Review Article - Interventional Cardiology (2012) Volume 4, Issue 1
Hemodynamic support in high-risk percutaneous coronary interventions and cardiogenic shock
- Corresponding Author:
- William W O’Neill
University of Miami Miller School of Medicine
Miami, FL, USA
E-mail: woneill@med.miami.edu
Abstract
Keywords
cardiogenic shock n high-risk percutaneous coronary intervention n Impella® n intra-aortic balloon pump n percutaneous left ventricular assist devices n TandemHeart®
Temporary percutaneous left ventricular (LV) assist devices (TPLVAD) have been approved for hemodynamic support in the setting of cardiogenic shock (CS) or for high-risk percutaneous coronary interventions (PCI). An ‘ideal’ TPLVAD is a device that can be rapidly inserted, provides adequate support, is simple to use, has few vascular complications and is easy to remove. TPLVADs can provide valuable time and help stabilize a ‘crashing and burning’ patient until recovery or prior to undertaking further definitive treatment measures.
CS is a state of end-organ hypoperfusion due to cardiac dysfunction. Suggestive hemodynamic parameters include persistent hypotension (systolic blood pressure <80–90 mmHg or mean arterial pressure 30 mmHg lower than baseline) with severe reduction in the cardiac index (CI; <1.8 l/min per m2 without support or <2.0–2.2 l/min per m2 with support) and adequate or elevated filling pressures [1]. Although a slight temporal decline in rates of CS has been observed with the introduction of routine PCI, CS still complicates approximately 5–8% of ST-elevation myocardial infarction (STEMI) and 2.5% of non-STEMI cases [2]. Patients developing CS have a very high mortality rate (>50%) during the index hospitalization [3]. TPLVAD have been used in the setting of CS to provide temporizing measures.
High-risk PCI does not have a universal definition. Nevertheless, most studies consider high-risk PCI to be interventions in patients with moderately depressed cardiac function, or in the setting of hemodynamic instability or due to the complex nature of the intervention (coronary or structural) [4,5]. In such settings, even brief episodes of myocardial ischemia or hypotension can set off a life threatening downward spiral of decreased cardiac output, coronary hypoperfusion, heart failure and hemodynamic collapse. Prophylactic use of hemodynamic support devices is therefore also employed in such cases, to prevent adverse outcomes [6,101].
Background
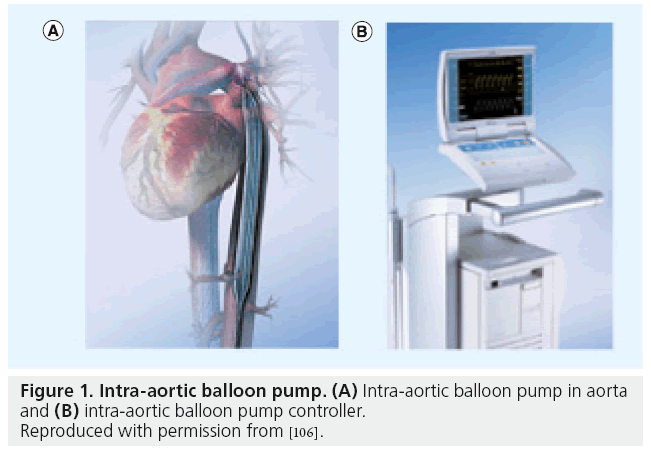
Hemodynamic support using extracorporeal membrane oxygenators has been described since the early 1950s [7]. Subsequently, the intra-aortic balloon pump (IABP) was introduced in the early 1960s and has been used increasingly since then (Figure 1). This was followed by the introduction of cardiopulmonary support systems in the 1970s, which used large femoral cannulas to pump femoral venous blood through an oxygenator and then back into the femoral artery. The high percentage of multiorgan complications, however, limited their widespread use. The Hemopump® (Medtronic, Inc., MN, USA) introduced in the 1980s was the first active forward-flow intraventricular pump; however, it failed to be readily adopted. The interim period was marked by widespread reliance on IABP, which became the workhorse of cardiac support along with extracorporeal membrane oxygenation (ECMO). Nevertheless, ECMO consists of combined cardiac and respiratory support and is more technically demanding. ECMO has been associated with a 40% survival benefit when used during CS as a bridge to transplant or destination therapy [8–11]. The details of ECMO as a cardiopulmonary adjunct are outside the scope of this article.
Figure 1: Intra-aortic balloon pump. (A) Intra-aortic balloon pump in aorta and (B) intra-aortic balloon pump controller.
Reproduced with permission from [106].
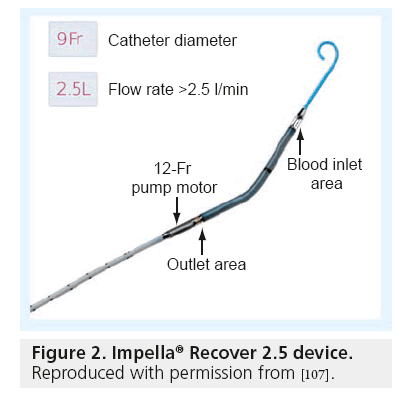
In the early 2000s, the TandemHeart® (Cardiac Assist, Inc., PA, USA) received US approval and has since been extensively used in the cardiac catheterization laboratory in the hands of experienced operators. More recently the Impella® device (Abiomed, Inc., MA, USA) has been introduced into the market and has gained rapid acceptance due to its ease of use (Figure 2). The Reitan catheter pump (CardioBridge GmbH, Hechingen, Germany) is an experimental intra-aortic axial pump that can provide flow rates of up to 20 l/min but is not yet commercially available in the USA [102].
