Research Article - Diabetes Management (2018) Volume 8, Issue 5
High rate of asymptomatic hypoglycemia in insulin-treated diabetes with severe chronic kidney disease: Utility of flash interstitial glucose monitoring
- *Corresponding Author:
- P Jean Ho
Department of Endocrinology
Diabetes Centre, Royal Prince Alfred Hospital
E-mail: Pamela.ho@health.nsw.gov.au
Abstract
Objective: Insulin-treated diabetes patients with severe chronic kidney disease (CKD) commonly have reduced hypoglycemia awareness that can potentiate hypoglycemia risk. We assessed in such patients: whether flash interstitial glucose monitoring (iGM) measures correlate with capillary blood glucose (cBGL) levels, the prevalence of asymptomatic hypoglycemia, and if iGM usage may help decrease hypoglycemia occurrence.
Methods: Thirteen adult insulin-treated, CKD stage 4 or 5 diabetes patients with reduced hypoglycemia awareness, from a tertiary hospital diabetes renal clinic, participated in a familiarization program with Freestyle Libre Flash Glucose Monitoring for two 14-d periods (P), and insulin treatment was adjusted as indicated between periods.
Results: Initial HbA1c was 7.2 ± 1.4%. The iGM correlated with cBGL (n=480, r=0.86, p<0.0001). By Clarke error grid analysis: 93.3% of data points were in combined Zones A and B, and 4 data points in Zone E. All hypoglycemia (iGM or cBGL < 3.9 mM) was asymptomatic. More hypoglycemic events were detected by iGM than by cBGL (7.9 ± 7.8 events/14 d vs. 0.4 ± 0.8, iGM vs cBGL, p<0.01 in P1, 5.4 ± 3.6 events/14 d vs. 0.3 ± 0.6, iGM vs cBGL, p<0.001 in P2). Hypoglycemia time in P1, 6.2 ± 6.2% (0%-20.0%), and number of events, did not change in P2. Ten patients recorded hypoglycemia in P1: their time in hypoglycemia decreased from 8.0% ± 5.9% to 3.2% ± 2.8% (p=0.01) in P2.
Conclusions: In this patient group, iGM detected high rates of hypoglycemia; its use may help to decrease hypoglycemia and improve patient safety.
Keywords
hypoglycemia, interstitial glucose monitoring, flash monitoring, blood glucose selfmonitoring, insulin-treated diabetes mellitus, chronic kidney disease, patient safety
Introduction
Material in this paper was presented as an abstract of the same title in the proceedings of the Australian Diabetes Society Annual Scientific Meeting, Perth 30 Aug - 1 Sep 2017. Patients with diabetes and severe renal impairment are prone to hypoglycemia, and are at increased risk of serious consequences of hypoglycemia, including acute myocardial infarction, cardiac arrhythmias, cognitive impairment, accidents and injury, and sudden death [1-3]. Multiple factors contribute to the increased risk of hypoglycemia in such patients and many are not modifiable, including severe renal impairment, decreased insulin clearance, impaired gluconeogenesis, and reduced appetite and food intake [4]. Insulin treatment, which is required in many of these patients for glycemic control, further exacerbates the risk of hypoglycemia. Additionally, in this patient group, poor hypoglycemia awareness, related to autonomic neuropathy and reduced counter regulatory hormone responses, is common, and results in hypoglycemia that is more likely to be unrecognized [5]. Thus, it is not surprising that the actual prevalence of occult hypoglycemia in insulin-treated patients with severe renal impairment is unknown. Yet the increased risk of hypoglycemia is one of the major barriers to achieving glycemic targets in this patient group. In addition to hidden hypoglycemia in the context of severe renal impairment, is the issue of an accurate measure of overall glycemic control. HbA1c is a poor index of glycemic control when red cell life cycle dynamics are altered by chronic kidney disease, associated anemia, and erythropoietic agent treatment [6]. Serum fructosamine may be useful in monitoring short-term glycemic control; however, its measurement is affected by hypoalbuminemia that is not uncommon in diabetes with severe CKD [7]. Furthermore, there is currently no standardization of the fructosamine assay or clear translation to average glycemia [8,9]. A simple and effective means to monitor glycemia, and to quantify and reduce the occurrence of occult hypoglycemia in these patients is therefore needed. The Freestyle Libre Flash Glucose Monitoring System (Abbott Diabetes Care, Doncaster, Australia) is a form of iGM that enables a patient’s interstitial glucose level to be measured every 15 minutes without finger pricking, over 14 days. The flash iGM may be useful in the management of patients with diabetes and reduced awareness of hypoglycemia to help identify hypoglycemic episodes, particularly asymptomatic episodes, and across those time periods to aid in recognition of possible precipitating factors. Given that background, we used the commercially available Freestyle Libre Flash Glucose Monitoring System in our ambulatory care of patients with long-standing, insulin-treated diabetes who had CKD stage 4 or 5 and decreased hypoglycemia awareness. This audit of the outcomes aimed to i) address whether cBGL correlates with flash iGM levels using the Freestyle Libre Flash Glucose Monitoring System; ii) describe the frequency of asymptomatic hypoglycemia in this cohort as detected by iGM; and iii) explore whether the incidence of hypoglycemia, especially asymptomatic hypoglycemia may be decreased by the use of short-term iGM to aid in the adjustment of glucose-lowering treatment in this cohort.
