Research Article - Interventional Cardiology (2020) Volume 12, Issue 3
Hex Induced segmental arterial mediolysis superimposed upon idiopathic pulmonary hypertension; Cross-talk of Serotonin with Norepinephrine?
- Corresponding Author:
- Richard E. Slavin Johns Hopkins Bay View Medical Center, Department of Pathology, Baltimore, Maryland, USA E-mail: reslavin@comcast.net
Abstract
Background: Segmental arterial mediolysis (SAM) is an infrequent arteriopathy initiated by norepinephrine released by the innervating peripheral sympathetic nervous system, that involves the large and medium-sized muscular arteries of the abdomen, retroperitoneum, heart and brain base. It extremely rarely develops in pulmonary arteries. Serotonin (5-HT) is an important vasoconstricting and remodeling agent in the genesis of idiopathic pulmonary hypertension.
Methods and findings: Terror stemming from a history of a hex putatively caused a massive release of catecholamines from the adrenal medulla – the norepinephrine representing another stimulus for pulmonary vasoconstriction. Since a presumed low density of alpha-1 adrenoceptor on the medial muscle of pulmonary arteries exists the cross talk of norepinephrine with 5-HT is theorized to have caused the toxic cytoplasmic calcium overload that transformed the norepinephrine induced vasoconstriction to vasospastic SAM. This was identified by the morphologically distinct features of SAM – vacuolization and apoptosis commencing in the outer media, gap formation and granulation tissue developing at the adventitial medial junction alterations superimposed on the morphologic changes of pulmonary hypertension. Cross talk of these two pressor agents also putatively accounted for the genesis of coronary artery SAM in newborn persistent pulmonary hypertension (NPPH) reported in an infant delivered from a pregnancy depressed mother putatively administered a selective serotonin-reuptake inhibitor.
Conclusion: Cross talk of norepinephrine with 5-HT accounts for the genesis of SAM in idiopathic pulmonary hypertension and in the coronary arteries in NPPH.
Keywords
Segmental arterial mediolysis •Idiopathic pulmonary hypertension •Norepinephrine serotonin Cross-talk •Hex •Neonatal persistent pulmonary hypertension •Coronary artery dissection
Introduction
An excessive release of norepinephrine associated with plastically elevated densities of adrenoceptors or through cross talking with other pressor agents has been implicated in a number of vascular, cardiac and renal conditions. The former entities include the following: 1. SAM, an apoptotic vasospastic disorder involving the large and medium sized muscular arteries innervated by the peripheral sympathetic nervous system most often observed in the abdominal cavity and retroperitoneum in the elderly, pericardial coronary arteries in infants, children and young adults and basilar cerebral arteries in adults [1,2]. The spastic arterial lesions generated in the injurious phase of SAM cause catastrophic hemorrhages in the abdominal cavity, retroperitoneum and brain base, focal renal infarcts and extensive pancreatic hemorrhages. 2. A series of non-inflammatory and nonatheromatous arterial diseases generated by the reparative response in arteries afflicted by injurious phase SAM. These include fibromuscular dysplasia, dissecting aneurysms, arterial stenosis, and persistent arterial aneurysms [3-5]. 3. Mesangial cell hyperplasia with focal segmental glomerulosclerosis generated by the norepinephrine stimulated release of mesangial precursors from the bone marrow that colonize the glomerular stalk and the coupling of norepinephrine with mesangial alpha-1 adrenoceptor causing their contraction, apoptosis and repair [6,7]. 4. Myocardial apoptosis and fine fibrosis developing in subjects with markedly elevated heart rates administrated Beta-receptor agonists having the ability to cause the manufacture and release of norepinephrine and clinical states that produce an excessive quantity of norepinephrine from the adrenal medulla [2,7]. The reported cross talk entity is Fibromuscular dysplasia of renal vein provoked by the cross talk of norepinephrine with endothelin-1 [8]. This article will show how norepinephrine can also cross talk with the pressor agent 5-HT which contributes to the genesis of pulmonary hypertension to create SAM in the pulmonary and coronary arteries.
