Research Article - Neuropsychiatry (2017) Volume 7, Issue 1
High Baseline Serum Asymmetric Dimethylarginine Level is Related to Delayed Psychomotor Development in Very Low Birth Weight Premature Infants
- Corresponding Author:
- Chih-Cheng Chen, MD
Section of Neonatology, Department of Pediatrics
Kaohsiung Chang-Gung Memorial Hospital,123 Ta-Pei Road
Niaosung, Kaohsiung, Taiwan, R.O.C.
Tel: 886-7-731-7123 ext. 8715
Fax: 886-7-733-8009
Email: charllysc@hotmail.com
Abstract
Objective:
Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of the endothelial nitric oxide synthase, is specifically related with cranial small vessel disease. We aim to investigate the baseline level of serum ADMA in correlation to neurodevelopmental outcomes in a cohort of very low birth weight premature infants.
Method:
A total of 50 very low birth weight (VLBW, <1500 g) preterm (gestational age ≦ 30 weeks) infants were included and assigned to a High or Low ADMA baseline level group according to the plasma ADMA levels obtained within 6-12 hours after birth. All patients were followed prospectively for the neurodevelopmental outcomes. Causes of morbidities were analysed and compared.
Results:
No differences in gestational age, birth body weight, 1’ and 5’ Apgar scores, gender prevalence, and serum creatinine level were noted between study groups. Premature infants with high ADMA baseline level had a higher incidence of periventricular echogenecity persisting longer than 2 weeks (p= 0.013), and a higher ratio of delayed psychomotor development at 6 and 12 months corrected age (p=0.041 and 0.012). ADMA baseline level is inversely correlated to the Psychomotor Developmental Index at 6 and 12 months corrected age within the high ADMA baseline level group (p=0.02, p=0.027 respectively). The occurrence of retinopathy of prematurity, chronic lung disease, and patent ductus arteriosus requiring medication intervention were of no significant differences between study groups.
Conclusion:
Premature infants with high ADMA baseline levels tended to have a higher ratio of delayed psychomotor development at corrected age of 6 and 12 months old. Further studies on perinatal factors related with high ADMA baseline levels are warranted.
Keywords
Asymmetric dimethylarginine baseline level, Psychomotor development, Very Low Birth Weight Premature
Introduction
Increasing cardiac output and decreasing peripheral resistance, the alterations in blood pressure and vascular reactivity to vasoconstrictors are some of the important changes that occur in the maternal vascular system during pregnancy [1]. And it is these changes that ensure adequate delivery of oxygen to the fetus. There are several factors, either of maternal or neonatal origin, which affect the nitric oxide (NO) metabolism and play a role in the pathogenesis of some serious disorders, such as hypoxic ischemic encephalopathy, persistent pulmonary hypertension, necrotizing enterocolitis and chronic lung disease [2-6].
Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of endothelial NO synthase, competes with L-arginine as a substrate for NO synthase. Higher plasma levels of ADMA have been implicated in the pathogenesis of the endothelial dysfunction, atherosclerosis [7-9], as well as increasing the risk of developing stroke [10], transient ischemic attack [11], and it is recently associated with image findings of smallvessel disease, specifically with the severity of white-matter abnormalities [12].
Elevated ADMA levels during pregnancy have been reported as an important pathophysiological factor in the development of preeclampsia [13,14]. Although, the effects of maternal factors or fetal problems during delivery on the ADMA levels are still not fully understood. Vida et al. [15] and Tsukahara et al. [16] investigated the plasma ADMA levels during the perinatal period and showed that ADMA might have an important role both in the control of the fetoplacental circulation and the neonate’s postnatal circulatory adaptations. Thus it is possible that the baseline ADMA level of the neonate might significantly reveal the severity of intraunterine endothelial dysfunction. Our study aimed to correlate baseline ADMA level to long-term neurodevelopment status in a cohort of very low birth weight premature infant.
Patient and Methods
▪ Subjects
Very low birth weight preterm infants delivered with Gestational age ≦30 weeks and birth body weight less than 1500 grams were recruited prospectively to participate in the study conducted at Kaohsiung Chang-Gung Memorial Hospital, Kaohsiung, Taiwan from June 2012 to May 2014. The exclusion criteria were as follows: neonates who required intense resuscitation, infants with craniosonographic images of interventricular hemorrhage or periventricular cystic leukomalacia, with congenital anomaly, metabolic disorders, and maternal drug abuse history were excluded from the study.