▪Methods
The commercially available Freestyle Libre Flash Glucose Monitoring System was used in a patient familiarization program for patients attending a diabetes renal clinic at a public tertiary referral hospital. Valid verbal consent was obtained from all participating patients as for all usual clinical management. The clinical treating team were familiar in iGM application but had not yet applied it systematically to patients in this dedicated clinic.
▪ Participants
Participants were from an established outpatient clinic for patients with diabetes and severe CKD. Thirteen adult patients (8F, 5M, aged 72 ± 10 y), (mean ± SD) who had type 1 (n=1) or 2 (n=12) diabetes of 24 ± 7 y (range 9-33 y) duration, participated. All had been receiving insulin at least twice daily for at least 1 month, and 1 patient was also receiving oral sulphonylurea therapy as gliclazide. Notably 92% of patients had a history of cardiovascular disease and 54% had a history of severe hypoglycemia (hypoglycemia requiring assistance) in the 6 months prior to the program. The most common insulin regimen was once or twice daily basal insulin with separate rapid acting insulin (n=10), although some participants (n=3) were receiving premixed insulin as at least part of their regimen. As is the practice in this clinic, all had received routine advice about prevention, detection and treatment of hypoglycemia.Eight patients had CKD stage 4 (eGFR 15-29 ml/min) and 5 patients had CKD stage 5 (eGFR < 15 or were receiving hemo- or peritoneal dialysis). Four patients were receiving hemodialysis while 9 had an eGFR 21 ± 4 mL/min/1.73 m2 (range 13-25). All patients were administered the hypoglycemia awareness questionnaire per Clarke [10] to determine their hypoglycemia awareness status. Based on that questionnaire, where normal awareness is defined as a score of 2 or lower, 11 participants had reduced awareness (score of 4 to 7 out of a maximum possible of 7), while 2 had borderline reduced awareness (score of 3). Thus, all participants had subnormal hypoglycemia awareness (score 4.5 ± 1.2; mean ± SD; range 3-7). FreeStyle Libre Flash iGM was performed as per the manufacturer’s instructions for two periods (P1 and P2) of 14 d each, consecutively where possible. The sensor was applied to the upper arm. Patients were asked to scan the sensor with the reader at least every 8 h as required by the manufacturer, and to check cBGL as per their usual routine. Patients were reviewed at the end of each of the 2 periods of monitoring and when technical problems arose. Insulin and/or gliclazide therapy was adjusted between periods using iGM results. Statistical analysis was performed on Excel, using paired t-testing, NCSS 2007 and PASS 12. Statistical significance was set at p<0.05. Data are shown as mean ± SD. Hypoglycemia range glucose refers to readings <3.9 mM, based on published clinical trials of iGM [11,12]. These studies used 2300 to 0600 h for detection of nocturnal hypoglycemia; after inquiring into our participants’ bedtimes we selected 2200 to 0600 h as a more clinically relevant time interval.
