Case Report - Neuropsychiatry (2017) Volume 7, Issue 5
Hippocampal Sclerosis in LGI1 and CSPR2 Positive Limbic Encephalopathy: Case Report
- Corresponding Author:
- JinMei Li
Assistant Professor, Department of Neurology, West China Hospital, Sichuan University, 610041 Chengdu, P.R. China
Tel: +86-18628280713
Abstract
Background:
Limbic encephalopathy (LE) is a sub-acute neuropsychiatric disorder characterized by the involvement of limbic structures. It was described to be associated with voltage-gated potassium channel (VGKC)–complex antibodies, particularly, leucine-rich glioma inactivated 1 (LGI1), but less frequently contactin associated protein 2 (CASPR2).
Case report:
We present a female patient with sub-acute memory impairment, followed by psychiatric symptoms and cognitive impairment. Further investigation reveals positive (VGKC)–complex antibodies. Her initial cranial MRI was normal and responded very well to the immunotherapy. Subsequent one year follow-up showed seizure control, psychiatric symptoms remission and some memory improvement. Despite both LGI1 and CASPR2 being negative, her MRI revealed wide cortex atrophy including bilateral hippocampal sclerosis.
Conclusion:
The clinical phenotype of patient with both LGI1 and CASPR2 positive is diverse, so early diagnosis and treatment is crucial, as they may improve the clinical outcome; however, the development of wide cortex atrophy including hippocampal sclerosis soon after acute phase is rare, and may indicate a bad prognosis in long-term. Further follow-up is needed to clarify the prognosis.
Keywords
Limbic encephalopathy (LE), Voltage gated potassium channel, Leucine-rich glioma inactivated protein 1 antibodies, Contactin associated protein-like 2 antibodies, Hippocampal sclerosis
Introduction
Limbic encephalopathy (LE) is a sub-acute autoimmune neuropsychiatric syndrome characterized by the inflammation of the limbic structures; other associated brain structures can also be involved in the pathological process [1]. Although the essential sign of limbic encephalopathy is severe short-term memory impairment, many clinical features may also be present such as confusion, sleep disturbance, seizures and psychiatric symptoms. The clinical features usually develop over a few weeks or months, and early detection of LE is difficult but crucial, as it may improve the clinical outcome [2].
Antibodies against VGKC–complex are involved in the pathogenesis of peripheral disorders and diseases involving the central nervous systemmost commonly Morvan’s syndrome and limbic encephalitis [1]. The antibodies bind mainly to extracellular antigens such as LGI1 and CASPR2 or Contactin-2, all of which are components of VGKC complexes [3]. LGI1-Ab was reported to be associated with amnesia, confusion, seizures, other reported features include hyponatremia and a good prognosis, while CASPR2-Ab was associated with peripheral presentations, probable risk for developing tumor and a poor prognosis [4-6].
Here we describe the clinical features of a female patient presented with LE, which is one of few cases that were reported to have both CASPR2- Ab and LGI1-Ab positive, and the first case to be reported with long-term memory impairment at onset and the development of hippocampal sclerosis during the recovery period. Her response to immunotherapy was very good and had achieved significant improvement with the treatment using intravenous immunoglobulins (IVIG). Through the detailed analysis of her clinical course, we aim to bring your attention to the diverse and overlapping clinical phenotype of LGI1-Ab and CASPR2-Ab in patients with LE as well as the effect of MRI findings during the recovery period on the overall outcome.