Case Presentation
This presentation represents a summation of the pertinent clinical aspects of a case previously reported in a clinicopathologic conference [9]. The patient, a 22-year old black female, was admitted because of shortness of breath, episodes of chest pain radiating to the right shoulder and scapula, and episodes of dizziness and syncope of six weeks duration. Her clinical work up was consistent with pulmonary hypertension. This was of the primary (idiopathic) type since other clinical states listed in the categories of conditions causing pulmonary hypertension were absent or ruled out. Associated with this diagnosis was a history of a hex. She stated that she had a serious problem and only had 3 days before her twenty-third birthday to solve it. “She was born on Friday the thirteenth in the Okefenokee Swamp delivered by a midwife who also delivered two other female children that day. The midwife told the mothers that the three girls were hexed and that the first would die before her sixteenth birthday, the second before her twenty-first birthday, and the third (the patient) before her twenty-third birthday.
The patent went on to tell her physician that the first girl was killed in an automobile accident before her sixteenth birthday. The second girl was quite fearful of the hex and on her twenty-first birthday called a friend and insisted on going out to celebrate the end of the hex. As they walked into a saloon a stray bullet hit the girl and killed her. The patient was terrified and believed she was doomed. She manifested increasing severe episodes of hyperventilation, a fall in her blood sugar to 40 mg. per 100 ml and on the day before her twenty-third birthday she died following an episode of hyperventilation, severe apprehension and profuse sweating. “ [reprinted with permission from the Clinicopathologic Conference Case Presentation (BCH # 469861), Johns Hopkins Medical Journal 120:3 (1967), 186-199, copyright 1967, The Johns Hopkins Press U.S.A.].
Morphologic Findings
Gross Description
The lungs were edematous, red brown in hue, and showed acute congestion most severely involving the right lower lobe. The elastic pulmonary arteries were dilated and exhibited atheromatous streaking. They were free of emboli.
The right heart was hypertrophied, the heart weighing 480 grams. Congenital abnormalities were not identified, the heart valves were normal, and all the cardiac chambers were free or recent, organizing or organized thrombi.
Microscopic Description
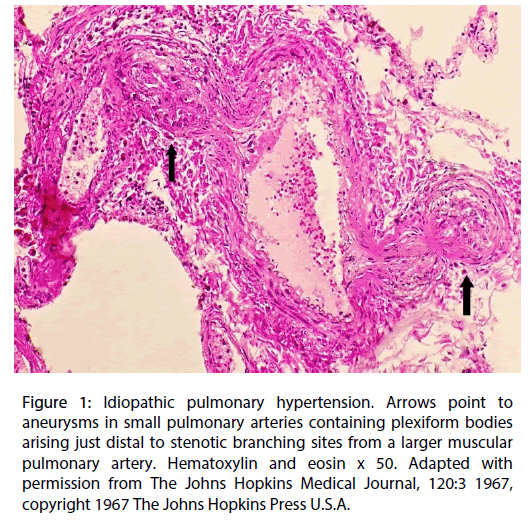
The large and small muscular pulmonary arteries showed medial hypertrophy with longitudinal smooth muscle extensions into arterioles, and a concentric varying aged neo intima composed of fibroblasts, endothelial and myoepithelial cells distributed in an extracellular matrix that progressed in some arteries towards intimal fibrosis with patchy concentric elastin deposition. Complexes of capillaries with both dilated and slit lumens formed plexiform bodies in the lumens of dilated small arteries or muscularized arterioles. The loss of medial muscle in these dilated arteries led to the formation of aneurysms principally originating just beyond their branching sites from small pulmonary arteries. These branch points were stenotic due either to muscular contraction and/or intimal plaque formation (Figure 1). The plexiform bodies could herniate into and through the aneurysms to create vascular communications with adjacent markedly dilated capillaries– angiomatoid bodies.
Figure 1: Idiopathic pulmonary hypertension. Arrows point to aneurysms in small pulmonary arteries containing plexiform bodies arising just distal to stenotic branching sites from a larger muscular pulmonary artery. Hematoxylin and eosin x 50. Adapted with permission from The Johns Hopkins Medical Journal, 120:3 1967, copyright 1967 The Johns Hopkins Press U.S.A.
