gentle” care. With experience, expertise, and appropriate attention to detail, these devices are safe and effective. There is great interest in next-generation guide extension devices that may overcome some of the limitations of the current first- generation devices. These tools have become valuable assets in continuing to improve the safety and success of percutaneous coronary and peripheral interventions.
References
- Ahmad M, Mehta P, Reddivari AKR, et al. Percutaneous coronary intervention. (Updated 2022 Jun 11). In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing. (2022)
- Chawla R, Ahmad W, Sharma V. Techniques to overcome difficulty in device deliverability to lesion in complex PCI. Curr Cardio Rev. 16(2): 117-124 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Prashant PU. Current and emerging catheter technologies for percutaneous transluminal coronary angioplasty. Res Rep Clin Cardiol. 5:213-226 (2014).
- Duong T, Christopoulos G, Luna M, et al. Frequency, indications, and outcomes of guide catheter extension use in percutaneous coronary intervention. J Invasive Cardio. 27(10): E211-215 (2015).
[Google Scholar] [PubMed]
- Chandra S, Tiwari A, Chaudhary G, et al. Guide catheter extension systems: Hype or a need? Indian Heart J. 73(5):535-538 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Mamas MA, Fath-Ordoubadi F, Fraser DG. Distal stent delivery with GuideLiner catheter: First in man experience. Catheter Cardiovasc Interv. 76(1): 102-111 (2010).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Kumar S, Gorong DA, Secco GG, et al. The GuideLiner “child” catheter for percutaneous coronary intervention–early clinical experience. J Invasive Cardiol. 22(10): 495-498 (2010).
[Google Scholar] [PubMed]
- Kovacic JC, Sharma AB, Roy S, et al. Guide liner mother-and-child guide catheter extension: a simple adjunctive tool in PCI for Balloon–Uncrossable chronic total occlusion. J Interventional Cardiol. 26(4): 343-350 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Furini FR, de Oliveira AT, Francisco RSB, et al. ‘Mother and Child’ Technique with a New Catheter: Initial Experience. Revista Brasileira de Cardio logia Invasiva. 20(2): 208-212 (2012).
- Maxwell YE. Adverse events report for guide extension catheter should spur cautious use. TCTMD. (2019).
- Sharma D, Shah A. Efficacy and safety of the Guide Liner mother-in-child guide catheter extension in percutaneous intervention. J Interv Cardiol. 30(1): 46-55 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Farooq V, Mamas MA, Fath-Ordoubadi F, et al. The use of a guide catheter extension system as an aid during trans radial percutaneous coronary intervention of coronary artery bypass grafts. Catheter Cardiovasc Interv. 78: 847-863 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Chan PH, Alegria-Barrero E, Foin N, et al. Extended use of the GuideLiner in complex coronary interventions. EuroIntervention. 11(3): 325-335 (2015).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- de Man FHAF, Tandjung K, Hartmann M, et al. Usefulness and safety of the GuideLiner catheter to enhance intubation and support of guide catheters: Insights from the Twenty GuideLiner registry. Euro Intervention. 8(3): 336-344 (2012).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Huang MS, Wu CI, Chang FH, et al. The efficacy and safety of using extension catheters in complex coronary interventions: A single center experience. Acta Cardiol Sin. 33(5):468-476 (2017).
[Google Scholar] [PubMed]
- Garcia-Guimaraes M, Cuesta J, Rivero F, et al. Mother-and-child catheter-facilitated optical coherence tomography: A novel approach to improve intracoronary imaging. Cardiol J. 23(6):647-651 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Stys AT, Stys TP, Rajpurohit N, et al. A novel application of GuideLiner catheter for thrombectomy in acute myocardial infarction: A case series. J Invasive Cardio. 25: 620-624 (2013).
[Google Scholar] [PubMed]
- Righetti S, Tresoldi S, Calchera I, et al. Innovative guide extension catheter trapping technique to retrieve a lost stent from a coronary artery. JACC Case Rep. 4(7):411-414 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Vo M, Brilakis ES. Faster, easier, safer: “GuideLiner reverse CART” for retrograde chronic total occlusion interventions. Catheter Cardiovasc Interv. 83(6): 933-935 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Hellig F, van Wyk P, Moosa MZ. The dos and don’ts of guide catheter extensions. The practicalities of effective and safe application of guide catheter extensions in the cath lab. Cardiol Interv. (2020).
- Waterbury TM, Sorajja P, Bell MR, et al. Experience and complications associated with use of guide extension catheters in percutaneous coronary intervention. Catheter Cardiovasc Interv. 88(7): 1057-1065 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Chang YC, Fang HY, Chen TH, et al. Left main coronary artery bidirectional dissection caused by ejection of GuideLiner catheter from the guiding catheter. Catheter Cardiovasc Interv. 82(3): E215-E220 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Waggoner T, Desai H, Sanghvi K. A unique complication of the GuideZilla guide extension support catheter and the risk of stent stripping in interventional and endovascular interventions. Indian Heart J. 67(4): 381-384 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Waterbury TM, Sarajja P, Gulati R. Stent loss associated with the use of GuideLiner catheter in percutaneous interventions. J Am College Cardiology. 64(11) Supplement TCT-125 (2014).
- Chen Y. Adverse events associated with the use of guide extension catheters during percutaneous coronary intervention: Reports from the Manufacturer and User Device Experience (MAUDE) database. Presented at CRT. (2019).
- Chen Y, Shah AA, Shlofmitz E, et al. Adverse events associated with the use of guide extension catheters during percutaneous coronary intervention: Reports from the Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 20(5): 409-412 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Shoda M, Yamamoto H, Tsukiyama Y, et al. Rare complications of Guide plus guide-extension catheter during complex percutaneous coronary intervention. J Cardiol Cases. 26(6): 399-403 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Andreou C, Karalis I, Maniotis C, et al. Guide extension catheter stepwise advancement facilitated by repeated distal balloon anchoring. Cardiovasc Revasc Med. 18(1): 66-69 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Fischell TA, Payne J, Wehde K, et al. A next generation guide extension system for percutaneous coronary intervention. Cardiovascular Revasc Medicine. 32: 50-55 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Ali M, Yagoub H, Ibrahim A, et al. Anchor-balloon technique to facilitate stent delivery via the GuideLiner catheter in percutaneous coronary intervention. Future Cardiol. 14(4): 291-299 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Murphy JC, Spence MS. GuideLiner® catheter-friend or foe? Catheter Cardiovasc Interv. 80(3): 447-445 (2012).
[CrossRef] [Google Scholar] [PubMed]