Case Report
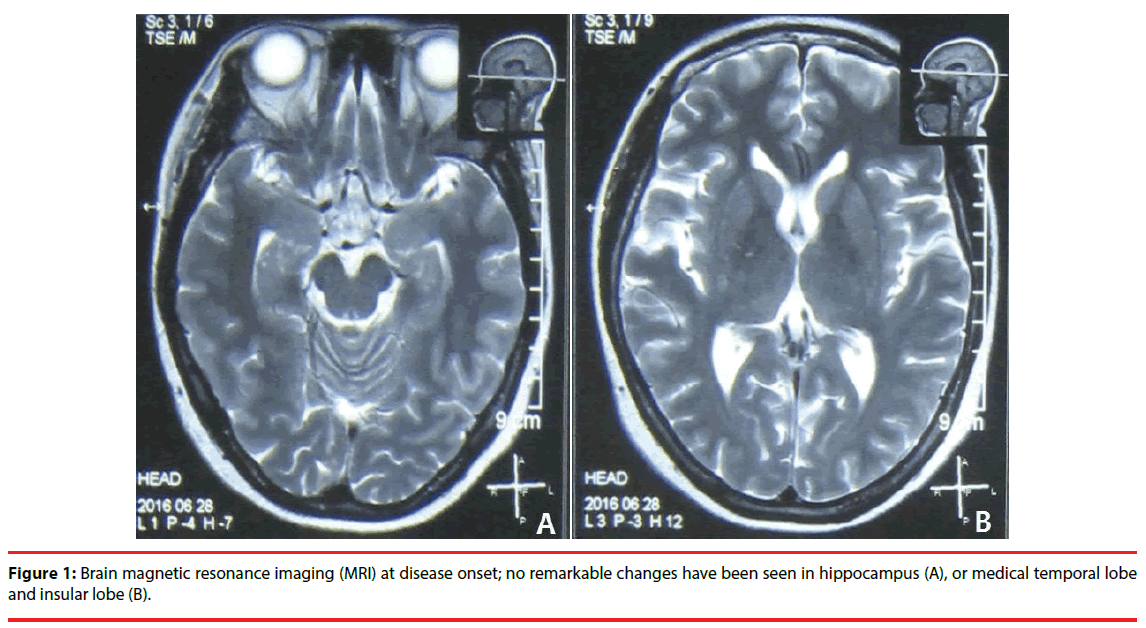
A 42 year-old right-handed Chinese woman was admitted to west china hospital with complains of memory impairment and abnormal episodic neuropsychiatric symptoms for two-months. At disease onset (2-months prior to the admission), she experienced abrupt deterioration of memory. Then she developed aura followed by generalized seizure. She also experienced hallucinations, confused thinking and abnormal psychiatric behavior associated with loss of consciousness. Brain magnetic resonance imaging (MRI) was normal (Figure 1), she was given antipsychotics (Seroquel 0.1 g bid) and anti-epileptic drug (Valproate 0.5 bid and Levtiracetam 0.75 bid); however, the patient didn’t improve and continued to have seizure, abnormal psychiatric behavior, but no hallucination, panic, persecutory delusion. She had no remarkable personal or family history except for hypertension so she was on antihypertensive drug (Levamlodipine).
Figure 1: Brain magnetic resonance imaging (MRI) at disease onset; no remarkable changes have been seen in hippocampus (A), or medical temporal lobe and insular lobe (B).
Neurological exam revealed conscious patient but with worsening in calculations, memory, comprehension and orientation. Her mini mental state examination (MMSE) score was 21/30, Montreal cognitive assessment scale (MoCA) could not be performed due to her severe psychiatric symptoms, interictal electroencephalography (EEG) didn’t show epileptic discharges or background slow waves (Figure 2).
Serum CASPR2-Ab was positive (1:32) and LGI1-Ab was strong positive (1:100), while cerebrospinal fluid (CSF) LGI1-Ab was weakly positive (1:10). Cerebrospinal fluid (CSF) analysis and culture were normal. Blood electrolytes were abnormal (Na: 132.8 mmol/L, K: 3.33 mmol/L, CL: 99 mmol/L). Onconeural antibodies were negative. Abdominal ultrasound and thorax CT, tumor markers, protein and immune electrophoresis were performed, all of these investigations mentioned previously were normal.