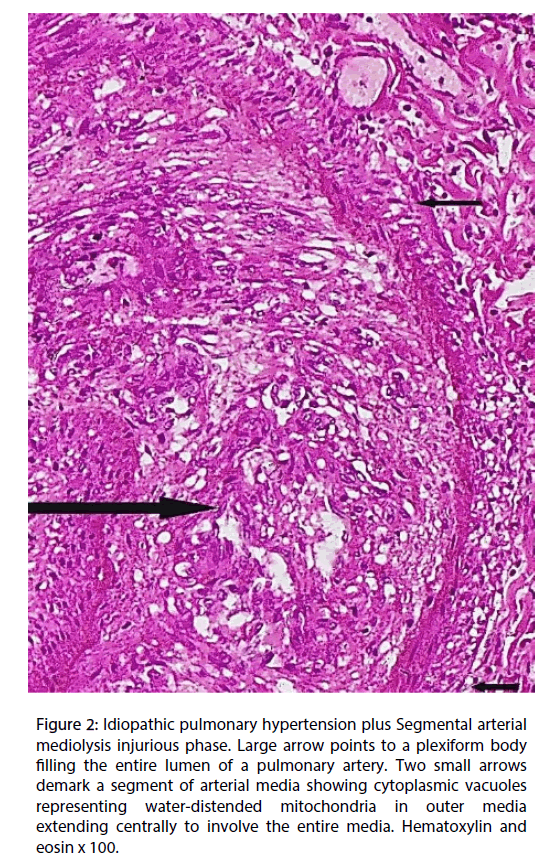
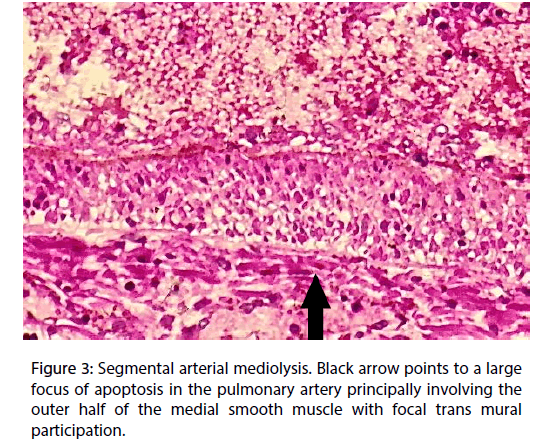
The outer medial muscle of the small muscular arteries, in areas free of inflammation, exhibited cytoplasmic vacuoles (Figure 2) and/or apoptosis (Figure 3) alterations that focally extended into the mid and inner media. Total medial loss created by these changes formed arterial gaps bordered by linear fibrin deposits.
Figure 2: Idiopathic pulmonary hypertension plus Segmental arterial mediolysis injurious phase. Large arrow points to a plexiform body filling the entire lumen of a pulmonary artery. Two small arrows demark a segment of arterial media showing cytoplasmic vacuoles representing water-distended mitochondria in outer media extending centrally to involve the entire media. Hematoxylin and eosin x 100. Figure
Figure 3: Segmental arterial mediolysis. Black arrow points to a large focus of apoptosis in the pulmonary artery principally involving the outer half of the medial smooth muscle with focal trans mural participation.
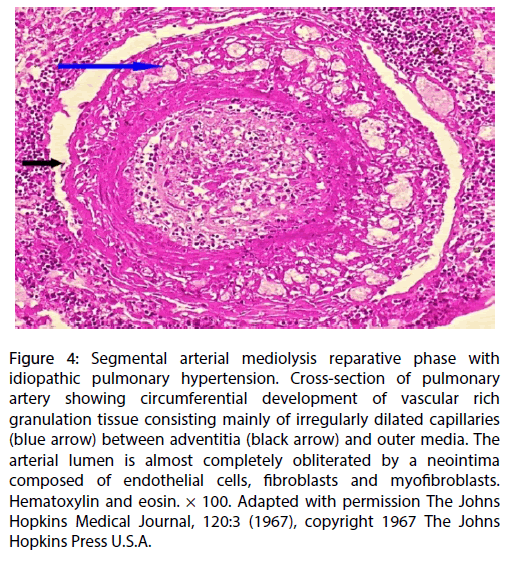
Granulation tissue containing irregularly dilated capillaries developed focally or circumferentially in varying sized spaces that formed between the outer media and adventitia of the muscular pulmonary arteries (Figure 4). These frequently but not always formed adjacent to areas of medial apoptosis. These capillaries were distinct from the angiomatoid bodies found in the lung parenchyma peripheral to the adventitia of the branched small pulmonary arteries and muscularized arterioles. Hemosiderin laded macrophages were evident in the various pulmonary air spaces.