The study candidates were grouped according to serum baseline ADMA levels obtained within 6-12 hours of life; the upper tertile of baseline values, the High ADMA baseline level group, and the lower two tertiles, the Low ADMA baseline level group (i.e., >0.895 mol/l vs. ≦ 0.895 mol/l) as listed in Table 1. The tertile cutoff values were derived from the 59 patients that constituted the database.
▪ Measurement of Serum ADMA, SDMA, and Arginine Concentration
ADMA, SDMA (Symmetric Dimethyl- Arginine), and Arginine was measured using commercially available ELISA kits (DLD Diagnostika GmbH, Hamburg, Germany). The blood samples taken from the premature infants were immediately placed on ice and centrifuged at 3000 rpm for 10 min at 48 0C. Plasma was immediately put in liquid N2, and stored at 2-8 °C before analysis; the levels of ADMA, SDMA, and Arginine were measured from aliquoted serum samples. The sensitivities of ADMA were 0.05mol/l. The intra-assay coefficient variation of ADMA was < 7.5%, respectively.
▪ Developmental Assessment
Developmental outcome of each candidate was assessed by the Bayley Scales of Infant Development II (BSID II) at 6, and 12 months corrected age respectively. A pediatric psychologist who was unaware of the craniosonographic finding was involved in the developmental assessment. The BSID II provided relevant information to aid in the diagnosis of developmental delay. There are two parameters within BSID II: the Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI). Mental index assesses cognition, language, and interpersonal social development. Motor index evaluates the fine and gross motor development. A child was considered to have significantly delayed development if the score was below 70; while a score between 85 and 114 was considered normal. The developmental outcomes of the infants that were studied in the two groups were compared and contrasted.
In this current study, antenatal steroids were defined as at least one dose of either betamethasone or dexamethasone given prenatally. Chorioamnionitis was diagnosed clinically by an obstetrician and recorded in the maternal records with at least one of the following: maternal fever >38.1°C, maternal leukocytosis, uterus tenderness, foul-smelling amniotic fluid and positive amniotic fluid culture. Periventricular echodensity (PVE) was defined as one or more areas of increased echodensities with at least one dimension greater than 1 cm in the periventricular region, seen in both coronal and sagittal planes and persisting for at least one week and after disappearance of these echodensities no cystic lesions and/or ventricular dilation were observed. And it is our institutional protocol to perform craniosonographic image assessment start from the 1st week after birth and then at a weekly intervals ‘til one month of age, then monthly interval thereafter and ended when two consecutive studies revealed a normal craniosonography.
▪ Statistical Analysis
Group means derived from the standard deviation of the mean and group differences were assessed by the use of the analysis of variance (ANOVA). The differences of continuous data between patients of the two groups were compared by two-sample t test or Mann- Whitney U test, when appropriate. Categorical data were compared by means of Chi-squre test or Fisher’s exact test. Variables included in the factor analysis were gestational age, gender, birth weight, Apgar score, and creatinine level were included as potential covariates, and those that differ statistically were included in the univariate analysis in stepwise logistic regression model. A P value of < 0.05 was considered to be significant. Statistical software Minitab 16 was used for analysis.
▪ Effective sample size
We determined the required sample sizes using Z-tests in order to achieve a 80% power, while maintaining the type 1 error rate at 5%. In order to obtain the required sample sizes in each group, we first computed the difference in the means and the pooled standard deviation using our data, and then obtained effect sizes (mean/ standard deviation) for each comparison. Based on the effect size, we obtained the required sample size of 10 in each group to achieve a 80% power with a prespecified type 1 error rate of 5%. In our study, we collected samples from 12 preterm infants with High baseline level Group, and 38 preterm infants with Low baseline level group respectively, which is higher than the required sample sizes in each group.
This study was approved by the institutional review board and the ethics committee of the Chang- Gung Memorial Hospital. Written informed consent was obtained from all participants before the commencement of the study.
▪ Demographic Data
Between June 2012 to May 2014, a total of fiftynine premature infants were initially enrolled into the study, but only 50 infants completed the study; two infants from High ADMA baseline level Group and one infant from Low ADMA baseline level Group had withdrawn from the study due to the occurrence of a grade 3 intraventricular hemorrhage during their stay in the hospital; one premature infant from Low ADMA baseline level Group and High ADMA baseline level Group respectively succumed to death due to sepsis during in-hospital period; two infants from Low ADMA baseline level Group and one infant from High ADMA baseline level Group were withdrawn from the study due to the distance of their house to the hospital and therefore could not make it back for the developmental survey; and one infant from Low ADMA baseline level Group had lost follow up after discharged from the hospital; thus, 50 out of 59 infants had completed the study.