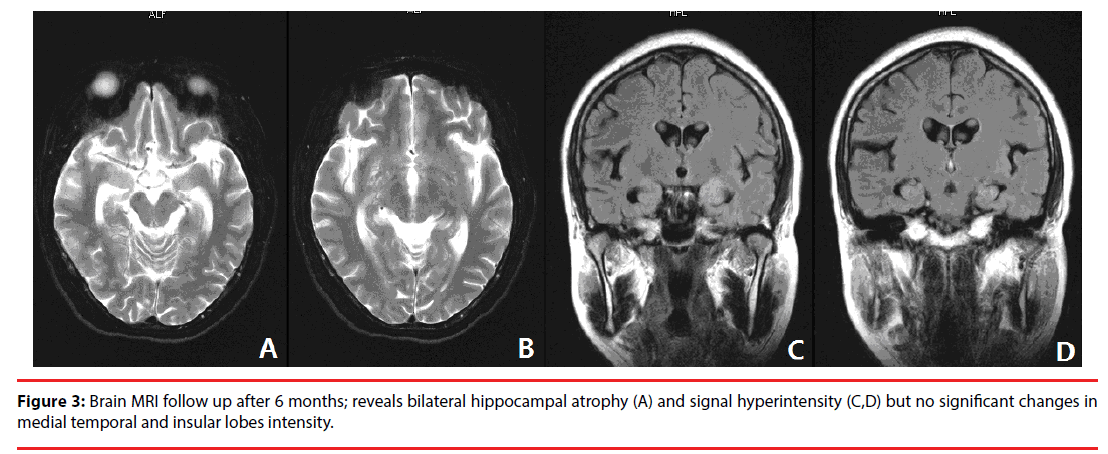
A diagnosis of VGKC- Ab associated LE was made. Treatment was initiated with intravenous immunoglobulin (IVIG) at a dose of 0.4 g/kg/ day for 5 consecutive days; Levtiracetam 0.5g bid was administrated to prevent epilepsy recurrence. During the course of hospitalization (2 weeks), the seizure was resolved, patient’s memory was slightly improved but neuropsychiatric symptoms and cognitive impairment were dramatically improved. Patient then was discharged with only Levtiracetam 0.5 bid and antihypertensive drug. The patient was followed up at the outpatient clinic, on a follow-up visit (at 4 months of discharge; 6 months after disease onset), except for memory impairment, her symptoms were improved significantly. Routine CSF, EEG, MRI, electrolytes, serum antibodies were performed, all the repeated tests were normal except for MRI, which reveals wide cortex atrophy including hippocampal sclerosis (Figure 3). On one year follow-up, MMSE, MoCA and Hamilton depression rating scale (HAMD) tests were performed to evaluate the patient’s condition, yielding scores of 25, 23 and 20 respectively. The tests show that the patient has severe short-term memory impairment; mild naming impairment, mild language and abstraction deficit as well as depression.
Figure 3: Brain MRI follow up after 6 months; reveals bilateral hippocampal atrophy (A) and signal hyperintensity (C,D) but no significant changes in medial temporal and insular lobes intensity.
Discussion
This disorder has traditionally been termed limbic encephalitis, but as more patients are diagnosed without routine CSF-based evidence of inflammation, or evidence of magnetic resonance imaging. The disorder may be better classified as a limbic encephalopathy (LE) [7]. LE is significantly more frequent associated to patients with LGI1 than in those with Caspr2 antibodies [3,8]. However, regarding our patient, both of LGI1 and Caspr2 antibodies were detected, with significantly high levels related to LGI1. It has been reported that LGI1-Ab associated with short-term memory and cognitive, seizure and hyponatremia, while Caspr2-Ab with peripheral nerve hyperexcitability (PNH), psychiatric features and insomnia [4-6]. Serum hyponatremia and High signs of hippocampus on MRI Flair scan are strongly supportive pointers towards the diagnosis; and many respond well to immunotherapy [9].
The encephalopathy associated with VGKC– complex antibodies shows a slight male preponderance and tends to affect those over 50 years of age (median 63 years) [9]. This patient is a young woman with significant memory and cognitive impairment, hallucination and seizure but still didn’t experience symptoms such as irregular small muscle contractions, painful cramps, itching, hyperhidrosis, dysautomomia and insomnia associated with Caspr2-Ab Positive. The reason for absence of neuromuscular (PNH) symptoms and for particular involvement of the limbic system in these patients is not clear. It is possible that VGKC antibodies in patients with neuromyotonia are specific for peripherally expressed special forms of VGKC, whereas patients with encephalitis have antibodies that are specific for special forms of VGKC that are more abundant in the CNS [10]. The majority of testing was unremarkable except for hyponatremia, which has been reported as an accompanying sign in LE cases with VGKC [7]. The association with underlying tumor has been recently reported to be as low as 20%-30% in cases with VGKC antibodies [3]. Tumor was not detected in our patient. Her presentation expanded the clinical phenotype of limbic encephalopathy which is believed to be more related to LGI1-Ab than Caspr2-Ab.
MRI changes in this patient was quiet interesting, her initial brain MRI did not show any definitive structural abnormalities to support an ongoing region-specific pathological process (Figure 1). In the past, one case with VGKC-LE reported that the initial MRI was unremarkable, 1 week later, abnormalities were detected [11]. This finding suggest that other cases reported with initially normal MRI scans may have shown typical temporal lobe signal changes later in the acute phase or during the recovery phase of the illness. More interestingly, during the recovery phase serious cortex atrophy especially hippocampus sclerosis was observed. The unexpected MRI change may have a far-reaching influence on prognosis and quality of life.