Figure 2: Impella® Recover 2.5 device.
Reproduced with permission from [107].
At present, there are three TPLVAD commercially available in the USA that are frequently used in the catheterization laboratory – the IABP, TandemHeart and the Impella Recover LP 2.5 (Figures 3 & 4). Each has its own set of advantages and challenges (Tables 1 & 2 & Box 1). In this review, we will attempt to summarize these devices and the literature supporting their use. Impella 5 is a larger verison of the Impella 2.5 and provides up to 5 l of cardiac output. Since it requires a subclavian cutdown and is mostly implanted by surgeons, its utility in the catheterization laboratory is limited.
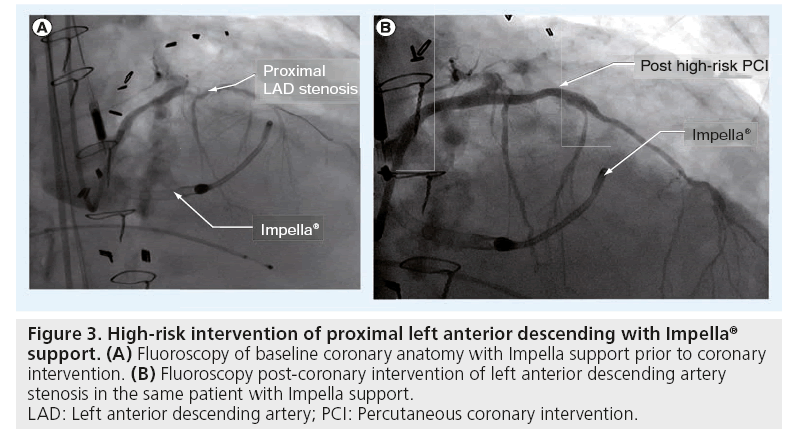
Figure 3: High-risk intervention of proximal left anterior descending with Impella® support. (A) Fluoroscopy of baseline coronary anatomy with Impella support prior to coronary intervention. (B) Fluoroscopy post-coronary intervention of left anterior descending artery stenosis in the same patient with Impella support.
LAD: Left anterior descending artery; PCI: Percutaneous coronary intervention.
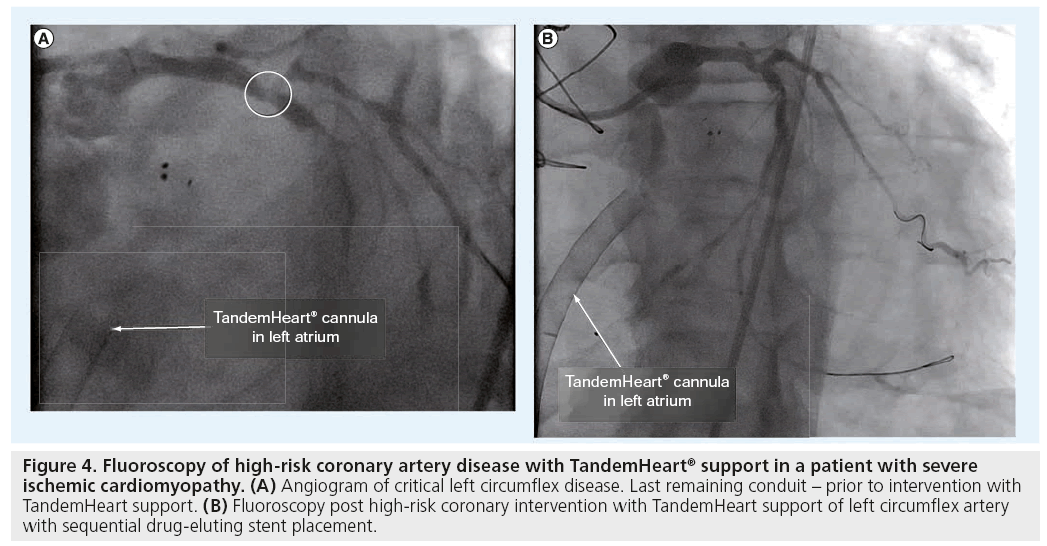
Figure 4: Fluoroscopy of high-risk coronary artery disease with TandemHeart® support in a patient with severe ischemic cardiomyopathy. (A) Angiogram of critical left circumflex disease. Last remaining conduit - prior to intervention with TandemHeart support. (B) Fluoroscopy post high-risk coronary intervention with TandemHeart support of left circumflex artery with sequential drug-eluting stent placement.
Review of evidence supporting TPLVAD use in CS
▪ IABP
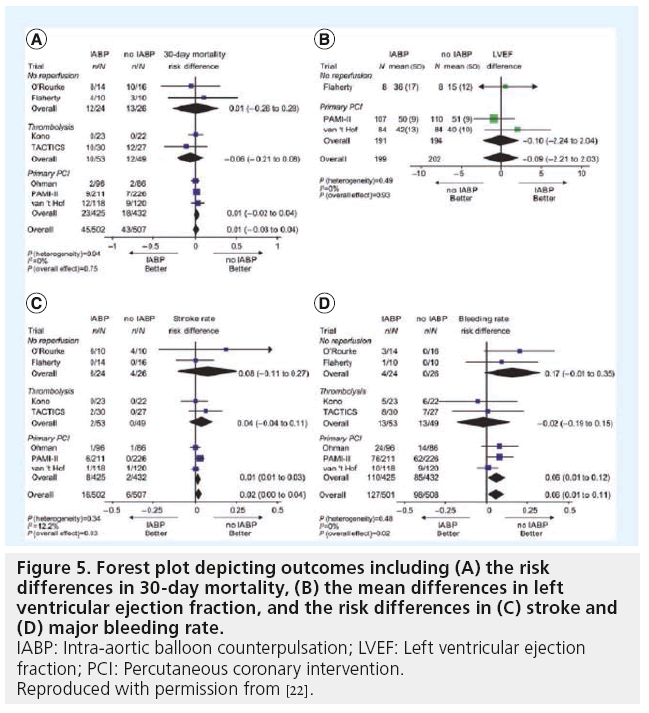
The current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on STEMI list IABP therapy as a Class IB recommendation in the setting of CS [12]. IABP therapy also received a Class IIA recommendation in the ACC/AHA guidelines for patients with non-STEMI and refractory ischemia [13]. The IABP is the most widely used support device in the USA in patients with acute myocardial infarction (MI). In the Benchmark registry involving over 22,000 patients, IABP use was successful in >97% of patients, with major complications arising in <3% of the cases [14].