Results
The enrolled infants’ characteristics are presented in Table 1. The patients’ characteristics were of no differences between the Low vs. High baseline level ADMA groups. Among these 50 premature infants, there were 21 female and 29 male infants with gestational age that ranged from 24 weeks to 34 weeks and birth body weight that ranged from 565grams to 1500 grams. There were 38 premture infants in Low ADMA baseline level Group and twelve in the High ADMA baseline level Group.
| Factors | L Group (n=38) | H Group (n=12) | p value |
|---|---|---|---|
| GA (weeks) | 29.5 ± 0.40 | 28.4 ± 0.50 | 0.30 |
| BBW (gram) | 1181.60 ± 32.30 | 1073.20 ± 52.30 | 0.94 |
| Apgar-1 | 5.3 ± 0.30 | 5.7 ± 0.40 | 0.90 |
| Apgar-5 | 7.5 ± 0.20 | 7.7 ± 0.30 | 0.73 |
| Antenatal steroid (n) | 22 | 5 | 0.35 |
| chorioamnionitis | 0 | 1 | - |
| PPROM | 1 | 2 | 0.20 |
| Pre-eclampsia | 3 | 1 | 0.71 |
| Diabetes mellitus | 2 | 1 | 0.68 |
| Smoking | 0 | 0 | - |
| hypertension | 0 | 0 | - |
| Gender (f/m) | 16/21 | 6/6 | 0.68 |
| CRP | 2.4 ± 0.30 | 3.1 ± 0.10 | 0.71 |
| Serum Cr. | 0.46 | 0.58 | 0.63 |
Table 1. Prenatal and Intrapartum factors of Low and High Baseline level ADMA Groups.
▪ Plasma ADMA, SDMA, Arginine levels
Plasma concentrations of ADMA, SDMA, arginine, and the arginine:ADMA ratio (AAR) are shown in Table 2. The plasma arginine concentration revealed no significant difference between both premature infants with Low or High ADMA baseline level Groups, p=0.1. Plasma SDMA was not different between the two groups. The AAR was significantly lowered (P=0.042) in premature infants with High baseline level ADMA.
| L Group | H group | |||||||
|---|---|---|---|---|---|---|---|---|
| mean | median | range | mean | median | range | P value | ||
| Arginine (umol/l) | 77.84+/-3.31 | 73.90 | 55.47-97.00 | 80.77+/-5.11 | 73.79 | 66.97-98.52 | 0.1 | |
| ADMA (umol/l) | 0.65+/-0.02 | 0.68 | 0.37-0.89 | 1.03+/-0.05 | 0.94 | 0.89-1.35 | <0.001 | |
| SDMA (umol/l) | 0.76+/-0.02 | 0.75 | 0.40-1.02 | 0.83+/-0.05 | 0.79 | 0.71-1.02 | 0.235 | |
| Arginine: ADMA | 115.06+/-5.41 | 119.99 | 62.87-152.98 | 86.65+/-3.93 | 90.77 | 77.93-93.82 | 0.042 | |
Table 2: Plasma concentration of arginine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and the arginine:ADMA ratio in premature infants with Low and High baseline level ADMA.