Figure 4: Segmental arterial mediolysis reparative phase with idiopathic pulmonary hypertension. Cross-section of pulmonary artery showing circumferential development of vascular rich granulation tissue consisting mainly of irregularly dilated capillaries (blue arrow) between adventitia (black arrow) and outer media. The arterial lumen is almost completely obliterated by a neointima composed of endothelial cells, fibroblasts and myofibroblasts. Hematoxylin and eosin. × 100. Adapted with permission The Johns Hopkins Medical Journal, 120:3 (1967), copyright 1967 The Johns Hopkins Press U.S.A.
Discussion
This case study reports the remarkable superimposition of one morphologic pressor response upon another. The patient died with right heart failure as a consequence of pulmonary hypertension complicated by extreme fright. Given the patient ’ s clinical presentation and the morphologic findings of plexiform bodies in the absence of accompanying cardiac, pulmonary or embolic lesions, the pulmonary hypertension was primary (idiopathic) in type [11]. Pulmonary hypertension does not have a single cause [12]. Rather a complex pathophysiology is implicated in its genesis. This situation is generated by the different signals sent to the pulmonary arteries by the various agents and clinical conditions causing the pulmonary hypertension and the heterogeneous response to these signals by the endothelial and smooth muscle cells of the pulmonary vessels. These responses cause the remodeling, obstruction or constriction of the pulmonary arteries alterations elevating the pulmonary artery vascular resistance an event that ultimately results in right heart failure and death.
Serotonin (5-HT) is currently considered as a very important agent contributing to the genesis of primary pulmonary hypertension [13]. This belief was founded on the outbreak of primary pulmonary hypertension in patients administered anorexigens, diet pills that increased 5-HT pulmonary concentration by inducing platelet release of 5-HT, restricting its reuptake and inhibiting the metabolizing enzyme monoamine oxidase activity [14]. Moreover, its remodeling and vasoconstrictive capabilities can create the characteristic lesions encountered in idiopathic pulmonary hypertension. The paracrine release of 5-HT produced by neuroendocrine cells at bronchial branching sites can generate stenosis of the adjacent pulmonary arteries at their branching sites by causing either vasoconstriction or neointimal plaque formation (Figure 1). With each ventricular contraction blood flow jetting through these stenotic arterial zones would meet a slower distal blood circulation that converts its high kinetic energy to the following dynamic alterations in the circulatory flowincreased pressure and eddying and retrograde currents. Such perturbations in blood flow upon striking the arterial wall could cause their dilatation, medial muscle loss and ultimate aneurysm formation [15]. The plexiform bodies developing in these dilated vessels are also putatively generated by a 5-HT remodeling signal released from their endothelial cells.
The second and much more recent pressor response was identified by the presence of the apoptosis and cytoplasmic vacuoles (water distended mitochondria) chiefly concentrated in the outer arterial medial muscle, the creation of arterial gaps bordered by a linear deposit of fibrin and the florid granulation tissue formation at the adventitial medial border (Figures 2-4). These are not morphologic injuries encountered in idiopathic pulmonary hypertension but are characteristic of SAM in it injurious and early reparative phases [1,2]. SAM develops in the muscular arteries innervated by the peripheral sympathetic nervous system. The medium sized and small pulmonary arteries are also innervated by this system. They receive signals from the pulmonary plexus. The stimuli initiating SAM are derived from iatrogenic sympathomimetic agonists or the endogenous release of supra physiological norepinephrine levels. Both stimuli rapidly initiate the release of excessive norepinephrine from varicosities on the efferent branches of the peripheral sympathetic nerves innervating targeted muscular arteries [2]. The latter stimulus undoubtedly was promoted by the patient ’ s extreme fear of the hex. Indeed, this was the principal reason for this case review re-examined in order to provide additional evidence that an exaggerated release of norepinephrine from the adrenal medulla could cause SAM [7].