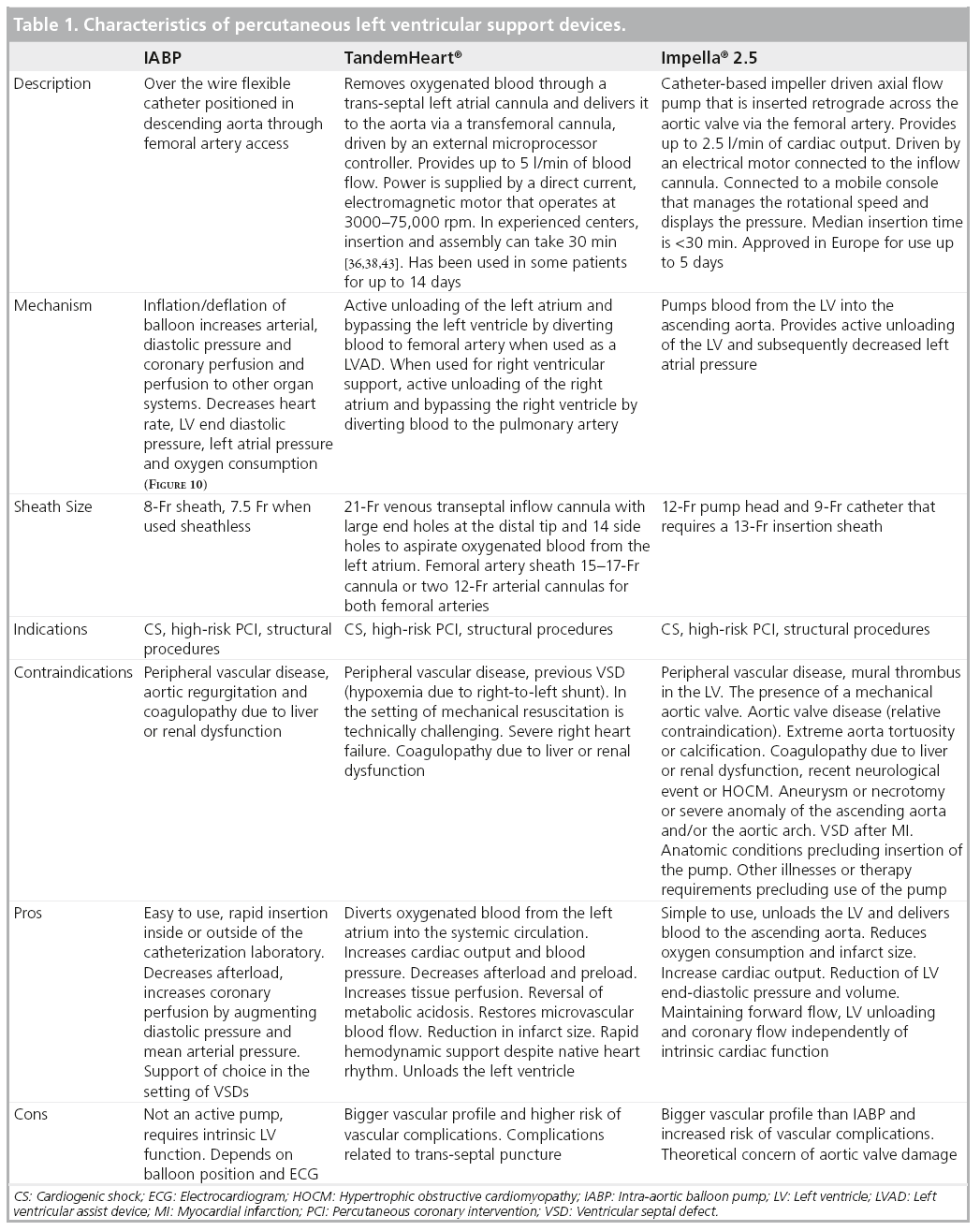
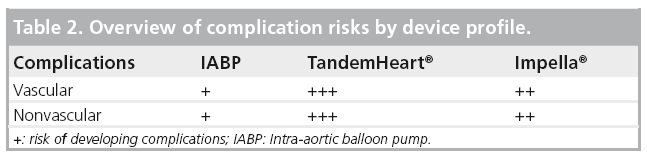
Various studies, including randomized trials, have investigated IABP use in MI and CS [15–21]. Recent metanalyses have confirmed the benefits of IABP in patients treated with thrombolysis. However, IABP therapy in adjunct to primary PCI did not demonstrate any benefits regarding 30-day mortality or changes in LV ejection fraction (EF; Figure 5 & 6) [22]. In addition, IABP use was associated with a significant 6% increase in 30-day mortality in patients treated with PCI with a nonsignificant increase in stroke and bleeding rates. It is worth noting that of the three randomized, controlled trials included in this metanalysis comparing IABP to no-IABP therapy during primary PCI for STEMI, two of them (Ohman et al. and Stone et al.) excluded patients with CS [17,18].The third study by Van’t Hof et al. included patients with CS. However, patients with STEMI and signs of CS who were assigned to standard treatment, crossover to IABP was prespecified by Van’t Hof [19]. In essence, all patients with CS were switched to IABP support. An ‘on treatment analysis’ was not mentioned and only results from ‘per protocol analysis’ were shown. This highlights the challenges of carrying out a randomized trial evaluating TPLVAD support in STEMI patients with CS. In conclusion, despite the results depicted in this metanalysis, there is still not enough evidence to qualify or disqualify the beneficial effects of IABP during CS.