▪ Developmental Outcomes
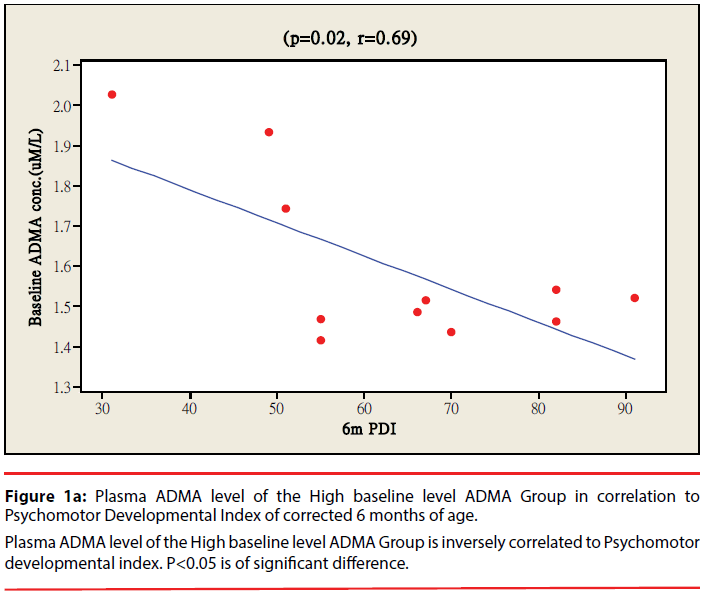
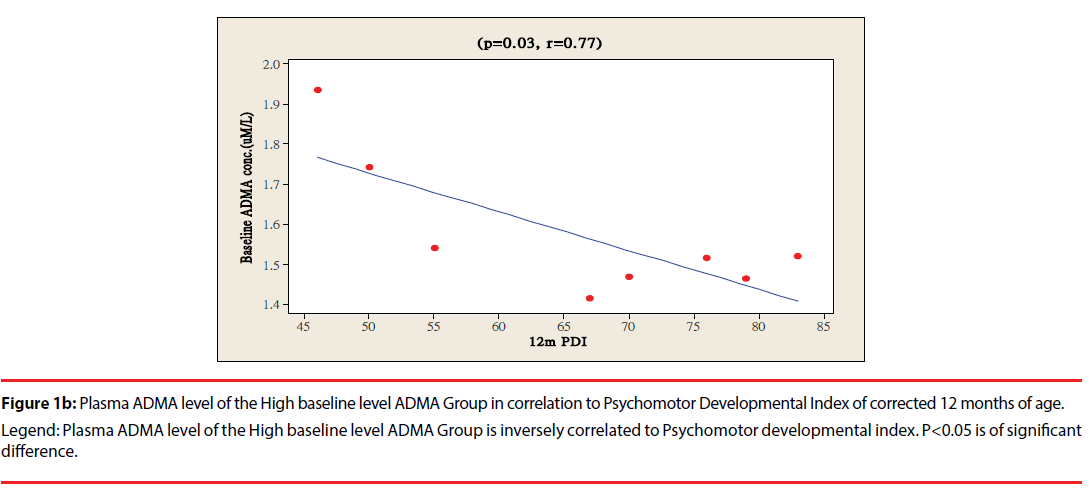
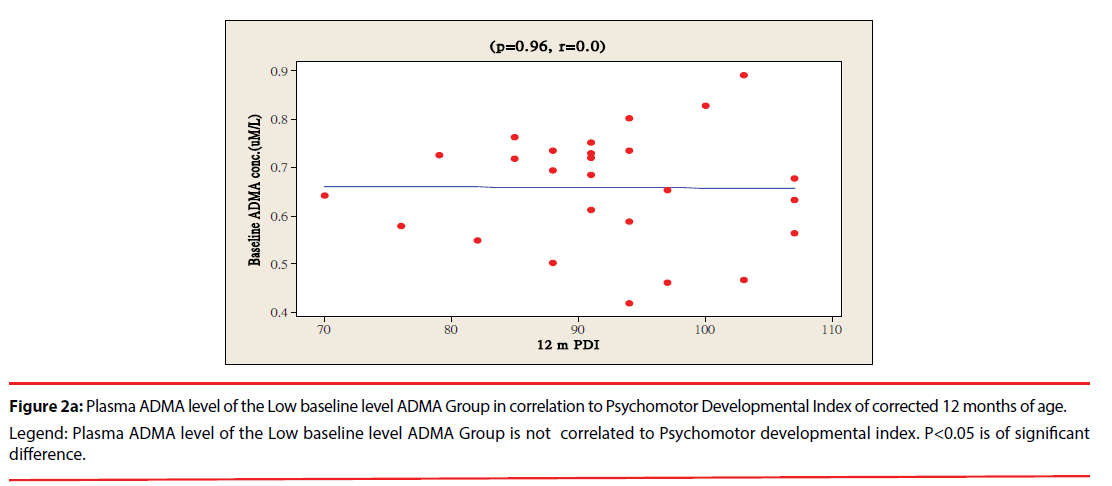
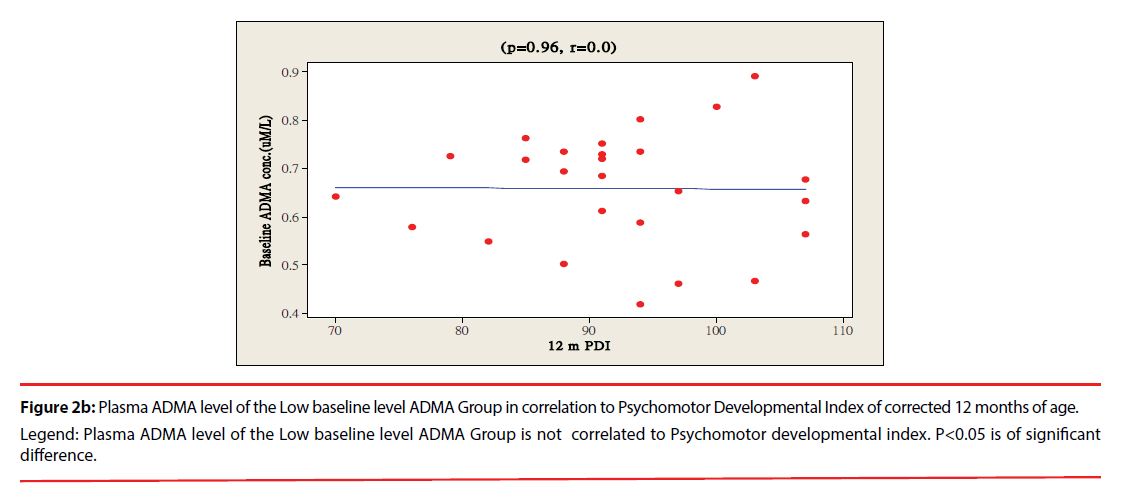
The ratio of premature infants with delayed mental development between high ADMA and low ADMA baseline level were of no significant differences at 6 and 12 months corrected age. Premature infants with High ADMA baseline level had a significantly higher ratio of delayed psychomotor development at corrected age of 6 and 12 months old as compared to premature infants with low ADMA baseline level, P=0.041 and 0.012 respectively (Table 3). Furthermore, the plasma ADMA levels of premature infants of the High baseline level group was significantly inversely correlated to the psychomotor developmental index at both 6 and 12 months corrected age, (p=0.020 and p=0.027 respectively, Figure 1a & 1b), however no correlations were observed between plasma ADMA levels and the psychomotor developmental index in the Low baseline level group, p=0.274 and p=0.964, Figure 2a & 2b.
| Outcome | L Group, n=38 | H Group, n=12 | P value | |
|---|---|---|---|---|
| Bayley Score | ||||
| 6 M MDI: delayed dev. (%) |
18 | 33 | 0.28 | |
| 6 M PDI: delayed dev. (%) |
44 | 75 | 0.04 | |