In SAM the liberated norepinephrine couples to zones of hyper dense alpha-1 adrenoceptor distributed on the cell membranes of medial smooth muscle. This hyper density is created by this receptor’s dynamic state controlled by a variety of exogenous and endogenous factors, such as age, sex, and prior exposure to appropriate iatrogenic agonists [16]. The normal genetic positions of this adrenoceptor can be overridden by these factors to create patchy zones of hyper density. The hyper dense receptor coupled with the abundant norepinephrine excessively activates a heterotrimeric G protein that signals the rapid development of a cascade of augmented biochemical events, which transforms vasoconstriction to vasospasm by creating a cytoplasmic calcium overload. This signals a powerful muscular contraction that shears the media from the adventitia and the development of calcium toxicity to mitochondria that induces mediolysis and/or apoptosis [17,18]. It also signals a rapid repair of these injuries by a florid proliferation of granulation tissue often containing irregularly dilated capillaries [1].
Pulmonary artery contraction caused by 5-HT is also dependent on myofilament Ca2+-sensitization and cytosolic calcium concentration [13]. Upon binding to its 5-HT2 receptors, 5-HT induces a rise in cytosolic calcium coming from the intracellular compartment, mainly the sarcoplasmic reticulum, and an influx of extracellular calcium both responsible for mitochondrial calcium increase. However, the density of alpha-1 adrenoceptor in the pulmonary arteries is putatively low [19] so that SAM in our patient ’ s pulmonary arteries is believed to have developed from the summation of biochemical events created by the cross talk of norepinephrine with 5-HT. The combined cytoplasmic entrance of calcium ions and its release from the sarcoplasmic reticulum incited by both pressor agents would overload the cytoplasmic calcium ion concentration rescinding the need for zones of hyper dense alpha-1 adrenergic receptor to create mitochondrial toxicity in the genesis of pulmonary artery SAM.
SAM is an uncommonly reported vascular disease, but its pulmonary occurrence is extremely rare. It is suspected, based on an animal study [19], that this is putatively ascribable to the low density of alpha-1 adrenoceptor on the medial muscle and/or a sluggish plastic response of one or more of this receptor ’ s subdivisions to conditions that would increase their density in other arterial systems. This deficiency offers an explanation for the absence of pulmonary SAM in cases of infant and childhood coronary artery SAM despite the communication of the pulmonary plexus with the cardiac plexus [20]. I am aware of only one case of pulmonary SAM reported by Lie’s in a small series of published cases of SAM [21]. This patient had a massive pulmonary hemorrhage putatively derived from a ruptured gap-aneurysm.
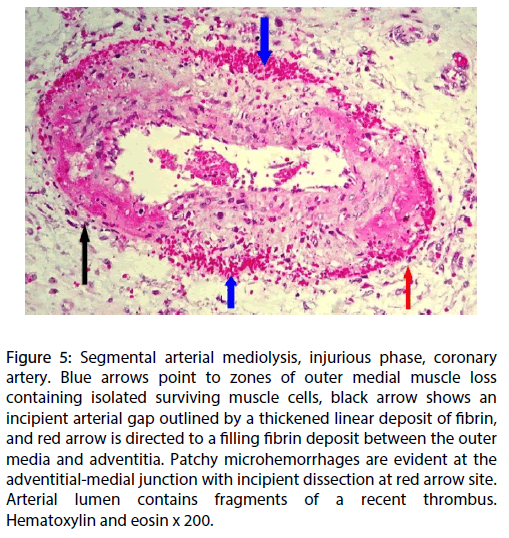
Can SAM develop in other arterial systems in patients with pulmonary hypertension? Slavin and coworkers reported a case of SAM involving the coronary arteries (Figure 5) in an infant with persistent pulmonary hypertension (PPHN) [10]. A recent review of the patient’s history revealed that the infant was delivered from a mother with a history of pregnancy depression. The fortuitous co-existence of these two pressor entities isn’t likely given the rarity of coronary artery SAM and the infrequency of PPHN, a disorder that occurs in about two of every 1000 births [22]. The report putatively linking the selective serotonin-reuptake inhibitor (SSRI) fluoxetine administered for the treatment of depression in mothers of infants born with PPHN [22] offers a possible explanation for the co-existence of these two entities. In this scenario the morphologic pulmonary artery alterations found in PPHN would by be created by 5- HT and stored pulmonary SSRI could result in an accumulation of non-metabolized pulmonary 5-HT that could be transported by paracrine means into the adjacent pericardial coronary arteries.