Figure 5: Forest plot depicting outcomes including (A) the risk differences in 30-day mortality, (B) the mean differences in left ventricular ejection fraction, and the risk differences in (C) stroke and (D) major bleeding rate.
IABP: Intra-aortic balloon counterpulsation; LVEF: Left ventricular ejection fraction; PCI: Percutaneous coronary intervention.
Reproduced with permission from [22].
Figure 6: Metanalysis comparing intra-aortic balloon pump support in ST-elevation myocardial infarction and cardiogenic shock stratified by the use of thrombolysis or primary percutaneous coronary intervention. (A) The risk differences in 30-day mortality for the each study, type of reperfusion therapy and for the overall analysis. The size of each square is proportional to the weight of the individual study. (B) The percentage of CABG and rescue PCI in the studies, as well as the weighted overall revascularization, categorized into IABP and no IABP groups. Single-coloured bars are used if separate figures for percutaneous coronary intervention and coronary artery bypass grafting could not be given. CABG: Coronary artery bypass grafting; IABP: Intra-aortic balloon counterpulsation; PCI: Percutaneous coronary intervention. Reproduced with permission from [22].
▪ TandemHeart
In a small randomized trial of patients with STEMI and CS, Thiele et al. showed up to 4 l of assisted cardiac support and improvement in CI and LV filling pressures with the use of TandemHeart [23]. Subsequent reports showed superior hemodynamic support when using TandemHeart versus IABP without an observable impact on 30-day mortality (Figure 7) [23,24]. Unfortunately, these hemodynamic benefits were accompanied by an increase in vascular complications, including access site bleeding and limb ischemia due to the large size cannulas. Appropriate selection of patients with vascular access to accommodate this device is necessary to prevent vascular complications. Despite the hemodynamic benefits observed with TandemHeart, the clinical data available to support its use in CS remains limited.
Figure 7: Change in hemodynamic parameters with TandemHeart® support as compared with intra-aortic balloon pump. (A) Change in CI, (B) Change in MAP, (C) Change in PCWP. CI: Cardiac index; IABP: Intra-aortic balloon pump; MAP: Mean arterial pressure; PCWP: Pulmonary capillary wedge pressure. Reproduced with permission from [23].
▪ Impella
The ISAR-SHOCK study randomized 26 patients with STEMI and CS to Impella 2.5 or IABP (Figures 8 & 9) [25]. The CI increased by 0.49 ± 0.46 l/min in the Impella group compared with 0.11 ± 0.31 l/min in the IABP group at 30 min (p = 0.02). However, there was no difference in 30-day mortality (46% in both groups). Lam et al. also showed improved microvascular indices in anterior STEMI patients treated with Impella 2.5 as compared with controls using sublingual side stream dark field microscopy [26].
Safety and feasibility in patients undergoing PCI with anterior STEMI was assessed in the nonrandomized AMC-MACH study, which showed a greater decrease in pulmonary capillary wedge pressure and improvement in LV function in patients who received Impella support compared with the IABP controls [27]. Longer follow-up from the study showed that three-day support with the Impella LP 2.5 was not associated with adverse effects on the aortic valve and lead to significantly improved recovery in the EF [28].
Figure 8: Change in (A) cardiac power, (B) serum lactate and (C) hemolysis in patients undergoing Impella® support compared with intra-aortic balloon pump.
*p < 0.05 between treatment groups at the specific time points.
IABP: Intra-aortic balloon pump.
Reproduced with permission from [25].
Figure 9: Change in organ dysfunction scores (A & B) and survival (B) in patients undergoing Impella® support as compared with intra-aortic balloon pump.
IABP: Intra-aortic balloon pump.
Reproduced with permission from [25].