Results
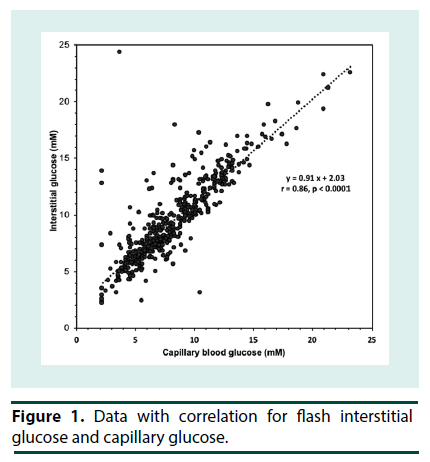
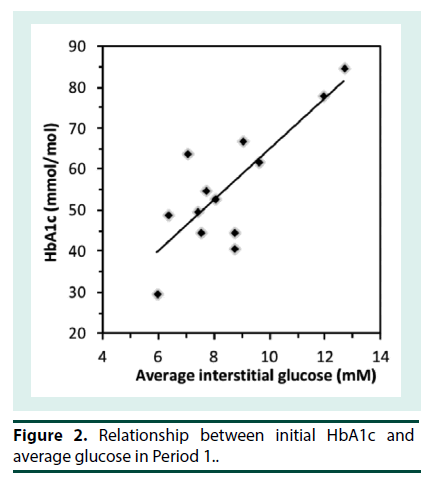
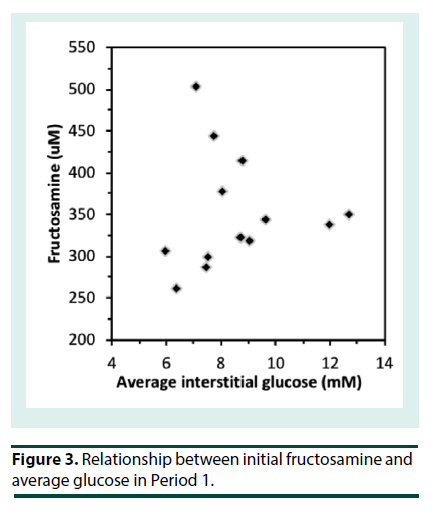
At the start of iGM, the average HbA1c was 7.2 ± 1.4% (range 4.9-9.9%) (56 ± 15 mmol/mol; range 30-85) and serum fructosamine was 351 ± 68 μM (range 260-503 μM; ref: 200-290). Patients routinely reported that the iGM was straightforward to use. Even though all sensors were applied at the clinic, there were 14 instances of complete sensor dislodgement. Six of them had been worn for 1-3 d and were replaced. Seven sensors were dislodged after 4-12 days and were not replaced and one sensor that was dislodged at 6 d was replaced. In addition, two sensors failed without dislodgement and were replaced. Only 3 patients had no dislodgements or sensor malfunction. The sensor maximally stores 8 h of iGM data; when the sensor is scanned, the previous 8 h of data is transferred to the scanner. The frequency of scanning was very variable among the patients. Where patients scanned less often than every 8 h, substantial iGM data was not recorded. Consequently, the number of iGM measurements obtained per patient over each 14-d period varied widely (990 ± 372, range 171 to 1359), out of a theoretical maximum of 1345 measurements per 14 d, and was similar in P1 and P2. The majority of undislodged sensors ceased sensing after 13 to 14 d. Due to sensor dislodgement or failure, P1 and P2 were consecutive in only 5 patients, and were separated by 3 to 15 d in the others. Thus, overall the average data capture efficiency was 990/1345 or 74%. The number of cBGL measurements performed by each patient over the 4 weeks (39 ± 32; range 9-106) varied widely, from approximately twice per week to nearly 4 times per day. Seven patients tested less than once daily, 3 patients tested once to twice daily while only 3 patients tested more than twice per day. This frequency of cBGL measurement reflects the clinical variation that pre-existed the use of the iGM in patients in this clinic. This patient cohort with highly morbid type 2 diabetes, did not adjust their own insulin therapy, but relied upon experienced clinicians in the clinic consultation reviews to advise between P1 and P2 in the adjustment of insulin therapy. The correlation between cBGL and iGM was determined by utilizing measures taken as proximate in time as possible, in all cases within 10 minutes of each other. Most of the cBGL measures were undertaken prior to main meals. Using this methodology, there was a statistically significant correlation between iGM and cBGL (n=480 data points, r=0.86, p<0.0001) (Figure 1). By Clarke error grid analysis [13], the 480 pairs of glucose measurements, using iGM compared with cBGL, is shown in Table 1. The combined zones A and B percentage was 93.3%, demonstrating that 93.3% of iGM measurements were acceptable compared with cBGL. In Zone E were 4 (0.8%) measurements, where iGM indicated hypoglycemia at 2.2- 2.7 mM when cBGL indicated hyperglycemia (12.8-24.3 mM) or vice versa (iGM 10.5 mM, cBGL 3.1 mM). Inspection of the iGM profile before and after each Zone E cBGL time point showed that the cBGL measurements were obviously erroneous (data not shown).The initial HbA1c across the group, at 7.2 ± 1.4% (56 ± 15 mmol/mol) correlated with average iGM in P1 (r=0.81, p<0.001; Figure 2). In contrast the initial fructosamine, at 351 ± 68 μM (ref: 200-290 μM), did not correlate with average iGM levels in P1 (r=0.04, Figure 3). The initial