| 12 M MDI: delayed dev. (%) |
5 | 25 | 0.49 | |
| 12 M PDI: delayed dev. (%) |
21 | 58 | 0.01 | |
| Disability: | ||||
| Cerebral palsy (%) | 0 | 2 | - | |
| Blindness (%) | 0 | 0 | - | |
| Hearing loss (%) | 0 | 0 | - | |
Table 3: Premature infants with Low baseline level ADMA vs. High baseline level ADMA in neurodevelopmental outcomes.
Figure 1b: Plasma ADMA level of the Low baseline level ADMA Group in correlation to Psychomotor Developmental Index of corrected 6 months of age.
Legend: Plasma ADMA level of the High baseline level ADMA Group is not correlated to Psychomotor developmental index. P<0.05 is of significant
difference.
Figure 2a: Plasma ADMA level of the Low baseline level ADMA Group in correlation to Psychomotor Developmental Index of corrected 12 months of age.
Legend: Plasma ADMA level of the Low baseline level ADMA Group is not correlated to Psychomotor developmental index. P<0.05 is of significant
difference.
Figure 2b: Plasma ADMA level of the Low baseline level ADMA Group in correlation to Psychomotor Developmental Index of corrected 12 months of age.
Legend: Plasma ADMA level of the Low baseline level ADMA Group is not correlated to Psychomotor developmental index. P<0.05 is of significant
difference.