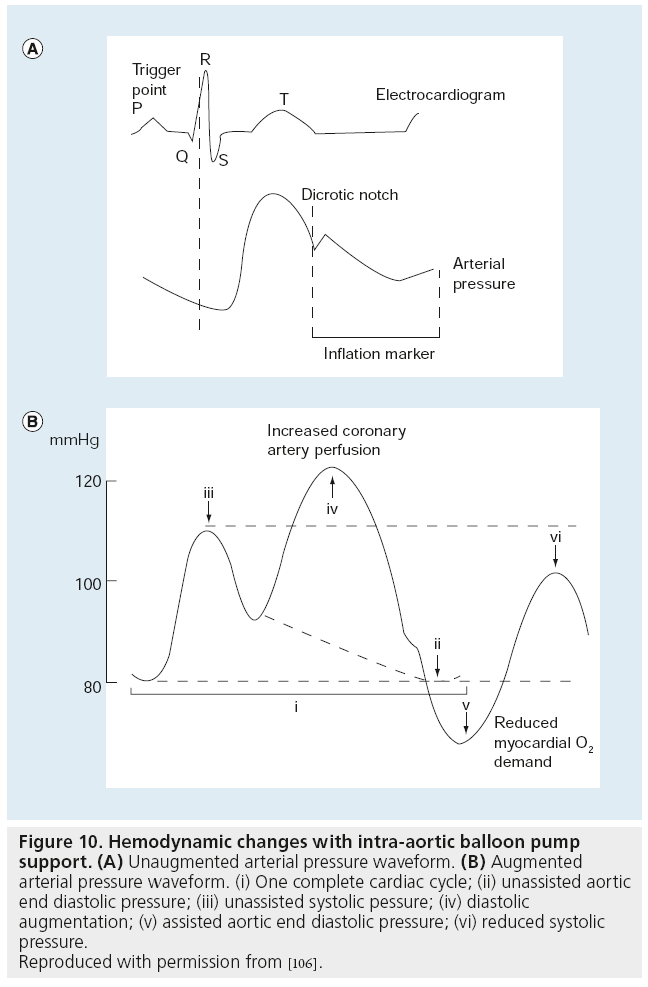
Figure 10: Hemodynamic changes with intra-aortic balloon pump support. (A) Unaugmented arterial pressure waveform. (B) Augmented arterial pressure waveform. (i) One complete cardiac cycle; (ii) unassisted aortic end diastolic pressure; (iii) unassisted systolic pessure; (iv) diastolic augmentation; (v) assisted aortic end diastolic pressure; (vi) reduced systolic pressure.
Reproduced with permission from [106].
In the acute MI cohort of the USPella registry, the Impella device was used mostly after conventional therapies had failed. It improved the CI from 1.9 to 2.5 l/min/m2, increased mean arterial pressure from 62 to 87 mmHg, decreased wedge pressure from 28 to 20 mmHg, decreased overall systemic vascular resistance and improved EF from 29 to 37%. Of the patients with acute MI and CS, a total of 58% were discharged compared with 89% of MI patients without CS [103].
More recently, the Academic Medical Center researchers have shown that in patients with STEMI and profound CS, survival was improved in patients who received immediate Impella 5.0 treatment, as well as in patients who were upgraded from 2.5 to 5.0 support, compared with patients who received only Impella 2.5 [29]. No randomized controlled trial has demonstrated superiority between IABP and Impella for long-term outcomes. The ongoing IMPRESS in STEMI trial is presently investigating the use of Impella versus IABP support in large anterior STEMI being treated with PCI [104].
Review of evidence supporting TPLVAD use in high-risk PCI
▪ IABP
The IABP is the most widely used support device for high-risk PCI in the USA [30]. Recently, the BCIS investigators randomized patients with low EF and severe multivessel disease to control versus prophylactic IABP strategy. In this trial, prophylactic IABP use did not reduce major adverse cardiac and cerebrovascular event (MACCE) or improve all cause mortality [31]. However, a trend in the reduction of mortality at 6 months was observed for the IABP arm. Another interesting observation was that operators were unsure whether support was required and in which patient, since half the patients received no support. Importantly, one-fifth of the patients in the ‘watchful waiting’ arm had to ‘crash’ to end up on IABP support. These patients had a worse outcome overall. Unfortunately, no predictive features for which patients are likely to ‘crash’ exists.
The results of the BCIS trial contradict prior nonrandomized or retrospective data demonstrating decreased MACCE in an elective versus provisional or rescue IABP strategy [32–34]. In summary, the BCIS trial demonstrated that when using prophylactic versus no planned IABP, there was no significant difference in terms of cardiovascular complication and 6-month mortality. There was a statistically significant difference in terms of major procedural complication in favor of the elective group, despite a trend towards a higher rate of bleeding and vascular complications in the elective group.
The recent CRISP-AMI trial addressed the use of IABP in patients with anterior wall STEMI without CS who were undergoing PCI [35]. Again there was no reduction in infarct size (as ssessed by cardiac MRI) or improvement in clinical outcomes at 6 months.
▪ TandemHeart
Vranckx et al. have demonstrated the efficacy of the TandemHeart for providing stable total LV support in the setting of high-risk PCI. In 23 patients followed over a 6-year period, TandemHeart insertion required an average of 35 min for implantation and resulted in significant reductions in LV filling pressures, pulmonary capillary wedge pressures and an increase in arterial pressure. However, the mortality of the cohort was modestly high at 22%, with vascular and bleeding complications in almost one-third of the patients [36]. Although the TandemHeart and Impella devices have not been compared head to head, Froesch et al. recently provided some insight into this [37]. In a single-center retrospective experience (n = 75) more TPLVADs were used in the setting of CS (n = 49) rather than highrisk PCI (n = 26). TandemHeart was also more likely to be used for CS. The Impella was more likely to be used for high-risk PCI. Mortality at 6 months was 23% after PCI and 55% after CS. Complication rates were also as high as one in three [2]. In a subsequent study, Vranckx et al. demonstrated a reduced procedural risk and excellent periprocedural circulatory support using TandemHeart in nine patients for left main PCI, who were otherwise declined for CABG [38]. Postprocedure vascular issues were observed in four of these patients. There have also been other smaller reports demonstrating similar procedural success at the expense of an increased rate of vascular complications [39–42]. Nevertheless, the more recent use of suture-mediated femoral artery preclosure may prevent vascular complications when used in conjunction with IABP devices [43].