HbA1c had a moderate correlation (r=- 0.53, p=0.04), while initial fructosamine was not significantly correlated (R=-0.21, p=0.26), each with percentage of time in hypoglycemia in P1, respectively. Backward regression analysis for the percentage time in hypoglycemia range and for the number of hypoglycemia events in P1 detected by iGM showed that patient age, gender, duration of diabetes, total daily insulin dose and baseline HbA1c did not predict either parameter of the occurrence of hypoglycemia. Glycemic parameters measured by iGM in P1 in comparison with P2 are shown in Table 2. In P1, average iGM (8.5 ± 2.0 mM) was on target but patients were at target glucose levels (5.0- 10.0 mM) for only approximately half the time. For all participants between P1 and P2, insulin dosage was adjusted as deemed appropriate by the same clinicians. Gliclazide (modified release) was ceased in the single patient who had been taking it during P1. By rationally utilizing the iGM data in P1, the average patient total daily insulin dose in P1 was reduced in P2 by 10% from 51.8 ± 33.3 (range 11-132) units daily in P1 to 46.5 ± 32.2 (range 2–127) units daily in P2 (p=0.0003). There was no statistically significant change in average iGM in P2 (8.4 ± 1.9 mM) compared with P1. For the entire cohort, the considerable percentage of time spent below target in each time period, into the hypoglycemic range, did not change significantly from P1 to P2 (16.3 ± 13.6% to 13.7 ± 8.5%, for P1 and P2 respectively). In this patient cohort, all hypoglycemic events (<3.9 mM) detected by iGM or cBGL throughout P1 and P2 were asymptomatic. As is often the case in people with diabetes receiving insulin therapy, the percentage of time spent in hypoglycemia by iGM was highly variable among the patients (0%-20.0%). More hypoglycemia events were detected by iGM than by cBGL in both P1 (7.9 ± 7.8 events vs. 0.4 ± 0.8 events, p<0.01) and P2 (5.4 ± 3.6 events vs. 0.3 ± 0.6 events, p<0.001). The average incidence of hypoglycemic events detected by iGM over 14 d in P1 was 7.9 ± 7.8 events and was not significantly changed in P2 (5.4 ± 3.6). There was a trend to a decrease in the average percentage of time in hypoglycemia from P1 (6.2 ± 6.2%; 0-20.0) to P2 (3.4 ± 2.7%; 0-7.3), which was not statistically significant (p=0.1). The percentages of time below, in, or above target, were also not significantly changed from P1 to P2 (Table 2). In post-hoc exploratory analysis, amongst the 10 patients who had experienced hypoglycemia by iGM in P1, the percentage of time in hypoglycemia decreased in P2 (8.0 ± 5.9% to 3.2 ± 2.8%, P1 vs. P2, p=0.01). Nine of these 10 patients recorded nocturnal hypoglycemia by iGM, and their percentage of nocturnal time in hypoglycemia decreased in P2 (13.0 ± 10.0% to 4.7 ± 5.8%, P1 vs. P2, p=0.02), and the percentage of hypoglycemic time that was nocturnal rather than occurring in the day time, also decreased (54.9 ± 14.8% to 32.0 ± 29.0%, P1 vs. P2, p=0.01). Post-hoc sample size analysis for hypoglycemia percentage time in range using a 2-sided 2-sample unequal-variance test showed that a sample size of n=23 would be required to achieve 80% power to reject the null hypothesis of equal means when the mean difference is 4.0 with SD of 5.9 for group 1 and 2.9 for group 2, with a significance level (alpha) of 0.05. No adverse effects including skin issues were encountered from wearing the iGM sensors, whether they were dislodged or worn continuously for 14 days.