Overall, premature infants with High baseline level ADMA had significantly higher ratio of delayed psychomotor development throughout a year of BSID II evaluation as compared to the infants with Low baseline level ADMA group. There are no significant difference in the occurrence of retinopathy, chronic lung disease, patent ductus arteriosus, and necrotizing enterocolitis between the 2 study groups (Table 4).
| Parameters | L Group, n=38 | H Group, n=12 | p value |
|---|---|---|---|
| PVE > 2 wks (%) | 21 | 58 | 0.01 |
| ROP (%) | 40 | 48 | 0.78 |
| CLD (%) | 35 | 44 | 0.99 |
| PDA (%) | 50 | 42 | 0.22 |
| NEC (%) | 10 | 9 | 0.88 |
Table 4: Parameters Related to Development in Premature Infants with Low vs. High Baseline Level ADMA.
Discussion
The primary finding of the present study was that elevated plasma baseline levels of ADMA is a powerful and independent predictor of adverse psychomotor development. To our knowledge, this is the first study which demonstrated that in VLBW premature neonates, if present with a higher baseline plasma level of ADMA (≥0.895 mol/l), would have a significantly poorer psychomotor development as compared to premature infants with lowered baseline plasma level of ADMA (≦0.895 mol/l) after adjustment for traditional neurodevelopmental influencing factors.
There are many clinical studies correlating elevated circulating levels of ADMA concentration in patients with cardiovascular risk factors and endothelial dysfunction [17-19]. High levels of baseline ADMA have been shown to be independently predictive of adverse outcomes in a variety of patient populations, such as those with peripheral vascular disease [20], acute coronary syndromes [21,22], and those with established coronary artery disease [23]. Our study demonstrated that premature infants with high baseline level of ADMA tended to have a delayed psychomotor development at one year of corrected age possibly due to higher association with PVE >2 wks. The significant higher association between PVE persisting greater than 2 weeks and higher serum ADMA level might be related to the greater contribution of increasing systemic vascular resistance [24] and imparing endothelial function in the brian; as demonstrated by Kielstein et al. [25] that ADMA increased vascular stiffness and decreased total cerebral perfusion.
Furthermore, like NO, ADMA is also a key regulator of vessels dilatation and resistance, and it is also an endogenous inhibitor of NOS which induces endothelial dysfunction by reversibly inhibiting NO production from L-arginine. The L-arginine to AAR is a key determinant of NOS activity and is a useful index for evaluating the effect of ADMA [26,27]. The decrease in AAR might be due to a decrease in L-arginine level or an increase in ADMA level. The significant elevation of ADMA could be a result of enhanced ADMA formation from high protein breakdown or reduced ADMA elimination. As in fetal hypoxia, the blood flow to the periphery (kidney, spleen, skin, and muscle, liver) is decreased, shifting the circulation towards vital organs [28]. Secondarily, dimethylarginine dimethylaminohydrolase (DDAH) metabolize ADMA and is excreted directly into the urine [29]. Decreased blood flow to the kidneys during hypoxia might have contributed to the impaired renal elimination of ADMA and thus resulted in higher plasma ADMA. In our study, the AAR was significantly lowered in high baseline level ADMA group; since the L-arginine level was of no significant difference between both Low and High baseline level ADMA groups, it is prudent to think that decreased AAR noted in preterm infants with high baseline level of ADMA might be a consequence of poor control of the fetoplacental circulation and the neonate’s postnatal circulatory adaptations as previously pointed out by Tsukahara et al. [15] and Vida et al. [16].
Conclusion
In conclusion, although the mechanism by which serum ADMA affects the psychomotor development of premature infants remains unclear, the present study demonstrated that elevated baseline levels of ADMA is a strong and independent predictor of delayed psychomotor development in VLBW premature infants. Further studies are needed to investigate the possible mechanism on how high baseline levels of serum ADMA influence psychomtoor development in premature infants.
Acknowledgments
This study was supported by a research grant (CMRPG8B0161&CMRPG8B0162) from the Chang-Gung Memorial Hospital to Dr. Chih- Cheng Chen. The authors have no conflicts of interest relevant to this article.
References
- Rosenfeld CR. Mechanisms regulating angiotensin II responsiveness by the uteroplacental circulation. Am. J. Physiol 281(4), 1025-1040 (2001).
- Richir MC, Siroen MP, van Elburg RM, et al. Low plasma concentrations of arginine and asymmetric dimethylarginine in premature infants with necrotizing enterocolitis. Br. J. Nutr 97(5), 906-911 (2007).