▪ Impella
The PROTECT I study was a prospective feasibility trial that investigated 20 patients with EF of <35% who underwent high-risk PCI of either unprotected left main artery or of the last patent coronary conduit with concomitant Impella support [6]. The device provided hemodynamic support and was safe to use. Similar results were also available in smaller studies in Europe [44]. The multicenter Europella registry involving 144 consecutive patients showed that high-risk PCI with Impella support was associated with low (5.5%) mortality with no MIs [45]. Major bleeding was 6% and vascular complications were 4%. These results were also reproduced on the other side of the Atlantic in the high-risk PCI subgroup of the USPella registry. Reported overall rate of MACCE was low at 6% and 30-day survival rate was 97% [103]. Real world experiences useing Impella for high-risk PCI have also been very promising [46].
Remmelink et al. shed further light on the hemodynamic support provided by Impella during high-risk PCI. Intracoronary measurements were performed in a nonstenotic coronary artery after PCI using adenosine-induced hyperemia at different Impella support levels. LV unloading by Impella led to increased aortic and intracoronary pressure, hyperemic flow velocity and coronary flow velocity reserve and decreased microvascular resistance. The increased coronary flow by Impella support is due to both increased perfusion pressure and decreased intramyocardial resistance from reduced LV stretch [47]. The same investigators, using a pressure conductance catheter, also demonstrated dose dependant decrease in end-diastolic wall stress and improved diastolic compliance by Impella LV unloading [48].
These encouraging results led to the PROTECT II trial, which randomized patients with high-risk PCI to IABP versus Impella 2.5. After an interim analysis the trial was stopped midway due to futility [49]. However, the final results from 447 patients showed a 21% reduction in major adverse events with Impella, compared with the IABP group (40.8 vs 51.4%; p = 0.029). This was driven mainly by a decrease in repeat revascularization. The Impella device provided better stability and support during PCI allowing the operator to perform longer and more complex procedures, with more aggressive use of atherectomy [105]. An even greater benefit was observed in the prespecified subgroup of high-risk PCI without atherectomy subgroup (88% of study), with the Impella device providing a 29% reduction of major adverse events over IABP. Hospital charges for all PROTECT II patients at 90 days were on average US$19,000 lower with Impella than with IABP use, providing a further economic stimulus [105]. The use of Impella in the setting of aortic stenosis and high-risk PCI has also recently been reported, especially in the setting of concomitant valvuloplasty [5,50].
Conclusion
Technical advances in coronary intervention devices have made PCI incredibly predictable and safe. These advances have allowed operators to intervene in complex, high-risk patients in a way that would not have been conceivable a decade ago. In order to further expand the boundaries of PCI, investigators are testing the utility of hemodynamic support devices as adjunctive therapy for patients at risk of hemodynamic collapse during PCI or in the setting of CS. Nevertheless, currently, the evidence to demonstrate any mortality benefit from the use of current hemodynamic support in the setting of CS and high risk PCI is not clear. However, the limitations of performing randomized trials in this acutely sick group of patients has to be taken into consideration.
Despite the lack of mortality benefits, the devices currently available have been shown to provide hemodynamic support. However, there is a dearth of truly randomized controlled data evaluating the use of hemodynamic support devices and the superiority of one TPLVAD over another for long-term mortality in patients undergoing primary PCI for high-risk MI with tenuous hemodynamics.
The recent ly completed BCIS and PROTECT II trials have attempted to scientifically study the two most widely available devices – the IABP and Impella 2.5. These trials were successful in identifying high-risk subgroups. One-month mortality was markedly higher than standard drug-eluting stent trials. Thus, an EF of <30% with last patent conduit, unprotected left main or severe triple-vessel disease can be used as a definition of ‘high-risk PCI’. Trends towards improved survival exist for IABP in the BCIS trial. Decreases in major adverse events occurred in patients not undergoing atherectomy in the PROTECT II trial. These trials will hopefully encourage further investigations in these high-risk patients.
At present, IABPs or Impella are not routinely indicated for high-risk PCI. However, because of their ease of use they can provide rapid support in the setting of CS. The data on TandemHeart are limited but the hemodynamic benefits in specific clinical scenarios have to be weighed against the risk of serious vascular complications.
Future perspective
As more complex interventions are being performed on sicker patients and technology continues to advance rapidly, the use of TPLVADs is predicted to increase. The indications will continue to expand to support more complex procedures, including structural heart disease and transcatheter valve therapies, as well as in the management of cardiomyopathies and regenerative cell therapy. We envision that in the near future, percutaneous devices with smaller profiles that are simpler to insert and that still provide effective hemodynamic support, will continue to be developed in parallel to the growth in high-risk procedures in the cardiac catheterization laboratory.
Nevertheless, the technological advances demand the need for further well-designed randomized studies that can be applied to this acutely ill population in order to truly define the role that TPLVADs will have in the future.
Financial & competing interests disclosure
WW O’Neill has received research support from ABIOMED, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Indications
▪ Temporary left ventricular (LV) assist devices are being increasingly used for hemodynamic support in patients with cardiogenic shock and in those undergoing high-risk percutaneous procedures.
Devices available
▪ The Intra-aortic balloon pump (IABP), Impella® Recover 2.5 and TandemHeart® are the three commonly used and approved percutaneous devices in the USA.
Device selection
▪ There are specific scenarios and indications in which to select one device over the other and the decision can be: selecting or escalating the support based on the hemodynamic requirements (i.e., IABP→Impella→TandemHeart). This is based on results from small studies and is not currently supported by data from randomized control trials.
▪ The IABP continues to be the easiest and fastest support, with the fewest complications compared with the rest.