Since limbic encephalopathy is a rare condition with no randomized-controlled trials to guide treatment, the best treatment is still unclear. a retrospective 4-year outcome follow-up suggested no difference between corticosteroids alone, corticosteroids with intravenous immunoglobulins (IVIG), or corticosteroids with IVIG and plasma exchange [12]. Another prospective study, reported that six of the seven patients were treated with IV methylprednisolone 1 g daily for 5 consecutive days, Symptoms were promptly and dramatically improved in the three who were treated within 2 months of symptom onset and minor in the three who were treated 9 months after symptom onset. Seizures resolved or became more responsive to treatment in all patients [10]. Our patient was treated with IVIG for 5 consecutive days, she showed quiet significant improvement in her condition after immunotherapy therapy, which is consistent with literature data [13]. On follow-up she continued to have gradual improvement but didn’t have a complete remission.
In a prospective study, hippocampal sclerosis were identified in 16 of 33 patients (48.5%) at follow-up imaging, ranging from 2 to 36 months but no patients were positive for both LGI1 and Caspr2 antibodies [14]. Interestingly, MRI was performed again (6 month after disease onset) and showed bilateral hippocampal atrophy with hyperintensity despite negative CSF and serum VGKC antibodies, which make this case the first case to be reported so far. It’s known that the hippocampus plays an essential role in all aspects of conscious, declarative memory, i.e. semantic memory for facts and concepts, episodic memory and spatial memory [14]. Interestingly, in a new study patients with temporal lobe epilepsy due to hippocampal sclerosis showed that besides episodic memory impairment, these patents had experienced significant memory deficits related to daily life activities [15]. Therefore, the followup MRI finding (hippocampal sclerosis) in our patient may explain the reason behind her slowly gradual improvement of memory impairment and may reflect on the overall outcome; MoCA and HAMD tests’ results increase the likelihood of suspected bad prognosis associated with developing hippocampal sclerosis in later stage. So further follow-up is required to clarify the prognosis [16].
Conclusion
Limbic encephalopathy associated with VGKC (LGI1 and Caspr2) Abs is potentially treatable condition. But, due to its unusual and diverse clinical features, it’s usually under-diagnosed, and hence under-treated.
Autoimmune antibodies should be screened in all patients with acute or sub-acute memory impairment regardless of the accompanying symptoms, since the early detection of LE followed by early treatment often lead to complete remission in a short time.
Repeated MRI is recommended during the recovery phase, the result may indicate longterm memory impairment in patients who develop hippocampal sclerosis due to VGKC encephalitis.
Conflicts of Interest
The Authors declare that they have no Conflicts of Interests.
References
- Vincent A, Bien CG, Irani SR, et al. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet. Neurol 10(8), 759-772 (2011).
- Anderson NE, Barber PA. Limbic encephalitis-a review. J. Clin. Neurosci 15(9), 961-971 (2008).
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 133(9), 2734-2748 (2010).
- Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann. Neurol 69(2), 303-311 (2011).
- Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann. Neurol 72(2), 241-55 (2012).
- Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet. Neurol 9(8), 776-785 (2010).
- Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 127(pt 3), 701-712 (2004).
- Klein CJ, Lennon VA, Aston PA, et al. Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA. Neurol 70(2), 229-234 (2013).
- Irani SR BC, Vincent A, Cockerell OC, et al. Immunotherapy‑responsive seizure‑like episodes with potassium channel antibodies. Neurology 71(20), 1647-1648 (2008).
- Thieben MJ, Lennon VA, Boeve BF, et al. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 62(7), 1177-1182 (2004).
- Merchut MP. Management of voltage-gated potassium channel antibody disorders. Neurol. Clin 28(4), 941-959 (2010).
- Irani SR, Gelfand JM, Al-Diwani A, et al. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann. Neurol 76(2), 168-184 (2014).
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann. Neurol 69(5), 892-900 (2011).
- Kotsenas AL, Watson RE, Pittock SJ, et al. MRI findings in autoimmune voltage-gated potassium channel complex encephalitis with seizures: one potential etiology for mesial temporal sclerosis. Am. J. Neuroradiol 35(1), 84-89 (2014).
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu. Rev. Neurosci 27(1), 279-306 (2004).
- Rzezak P, Lima EM, Gargaro AC, et al. Everyday memory impairment in patients with temporal lobe epilepsy caused by hippocampal sclerosis. Epilepsy. Behav 69(1), 31-36 (2017).