| ERROR GRID ZONE | GLUCOSE MEASUREMENT PAIRS (% DISTRIBUTION) |
|---|---|
| A-Both less than 3.9 mM, or iGM/cBGL=0.8 to 1.2 | 72.7 |
| B-Both 4.0-4.9 mM, 5.0-10.0 mM, or >10.0 mM, or management would lead to benign consequence | 20.6 |
| C-Treatment might cause glucose to be <3.9 mM or >10.0 mM | 0.4 |
| D-cBGL outside, but iGM inside 5.0-10.0 mM | 5.4 |
| E-cBGL and iGM opposite; treatment would be opposite to what was correct | 0.8 |
iGM, interstitial glucose monitoring. SD, standard deviation
Table 1. Results of Clarke Error Grid analysis10 of interstitial glucose monitoring (iGM) measurements against capillary blood glucose (cBGL) measurements.
| MEAN ± SD (RANGE) | PERIOD 1 | PERIOD 2 | P VALUE ON T-TEST |
|---|---|---|---|
| Average interstitial glucose (mM) | 8.5 ± 2.0 (5.9-12.6) |
8.4 ± 1.9 (5.9-13.1) |
0.8 |
| % time below 3.9 mM |
6.2 ± 6.2 (0-20.0) |
3.4 ± 2.7 (0-7.3) |
0.1 |
| % time below 5 mM |
16.3 ± 13.6 (0-41.0) |
13.7 ± 8.5 (0.8-29.3) |
0.4 |
| % time at 5.0-10.0 mM (target) |
54.9 ± 11.8 (30.2-69.8) |
59.6 ± 13.2 (33.2-72.6) |
0.2 |
| % time above 10.0 mM |
28.7 ± 19.2 (7.3-69.8) |
26.7 ± 19.0 (1.0-66.1) |
0.6 |
| % time 2200 h to 0600 h below 3.9 mM | 9.8 ± 10.4 (0-28.9) |
4.6 ± 6.0 (0-18.2) |
0.1 |
| % time below 3.9 mM that is 2200 h to 0600 h | 41.2 ± 27.87 (0-78.7) |
30.6 ± 31.7 (0-90.0) |
0.3 |
| No. of events below 3.9 mM | 7.9 ± 7.8 (0-29.0) | 5.4 ± 3.6 (0-12.0) | 0.2 |
Table 2. Glycemic parameters by iGM in Period 1 and Period 2. Data are shown as mean ± SD (range).
Discussion
The Freestyle Libre Flash Glucose Monitoring System has been shown in randomized controlled trials to decrease the time spent in hypoglycemia in patients with type 1 [14] and type 2 diabetes on multiple times daily insulin regimens [11]. This flash iGM system is currently widely available and has gained approval for reimbursement by at least 19 national health services when used in adult patients with diabetes using insulin, including in the US and UK [15,16]. Due to its ability to assist in reducing both time in hypoglycemia and hyperglycemia, goals that are difficult to achieve by existing practices of diabetes education, self-monitoring of capillary blood glucose (SMBG) and HbA1c, and to its ease of use and affordability, compared with non-flash devices for continuous glucose monitoring, interest in this diabetes management tool is increasing. However, it ideally requires validation before it is used in particular patient groups, compared with established capillary BGL monitoring and HbA1c measures. Insulin-treated patients with severe CKD are prone to hypoglycemia and particularly asymptomatic hypoglycemia [3] and thus they may be expected to particularly benefit from flash iGM. However, there is a lack of published information on its utility in such patients, with to our knowledge no systematic reporting in such a cohort. Therefore, we studied a serial group of such patients in our familiarization program. Flash iGM levels correlated well with cBGL measurements, and 93% of iGM measurements were acceptable compared with cBGL. Thus, flash iGM is a feasible alternative to SMBG in patients with diabetes who have severe CKD. This agrees with studies of flash iGM in patients with type 1 and type 2 insulin-treated diabetes not selected for nephropathy, where 99% of paired iGM and cBGL measurements were observed in combined Zones A and B [17] or 86% were located in Zone A [18]. Furthermore, using iGM, we demonstrated a high incidence of asymptomatic hypoglycemia in our cohort, and a reduction in hypoglycemia time when iGM was used to guide decision making in glucose lowering treatment. Until recently, apart from continuous glucose monitoring which remains financially and technically out of reach for many patients, SMBG has been the only day-to-day glucose monitoring tool readily available to patients with diabetes treated using multiple insulin injections. However, the need to capillary finger-prick is often a significant barrier to SMBG. This difficulty in the real world is confirmed in our patient series receiving insulin therapy, half of whom did SMBG less than once daily, and in whom only a quarter tested more than twice a day, when they were asked to perform it in their normal routine, at least twice daily. In contrast, iGM can provide real-time glucose measurement on demand without capillary finger pricking, as well as record an ambulatory glucose profile (AGP) for later review. In the hands of our patients, a suboptimal overall 74% data capture for the AGP was achieved. With future iGM technology advances, hopefully the sensor interval can be increased to every 12 hours or longer, and this difficulty with patient adherence to scanning may then be overcome. Using iGM, this is the first series to document the incidence of asymptomatic hypoglycemia in a group of insulin-treated diabetes patients who have severe CKD and known poor hypoglycemic awareness. The asymptomatic hypoglycemia occurred approximately once every second day, or up to 20% of the time, and was uncommonly detected by the patient’s