▪ During high-risk procedures, the device selected will depend on the operators familiarity and comfort level with each device as well as with how much support is desired, depending on the patients intrinsic cardiac reserve and nature of planned intervention.
▪ In the setting of peripheral vascular disease the device with the lowest vascular profile should be initially selected (IABP→Impella→TandemHeart) in order to avoid vascular complications. Caution should be taken in the cases of Impella and TandemHeart use.
Caution
▪ Temporary LV assist devices have not been decisively shown to have mortality benefits and can be associated with increased complications, depending on their vascular profile. Nevertheless, they do provide crucial hemodynamic support.
▪ The use of percutaneous LV assist devices should be performed only in centers with trained staff to be able to manage devices during and after procedure.
Future perspective
▪ Simple to insert percutaneous devices with smaller vascular profiles, which provide effective hemodynamic support, will continue to be developed with the growth in high-risk procedures in the cardiac catheterization laboratory.
References
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 117(5), 686–697 (2008).
- Badheka AO, Cohen MG. Percutaneous left ventricular assist devices: still waiting for the final word. Catheter. Cardiovasc. Interv. 78(2), 314–315 (2011).
- Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 119(9), 1211–1219 (2009).
- Harjai KJ, O’Neill WW. Hemodynamic support using the Impella 2.5 catheter system during high-risk percutaneous coronary intervention in a patient with severe aortic stenosis. J. Interv. Cardiol. 23(1), 66–69 (2010).
- Londono JC, Martinez CA, Singh V, O’Neill WW. Hemodynamic support with Impella 2.5 during balloon aortic valvuloplasty in a high-risk patient. J. Interv. Cardiol. 24(2), 193–197 (2011).
- Dixon SR, Henriques JP, Mauri L et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial US experience. JACC 2(2), 91–96 (2009).
- Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA 203(2), 113–118 (1968).
- Combes A, Leprince P, Luyt CE et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit. Care Med. 36(5), 1404–1411 (2008).
- Younger JG, Schreiner RJ, Swaniker F, Hirschl RB, Chapman RA, Bartlett RH. Extracorporeal resuscitation of cardiac arrest. Acad. Emerg. Med. 6(7), 700–707 (1999).
- Smedira NG, Blackstone EH. Postcardiotomy mechanical support: risk factors and outcomes. Ann. Thoracic Surg. 71(Suppl. 3), S60–S66; discussion S82–S85 (2001).
- Pagani FD, Aaronson KD, Swaniker F, Bartlett RH. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device. Ann. Thoracic Surg. 71(Suppl. 3), S77–S81; discussion S82–S85 (2001).
- Kushner FG, Hand M, Smith SC Jr et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 54(23), 2205–2241 (2009).
- Wright RS, Anderson JL, Adams CD et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 57(19), e215–e367 (2011).
- Stone GW, Ohman EM, Miller MF et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: the benchmark registry. J. Am. Coll. Cardiol. 41(11), 1940–1945 (2003).
- O’Rourke MF, Norris RM, Campbell TJ, Chang VP, Sammel NL. Randomized controlled trial of intraaortic balloon counterpulsation in early myocardial infarction with acute heart failure. Am. J. Cardiol. 47(4), 815–820 (1981).
- Kono T, Morita H, Nishina T et al. Aortic counterpulsation may improve late patency of the occluded coronary artery in patients with early failure of thrombolytic therapy. J. Am. Coll. Cardiol. 28(4), 876–881 (1996).
- Ohman EM, George BS, White CJ et al. Use 0f aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction. Results of a randomized trial. The Randomized IABP Study Group. Circulation 90(2), 792–799 (1994).
- Stone GW, Marsalese D, Brodie BR et al. A prospective, randomized evaluation of prophylactic intra-aortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty. Second Primary Angioplasty in Myocardial Infarction (PAMI-II) Trial Investigators. J. Am. Coll. Cardiol. 29(7), 1459–1467 (1997).
- Van’t Hof AW, Liem AL, De Boer MJ, Hoorntje JC, Suryapranata H, Zijlstra F. A randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarction. Eur. Heart J. 20(9), 659–665 (1999).
- Flaherty JT, Becker LC, Weiss JL et al. Results of a randomized prospective trial of intraaortic balloon counterpulsation and intravenous nitroglycerin in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 6(2), 434–446 (1985).
- Ohman EM, Nanas J, Stomel RJ et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS Trial. J. Thromb. Thrombolysis 19(1), 33–39 (2005).
- Sjauw KD, Engstrom AE, Vis MM et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur. Heart J. 30(4), 459–468 (2009).
- Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 26(13), 1276–1283 (2005).
- Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart® percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am. Heart J. 152(3), 469 e461–e468 (2006).
- Seyfarth M, Sibbing D, Bauer I et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 52(19), 1584–1588 (2008).
- Lam K, Sjauw KD, Henriques JP, Ince C, De Mol BA. Improved microcirculation in patients with an acute ST-elevation myocardial infarction treated with the Impella LP2.5 percutaneous left ventricular assist device. Clin. Res. Cardiol. 98(5), 311–318 (2009).
- Sjauw KD, Remmelink M, Baan J Jr. et al. Left ventricular unloading in acute ST-segment elevation myocardial infarction patients is safe and feasible and provides acute and sustained left ventricular recovery. J. Am. Coll. Cardiol. 51(10), 1044–1046 (2008).
- Engstrom AE, Sjauw KD, Baan J et al. Long-term safety and sustained left ventricular recovery: long-term results of percutaneous left ventricular support with Impella LP 2.5 in ST-elevation myocardial infarction. EuroIntervention 6(7), 860–865 (2011).