- Ergun O, Ergun G, Oktem G, et al. Enteral resveratrol supplementation attenuates intestinal epithelial inducible nitric oxide synthase activity and mucosal damage in experimental necrotizing enterocolitis. J. Pediatr. Surg 42(10), 1687-1694 (2007).
- Maxwell AJ. Mechanisms of dysfunction of the nitric oxide pathway in vascular diseases. Nitric. Oxide 6(2), 101-124 (2002).
- Davis JM. Role of oxidant injury in the pathogenesis of neonatal lung disease. Acta. Paediatr. Suppl 91(437), 23-25 (2002).
- Shi Y, Pan F, Li H, et al. Role of carbon monoxide and nitric oxide in newborn infants with postasphyxial hypoxic-ischemic encephalopathy. Pediatrics 106(6), 1447-1451 (2000).
- Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler. Thromb. Vasc. Biol 20(9), 2032–2037 (2000).
- Vallance P, Leone A, Calver A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339(8793), 572-575 (1992).
- Suda O, Tsutsui M, Morishita T, et al. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin–angiotensin system and oxidative stress. Arterioscler. Thromb. Vasc. Biol 24(9), 1682-1688 (2004).
- Wanby P, Teerlink T, Brudin L, et al. Asymmetric dimethylarginine (ADMA) as a risk marker for stroke and TIA in a Swedish population. Atherosclerosis 185(2), 271-277 (2006).
- Yoo JH, Lee SC. Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke. Atherosclerosis 158(2), 425-430 (2001).
- Khan U, Hassan A, Vallance P, et al. Asymmetric dimethylarginine in cerebral small vessel disease. Stroke 38(2), 411-413 (2007).
- Savvidou MD, Hingorani AD, Tsikas D, et al. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 361(9368), 1511-1517 (2003).
- Speer PD, Powers RW, Frank MP, et al. Elevated asymmetric dimethylarginine concentrations precede clinical preeclampsia, but not pregnancies with smallfor-gestational age infants. Am. J. Obstet. Gynecol 198(1), 112-117 (2008).
- Maeda T, Yoshimura T, Okamura H. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, in maternal and fetal circulation. J. Soc. Gynecol. Invest 10(1), 2-4 (2003).
- Vida G, Sulyok E, Ertl T, et al. Plasma asymmetric dimethylarginine concentration during the perinatal period. Neonatology 92(1), 8-13 (2007).
- Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98(18), 1842-1847 (1998).
- Lundman P, Eriksson MJ, Stuhlinger M, et al. Mild to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J. Am. Coll. Cardiol 38(1), 111-116 (2001).
- Surdacki A, Nowicki M, Sandmann J, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine inmenwith essential hypertension. J. Cardiovasc. Pharmacol 33(4), 652-628 (1999).
- Boger RH, Bode-Boger SM, Thiele W, et al. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 95(8), 2068-2074 (1997).
- Krempl TK, Maas R, Sydow K, et al. Elevation of asymmetric dimethylarginine in patients with unstable angina and recurrent cardiovascular events. Eur. Heart. J 26(18), 1846-1851 (2005).
- Cavusoglu E, Ruwende C, Chopra V, et al. Relationship of baseline plasma ADMA levels to cardiovascular outcomes at 2 years in menwith acute coronary syndrome referred for coronary angiography. Coron. Artery. Dis 20(2), 112-117 (2009).
- Schnabel R, Blankenberg S, Lubos E, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ. Res 97(5), e53-e59 (2005).
- Kielstein JT, Impraim B, Simmel S, et al. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation 109(2), 172-177 (2004).
- Kielstein JT, Donnerstag F, Gasper S, et al. ADMA increases arterial stiffness and decreases cerebral blood fl ow in humans. Stroke 37(8), 2024-2029 (2006).
- Bode-Boger SM, Boger RH, Kienke S, et al. Elevated L-arginine/dimethylarginine ratio contributes to enhanced systemic NO productionmby dietary L-arginine in hypercholesterolemic rabbits. Biochem. Biophys. Res. Commun 219(2), 598-603 (1996).
- Kielstein JT, Zoccali C. Asymmetric dimethylarginine: a cardiovascular risk factor and a uremic toxin coming of age? Am. J. Kidney. Dis 46(2), 186-202 (2005).
- Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp. Biochem. Physiol. A Mol. Integr. Physiol 119(3), 717-723 (1998).
- Teerlink T. ADMA metabolism and clearance. Vasc. Med 10(1), S73-S81 (2005).