routine cBGL testing. Thus, our series strongly suggests that flash iGM is valuable in detecting occult hypoglycemia in both type 1 and type 2 insulin-treated patients with severe CKD. To date to our knowledge there have been no iGM studies examining series of patients with diabetes receiving insulin therapy who have reduced hypoglycemia awareness. The main randomized controlled trials using iGM excluded patients with clinical hypoglycemia awareness [11,14]. This patient series utilized the hypoglycemia awareness questionnaire of Clarke [10,13] which is a clinically useful assessment tool that predicts severe hypoglycemia risk. The long diabetes duration of the patients studied, all with late stage CKD predisposes them to having hypoglycemia unawareness and increased risk of severe hypoglycemia. Despite the patients being under regular specialist care, the high rate of undetected hypoglycemia in this cohort by iGM, likely reflects the severe patient phenotype in diabetes renal complication and diabetes duration. Hypoglycemia is known to increase the risk of cardiovascular events due to increased release of catecholamines and inflammatory cytokines, leading to an increase in myocardial workload, cardiac arrhythmia, increased blood coagulability and endothelial injury [19]. Recurrent hypoglycemia leads to deterioration in hypoglycemia awareness, further increasing the risk of hypoglycemia [5] and death [20]. It is imperative therefore in patients with reduced hypoglycemia awareness to detect, reduce and prevent hypoglycemia. Fokkert [18] showed that iGM measurements were lower than cBGL in the hypoglycemia range in a series of type 1 and type 2 diabetes patients, and suggested that patients check cBGL when a low iGM measurement is observed. In contrast, in the current series, iGM levels were generally higher than cBGLs as shown by the positive y-intercept (2.03 mM) of the graph of iGM against cBGL. Thus, our findings may be an underestimate of the incidence of hypoglycemia in these patients. Further studies with larger patient numbers may more accurately define the correlation between interstitial and capillary glucose in these patients. Even so, far more hypoglycemic events were detected by iGM than by SMBG. Thus, by assessing the AGP, flash iGM is far superior to SMBG in detecting asymptomatic hypoglycemia in insulin-treated patients with severe CKD. In the current series, the only patients who were excluded were those who were thought to lack the cognitive capacity to scan the sensor in addition to perform SMBG according to their usual schedule. Apart from this consideration, and the relatively small size of the patient sample, the participants could be representative of other insulin-treated patients with diabetes who have severe CKD in the ambulant clinic. The backward regression analysis suggests that it would be not be possible to predict the percentage time in hypoglycemia range or the number of hypoglycemia events from age, duration of diabetes, total insulin dose or baseline HbA1c in patients with decreased hypoglycemia awareness similar to those examined. As in the real world, a large majority of patients examined had type 2 diabetes and were not willing to undertake more frequent SMBG. They were found to have significant asymptomatic hypoglycemia duration and event numbers, and thus were susceptible to the dangers of experiencing occult hypoglycemia. Furthermore, their low adherence to SMBG may have implications for other areas of their self management such as diet and medication adherence. In nearly all cases, patients did not use iGM as an approach to adjust insulin therapy in real-time, but the iGM was utilized more as a profiling diagnostic tool across the monitoring period P1, with AGP data then being utilized after P1 by the clinical team with the patient present, to explore glycemic trends and then prescribe adjustments to insulin therapy. Thus, the AGP was a particularly useful tool in this patient group. This clinical approach is in contrast to the IMPACT [14] and REPLACE [11] studies in type 1 and type 2 insulin-treated diabetes respectively, where patient self-adjustment of insulin therapy in response to iGM real-time, between health care professional reviews, was the most common form of insulin adjustment observed. In our model, close patient follow-up is required for trouble-shooting, monitoring of glucose profiles and adjustment of glucose lowering treatment. Each patient requires good access to and continuity of care. In our series and routine clinic practice, this is provided by a physician and diabetes educator for these ambulatory patients. If resources were available, the use of a care manager would enable more such patients to benefit from the device. We also investigated whether short-term use of iGM could improve glycemic control in our patients, especially in reducing the occurrence of hypoglycemia. Indeed, in the whole cohort, there was no change in the number of hypoglycemic events, or percentage of time spent in hypoglycemia, in contrast to significant reductions