- Engstrom AE, Cocchieri R, Driessen AH et al. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: The Academic Medical Center intensive care unit experience. Crit. Care Med. 39(9), 2072–2079 (2011).
- Cohen M, Urban P, Christenson JT et al. Intra-aortic balloon counterpulsation in US and non-US centres: results of the Benchmark Registry. Eur. Heart J. 24(19), 1763–1770 (2003).
- Perera D, Stables R, Thomas M et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA 304(8), 867–874 (2010).
- Mishra S, Chu WW, Torguson R et al. Role of prophylactic intra-aortic balloon pump in high-risk patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 98(5), 608–612 (2006).
- Briguori C, Sarais C, Pagnotta P et al. Elective versus provisional intra-aortic balloon pumping in high-risk percutaneous transluminal coronary angioplasty. Am. Heart J. 145(4), 700–707 (2003).
- Brodie BR, Stuckey TD, Hansen C, Muncy D. Intra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in high-risk patients with acute myocardial infarction. Am. J. Cardiol. 84(1), 18–23 (1999).
- Patel MR, Smalling RW, Thiele H et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: The CRISP AMI Randomized Trial. JAMA doi:10.1001/jama.2011.1280 (2011) (Epub ahead of print).
- Vranckx P, Meliga E, De Jaegere PP, Van Den Ent M, Regar ES, Serruys PW. The TandemHeart®, percutaneous transseptal left ventricular assist device: a safeguard in high-risk percutaneous coronary interventions. The six-year Rotterdam experience. EuroIntervention 4(3), 331–337 (2008).
- Froesch P, Martinelli M, Meier P et al. Clinical use of temporary percutaneous left ventricular assist devices. Catheter. Cardiovasc. Interv. 78(2), 304–313 (2011).
- Vranckx P, Schultz CJ, Valgimigli M et al. Assisted circulation using the TandemHeart during very high-risk PCI of the unprotected left main coronary artery in patients declined for CABG. Catheter. Cardiovasc. Interv. 74(2), 302–310 (2009).
- Al-Husami W, Yturralde F, Mohanty G et al. Single-center experience with the TandemHeart percutaneous ventricular assist device to support patients undergoing high-risk percutaneous coronary intervention. J. Invas. Cardiol. 20(6), 319–322 (2008).
- Gimelli G, Wolff MR. Hemodynamically supported percutaneous coronary revascularization improves left ventricular function in patients with ischemic dilated cardiomyopathy at very high risk for surgery: a single-center experience. J. Invas. Cardiol. 20(12), 642–646 (2008).
- Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: ‘TandemHeart®’ for high-risk coronary intervention. Catheter. Cardiovasc. Interv. 65(3), 346–352 (2005).
- Kar B, Forrester M, Gemmato C et al. Use of the TandemHeart percutaneous ventricular assist device to support patients undergoing high-risk percutaneous coronary intervention. J. Invas. Cardiol. 18(4), A6 (2006).
- Rajdev S, Krishnan P, Irani A et al. Clinical application of prophylactic percutaneous left ventricular assist device (TandemHeart) in high-risk percutaneous coronary intervention using an arterial preclosure technique: single-center experience. J. Invas. Cardiol. 20(2), 67–72 (2008).
- Henriques JP, Remmelink M, Baan J Jr. et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am. J. Cardiol. 97(7), 990–992 (2006).
- Sjauw KD, Konorza T, Erbel R et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J. Am. Coll. Cardiol. 54(25), 2430–2434 (2009).
- Alasnag MA, Gardi DO, Elder M et al. Use of the Impella 2.5 for prophylactic circulatory support during elective high-risk percutaneous coronary intervention. Cardiovasc. Revasc. Med. 12(5), 299–303 (2011).
- Remmelink M, Sjauw KD, Henriques JP et al. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter. Cardiovasc. Interv. 70(4), 532–537 (2007).
- Remmelink M, Sjauw KD, Henriques JP et al. Effects of mechanical left ventricular unloading by Impella on left ventricular dynamics in high-risk and primary percutaneous coronary intervention patients. Catheter. Cardiovasc. Interv. 75(2), 187–194 (2010).
- Syed AI, Kakkar A, Torguson R et al. Prophylactic use of intra-aortic balloon pump for high-risk percutaneous coronary intervention: will the Impella LP 2.5 device show superiority in a clinical randomized study? Cardiovasc. Revasc. Med. 11(2), 91–97 (2010).
- Martinez CA. Hemodynamic Support with Impella 2.5 in High-Risk Interventions for Patients with Aortic Stenosis. Presented at: The 60th Annual Scientific Sessions of the American College of Cardiology. New Orleans, LA, USA, 2–5 April, 2011. ▪ Websites
- Protect II, A Prospective, Multicenter, Randomized Controlled Trial (PROTECT II). www.clinicaltrials.gov/ct2/show/ nct00562016
- Cardiobridge. www.cardiobridge.com
- Cath Lab Digest. www.cathlabdigest.com/abiomed-presentsresults- from-two-studies-uspella-and-nach-ii
- Nederlands Trial Register. www.trialregister.nl/trialreg/admin/rctview. asp?tc=1079
- Abiomed Announces New PROTECT II Data at 2011 SCAI Scientific Sessions. www.cathlabdigest.com/abiomedannounces- new-protect-li-data-2011-scaiscientific- sessions
- Maquet Cardiac Arrest Getinge Group. http://ca.maquet.com
- Abiomed. Impella® 2.5. www.abiomed.com/products/impella-2-5