Figure 5: Segmental arterial mediolysis, injurious phase, coronary artery. Blue arrows point to zones of outer medial muscle loss containing isolated surviving muscle cells, black arrow shows an incipient arterial gap outlined by a thickened linear deposit of fibrin, and red arrow is directed to a filling fibrin deposit between the outer media and adventitia. Patchy microhemorrhages are evident at the adventitial-medial junction with incipient dissection at red arrow site. Arterial lumen contains fragments of a recent thrombus. Hematoxylin and eosin x 200.
The cross talk of the coronary 5-HT coupled with a norepinephrine release stimulus in the coronary arteries could generate the cytoplasmic calcium ion overload in the coronary medial muscle to produce SAM. The diagnosis of coronary artery SAM, in surviving infants with PPHN, would principally depend on the radiologic identification of the sequelae created in SAM ’ s reparative phase since bleeding from gap-aneurysms formed in SAM’s injurious phase, the chief presentation of SAM, rarely occurs in coronary arteries and the mediolysis or apoptosis created in this phase may rapidly repair so that their angiographic presentations may completely resolve [4] or the sequelae of SAM may develop. These are persistent aneurysms, luminal stenosis, fibromuscular dysplasia and dissecting hematomas [3,4,5], the latter reported in coronary artery SAM [23]. These dissections can occur weeks, months or even years after the onset of SAM and may present as spontaneous coronary artery dissections [2]. Physicians following surviving infants with PPHN, born from mentally depressed mothers administered SSRI, should consider this possibility if they develop cardiac symptoms, not ascribable to congenital heart disease, in childhood or as young adults.
Conclusion
The supraphysiologic release of norepinephrine from the adrenal medulla induced by the fear of the hex, that cross talked with the pressor agent 5-HT implicated in the genesis of idiopathic pulmonary hypertension, generated SAM in the muscular pulmonary arteries of this case. This cross talk created SAM by bypassing the need for a hyper density of the alpha1-adrenergic receptor in order to generate a toxic cytoplasmic calcium overload in the medial smooth muscle. Lesions of SAM, so generated, were superimposed on the hypertensive pulmonary lesions. The norepinephrine induced vasospastic response was distinct and differed from that found in pulmonary hypertension in that it originated at the adventitial medial interface rather than at the intima. It was identified by cytoplasmic vacuoles and apoptosis principally involving the outer medial muscle, focal formation of arterial gaps bordered by a linear deposit of fibrin and the florid overgrowth of granulation tissue at the adventitial medial junction.
SAM occurring in the coronary arteries of infants and children is not associated with pulmonary artery SAM an observation putatively attributable to the low density or low plasticity potential of alpha-1 adrenoceptors on the medial muscle of the muscular pulmonary arteries.
Coronary artery SAM developing in infants with PPHN delivered from depressed mothers treated with SSRI is putatively generated by the paracrine cross talk of stored non metabolized pulmonary 5-HT with released norepinephrine in the media of the pericardial coronary arteries. SAM in surviving children would be clinically recognized mainly in its reparative phase as dissecting hematomas presenting weeks, months or years after the onset of SAM.
Acknowledgments
The author wishes to express his appreciation to Jason Slavin for his assistance in creating and formatting the digital images, to Beth Caldwell and Kelly Fercovich of the Legacy library system for their help with the references and to Shannon McCullough of The Johns Hopkins Press for obtaining permission for use of reprint material. ORCID identifying Number: 0000-0002-2774-2595
Figures 1 to 4 utilized in this article were derived from microphotographs of pulmonary vascular lesions in reference 9. Figures 1 and 4 were adapted from photographs in this article; Figures 2 and 3 were not previously published. Figure 5 was selected from unpublished photographs of coronary artery SAM whose journal origin is reference 10.
Conflict of interest
There was no conflict of interest.
Funding
No funding agent played a role in the preparation or writing of this manuscript.