in time in hypoglycemia in longer-term studies [11,12]. It is possible that if iGM was continued for a longer period, allowing time for more adjustments of glucose lowering treatment, hypoglycemia could be decreased in the group as a whole, as data appeared to be trending in that direction. In our short-term program, the influence of dietary changes on the occurrence of hypoglycemia in the two periods cannot be ruled out. However, the participants did not appear to have altered their diet from P1 to P2, from their recall and from routine food diaries in those who maintained them. In post-hoc analysis, among the ten patients who had any hypoglycemia detected in P1, the percentage of time spent in hypoglycemia in P2 decreased significantly. Further, 9 of 10 of these patients had nocturnal hypoglycemia detected and their percentage of nocturnal time in hypoglycemia, as well as their percentage of time in hypoglycemia that was nocturnal, also decreased. These observations reinforce the suggestion that in those with any detectable hypoglycemia at baseline, iGM could be useful in decreasing hypoglycemia and nocturnal hypoglycemia. It has been suggested that 12-15 days of monitoring every 3 months is suitable for assessment of overall glycemic control [21]. From the perspective of hypoglycemia occurrence, as access to flash iGM is limited, an initial 2-week iGM could be performed in this group of high-risk patients to screen for hypoglycemia. The patient could be reviewed after one week, so that glucose lowering therapy can be adjusted if required, and then be reviewed again at 2 weeks to assess the impact of any changes made after the first week. Additional iGM might then be performed only for those patients requiring further adjustment of glucose lowering treatment to aid avoidance of hypoglycemia, itself potentially detected by subsequent use of diagnostic iGM. The post-hoc sample size analysis indicates that if a definitive study were to be undertaken, then at least 23 subjects similar to those examined would need to enrol in a randomized controlled trial to determine if time in hypoglycemia range improves. Whilst HbA1c has been widely accepted as the gold standard measure of glycemic control in diabetes management, serum fructosamine is an indicator of shorter term glycemic control over 2-4 weeks [22]. Interestingly, in our small group of patients, HbA1c, but not fructosamine, was reasonably well correlated with the average iGM in P1. The reasons for this difference are not fully apparent but are likely to be multifactorial. HbA1c is influenced by a variety of factors, a number of which were likely to be present in our patients, such as CKD-related decrease in red cell lifespan, renal anemia, administration of erythropoietic agents, iron deficiency and large glucose excursions over shorts period of time [7,23]. Fructosamine is unaffected by hemoglobin-related factors, but could be influenced by conditions which affect serum proteins, especially albumin, the most prevalent serum protein [24]. Unlike HbA1c, there is no consensus about fructosamine assay standardization or its clinical utility. Glycated albumin, has been proposed as an alternative to fructosamine, [23] but its level is also affected by a number of serum protein related factors that are likely to affect fructosamine measurement. Within our ambulatory clinic, glycated albumin was not part of usual clinical care and was not performed in the familiarization program. We acknowledge a number of limitations in the current series of “free-living” patients, including the small number of patients, incomplete iGM data due to failure to scan the sensor or to sensor failure/dislodgement, the low and variable frequency of SMBG, and incomplete dietary monitoring. However, some of these limitations are to be expected in the real world, and as such, our findings may reflect the utility of iGM in such a setting. In addition, 74% of total potential data capture was realized in this patient cohort who have long diabetes duration and high attendant comorbidity.
Conclusions
Interstitial GM is valuable in detecting commonly occult hypoglycemia in insulin-treated patients with diabetes who have severe CKD, and we recommend its use in such patients who are ready to use the technology. Adjustment of glucose lowering treatment with the aid of iGM would be expected to decrease occult hypoglycemia and to improve patient safety. Patient access to glucose levels without finger pricking was a valuable asset of FreeStyle Libre Flash Glucose Monitoring System. However, the need to scan the sensor at least every 8 hours in order to store the data for later clinic review was a significant hurdle for this group of challenging patients. Technical improvements to decrease sensor dislodgement and failure would also be invaluable. This study advocates for some form of automated glucose monitoring such as iGM to be rendered financially affordable for diabetes patients at high risk of hypoglycemia, such as those with severe CKD who commonly have reduced hypoglycemic awareness.
Acknowledgements
The FreeStyle Libre Flash Glucose Monitoring System equipment (readers and sensors) was kindly provided by Abbot Diabetes Care Australasia Pty Ltd, Doncaster VIC.
Author Disclosure Statement
SMT and MJM are members of an Abbott national advisory board for the FreeStyle Libre Flash Glucose Monitoring System and MJM is in the Speakers Bureau of that board.
References
- Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabete. Metab. Res. Rev. 24(2), 87-92 (2008).
- Kong AP, Yang X, Luk A, et al. Hypoglycaemia, chronic kidney disease and death in type 2 diabetes: The Hong Kong diabetes registry. BMC. Endocr. Disord. 14, 48 (2014).
- Moen M, Zhan M, Hsu V, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 4(6), 1121–1127 (2009).
- Hettige T, Cooper M. Hypoglycaemia in patients with diabetes mellitus and renal impairment. Diab. Vasc. Dis. Res. 14(2), 166–168 (2017).
- Seaquist E, Anderson J, Childs B, et al. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes. Care. 36(5), 1384-1395 (2013).
- Vos F, Schollum J, Coulter C, et al. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton). 17(2), 182-8 (2012).
- Danese E, Montagnana M, Nouvenne A, et al. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 9(2), 169-76 (2015).
- American Diabetes Association. Glycemic Targets in Standards of Medical Care in Diabetes-2018. Diabetes. Care. 41(Suppl 1), S55–S64 (2018).
- Nansseu J, Fokom-Domgue J, Noubiap J, et al. Fructosamine measurement for diabetes mellitus diagnosis and monitoring: a systematic review and meta-analysis protocol. BMJ Open. 5(5), e007689 (2015).
- Clarke W, Cox D, Gonder-Frederick L, et al. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes. Care. 18(4), 517–522 (1995).
- Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: A multicenter, open-label randomized controlled trial. Diabetes. Ther. 8(1), 55–73 (2017).
- Haak T, Hanaire H, Ajjan R, et al. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes. Ther. 8(3), 573–586 (2017).
- Clarke W, Cox D, Gonder-Frederick L, et al. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes. Care. 10(5), 622–628 (1987).
- Bolinder J, Antuna R, Geelhoed-Duijvestijn, et al. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 388(10057), 2254–2263 (2016).
- Abbott. Abbott's Revolutionary Continuous Glucose Monitoring System, FreeStyle® Libre, http://abbott.mediaroom.com/2018-01-04-Abbotts-Revolutionary-Continuous-Glucose-Monitoring-System-FreeStyle-R-Libre-Now-Available-To-Medicare-Patients.
- Abbott. FreeStyle® Libre Flash Glucose Monitoring Technology Gains Reimbursement Approval from the United Kingdom's National Health Service. http://abbott.mediaroom.com/2017-09-13-FreeStyle-R-Libre-Flash-Glucose-Monitoring-Technology-Gains-Reimbursement-Approval-from-the-United-Kingdoms-National-Health-Service.
- Bailey T, Bode B, Christiansen M, et al. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes. Technol. Ther. 17(11), 787–794 (2015).
- Fokkert M, Van Dijk P, Edens M, et al. Performance of the freestyle libre flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ. Open. Diabetes. Res. Care. 5(1), e000320 (2017).
- Yakubovich N, Gerstein H. Serious cardiovascular outcomes in diabetes: The role of hypoglycemia. Circulation. 123(3), 342–348 (2011).
- Cryer P. Death during intensive glycemic therapy of diabetes: Mechanisms and implications. Am. J. Med. 124(11), 993–996 (2011).
- Xing D, Kollman C, Beck R, et al. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes. Technol. Ther. 13(3), 351–358 (2011).
- Malmstrom H, Walldius G, Grill V, et al. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies--cross-sectional and longitudinal experience from the AMORIS cohort. Plos. One. 9(10), e111463 (2014).
- Ribeiro R, Macedo M, Raposo J. HbA1c, fructosamine, and glycated albumin in the detection of dysglycaemic conditions. Curr. Diabetes. Rev. 12(1), 14–19 (2016).
- Nouya A, Nansseu J, Moor V, et al. Determinants of fructosamine levels in a multi-ethnic Sub-Saharan African population. Diabetes. Res. Clin. Pract. 107(1),123–129 (2015).