References
- Slavin RE. Segmental arterial mediolysis: A clinical-pathologic review, its role in fibromuscular dysplasia and description and differential diagnosis of the masquerader-muscular artery cystic necrosis. WJCD. 3(1): 64-81 (2013).
- Slavin RE. Segmental arterial mediolysis-a vasospastic arteriopathy: a morphologic survey and proposed role of norepinephrine coupled with hyperdense alpha-1 adrenoceptor in its pathogenesis. EC Cardiol. 5(7): 445-466 (2018).
- Slavin RE, Saeki K, Bhagavan B, et al. Segmental arterial mediolysis: A precursor to fibromuscular dysplasia? Mod Pathol. 8(3): 267-294 (1995).
- Slavin RE, Gonzalez JC, Manchin JM, et al. Segmental arterial mediolysis, reparative phase: An analysis and case report showing conversion to fibromuscular dysplasia with renal infarction. WJCD. 4(2): 50-60 (2014).
- Slavin RE. Segmental arterial mediolysis: course, sequelae, prognosis, and pathologic-radiologic correlation. Cardiovasc Pathol.18(6): 352-360 (2009).
- Leifsson PS, Slavin RE. Segmental arterial Mediolysis in pigs presenting with renal infarct. Vet Pathol. 52(6): 1157-1162 (2015).
- Slavin RE, Leifsson PS. Segmental arterial mediolysis with mesangial hyperplasia: a review with supplementary comments concerning its pathogenesis. Inter Cardiol. 9(5): 181-190 (2017).
- Slavin RE, Inada K. Segmental arterial mediolysis with accompanying venous angiopathy: a clinical pathologic review, report of 3 new cases and comments on the role of endothelin-1 in its pathogenesis. Int J Surg Pathol. 15(2): 121-134 (2007).
- Friesinger GC, Slavin RE. Clinicopathologic Conference. Case Presentation. The Johns Hopkins Med J.120(3):186-199 (1967).
- Slavin RE, Cafferty L, Cartwright J. Segmental mediolytic arteritis: A clinicopathologic and ultrastructural study of 2 cases. Am J Surg Pathol. 13(7): 558-568 (1995).
- Primary pulmonary hypertension. Executive summary from the world symposium-primary pulmonary hypertension 1998, Evian France: World Health Organization.
- Travis WD, Colby TV, Koss MN, et al. Pulmonary hypertension and other vascular disorders. In: ed. King DW. Non-neoplastic disorders of the lower respiratory tract. Atlas of Nontumor Pathology, First series, Fascicle 2, American Registry of Pathology and the Armed Forces Institute of Pathology, Washington DC. 768-778 (2002).
- Genet N, Billaud M, Rossignol R, et al. Signaling pathways linked to serotonin-induced superoxide anion production: A physiological role for mitochondria in pulmonary arteries. Front Physiol.
- Rothman RB, Ayestas MA, Dersch CM, et al. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation.100: 869-875 (1999).
- Holman E. The obscure physiology of poststenotic dilatation: Its relation to the development of aneurysms. J Thorac Cardiov Surg.28: 109 (1954).
- Slavin RE. Segmental arterial mediolysis: a review of a proposed vascular disease of the peripheral sympathetic nervous system-a density disorder of the alpha-1 adrenergic receptor? J Cardiovasc Dis Diagn. 3(2): 1-7 (2015).
- Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol. 287(4): 817-833 (2004).
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 3005: 626-629 (2004).
- Shaul PW, Magness RR, Muntz KH, et al. Alpha1-Adrenergic receptors in pulmonary and systemic vascular smooth muscle alterations with development and pregnancy. Circulation Res. 67(5): 1193-1200 (1990).
- Berry MM, Sandring SM, Bannister LH. Nervous system. In: William PL. Gray’s Anatomy 38th ed. New York, Edinburgh London Tokyo Madrid and Melbourne: Church Livingston. 1995: 1307.
- Lie JT. Systemic, Cerebral, and pulmonary segmental mediolytic arteriopathy villainous masqueraders of vasculitis. Cardiovasc Pathol. 5(6): 305-314 (1996).
- Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 354: 579-587 (2006).
- Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Human Pathol.18(6): 654-656 (1987).