Special Report - Imaging in Medicine (2010) Volume 2, Issue 3
How the Institute of Medicine report on comparative effectiveness research will impact imaging research and practice
Justine Seidenfeld†1 & Wade M Aubry1,21Center for Medical Technology Policy, 401 East Pratt Street, Suite 631, Baltimore, MD 21230, USA
2University of California San Francisco, Philip R Lee Institute for Health Policy Studies, 3333 California Street 265, San Francisco, CA 94118, USA
- Corresponding Author:
- Justine Seidenfeld
Center for Medical Technology Policy
401 East Pratt Street, Suite 631
Baltimore, MD 21230, USA
Tel: +1 410 547 2687
Fax: +1 410 547 5088
E-mail: justine.seidenfeld@cmtpnet.org
Abstract
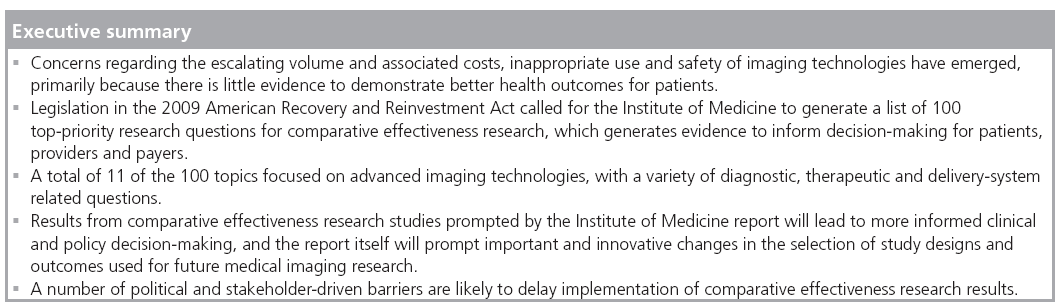
Medical imaging research questions comprise 11 of the 100 topics highlighted by the Institute of Medicine’s report on priorities in comparative effectiveness research (CER). The current report was solicited by Congress in the 2009 American Recovery and Reinvestment Act to provide guidance for the distribution of US$1.1 billion in new funding for CER studies. Besides suggesting cautious use of imaging technologies that lack sufficient evidence of effectiveness, the report will impact future research by supporting the use of comparative study designs, more appropriate outcomes and a greater emphasis on subpopulation analysis. Results from CER on medical imaging technologies has significant potential to help end users of evidence, such as patients, providers and payers, make well-informed clinical and policy decisions.
Keywords
comparative effectiveness research ▪ coverage with evidence development ▪ decision-maker ▪ imaging technology ▪ Institute of Medicine ▪ priorities
Imaging technologies are recognized as remarkable tools that aid physicians in their capacity to diagnose a variety of diseases in a less invasive manner. In the wake of rapid uptake of new imaging technologies in the healthcare delivery system, a number of concerns have emerged and have prompted reassessment of their appropriate use. The volume and associated costs of diagnostic imaging tests has skyrocketed in the last decade, far outstripping the rates of other medical services, including alternative types of diagnostic tests [1]. Additionally, there is substantial geographic variation in the volume of services, raising questions of medical necessity for some of these tests [101]. Other commonly cited reasons for the escalating use of diagnostic imaging tests include the competing (and often redundant) use of imaging technologies by radiologists and other specialists (particularly cardiologists [2]), the use of imaging tests as defensive medicine to avoid possible malpractice suits for failure to diagnose, high patient demand and conflicts of interest from highly profitable revenue streams for physicians who have a financial interests in imaging technologies and facilities.
Both private and public health insurers have expressed concern regarding escalating imaging services, primarily because there is little reliable data to demonstrate a direct connection between the increasing use of advanced imaging technologies and better health outcomes for patients [3]. Furthermore, recent concerns regarding the safety issues related to excessive radiation exposure are forcing them to reexamine the risks and potential benefits from the use of these technologies [4–7]. For example, payers are using a number of policy and coverage tools to try to curb inappropriate utilization. Congress began to regulate Medicare payments for imaging services in 2005, leading to a substantial reduction in the rise of imaging costs. However, pushback from the industry has managed to derail a number of additional pieces of legislation that would have further reduced Medicare spending. One example of this is the Medicare Improvements for Patients and Providers Act of 2008, in which Congress mandated that advanced imaging test suppliers must be accredited by the Department of Health and Human Services (DHHS)-affiliated organizations [1]. Actions like these, along with increasing the amount of time an imaging facility is open in order to lower the cost per service, hold great potential to encourage more appropriate use of imaging services while lowering costs. In the private sector, health plans have adopted a number of tools to control imaging usage, such as outsourcing utilization review and requiring prior authorization for nonemergency outpatient imaging tests. Many physicians object to submitting their clinical decisions to an independent authorization agency, so a number of review organizations are trying alternative solutions, such as computerized decision-support programs that can make recommendations and notify physicians of duplicative imaging test orders [8]. It remains to be seen how effective these decision-support programs are.
Meanwhile, imaging volume continues to rise unabated, with little evidence of beneficial health outcomes for patients.
Comparative effectiveness research & the Institute of Medicine report
Comparative effectiveness research (CER) has been hailed as an important approach to generate evidence of effectiveness in real-world care settings, eliminating many of the uncertainties surrounding the appropriate use of advanced imaging technologies. The central idea behind CER is that decision-makers (e.g., patients, clinicians, payers and policymakers) should have a greater role in guiding the activities of the clinical research enterprise, driving research questions and designs that can answer the most pressing questions found in everyday clinical practice. The passage of the 2009 American Recovery and Reinvestment Act (ARRA), investing US$1.1 billion for new research, has resulted in significant attention in CER as a crucial part of the national dialog on how to improve the US healthcare system. Of this federal investment in CER, $400 million was allocated to the NIH, $300 million to Agency for Healthcare Research and Quality (AHRQ) and $400 million directly to the Office of the Secretary in the DHHS. While the NIH and AHRQ money is being distributed by a variety of grant mechanisms, the final distribution of the DHHS allocation is in development and will likely target areas of CER not covered by the NIH and AHRQ.
The ARRA legislation charged the Institute of Medicine (IOM) with the task of soliciting stakeholders for advice and generating a list of 100 top-priority research questions for CER [102]. The IOM committee, which submitted its report to Congress on 30th June 2009, defined CER as “the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat and monitor a clinical condition, or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers and policymakers to make informed decisions that will improve healthcare at both the individual and population levels” [9]. This definition expresses the decision-makerdriven nature of CER, as well as the new push to directly compare different clinical interventions, both prospectively and retrospectively.
However, there is a growing consensus that, in addition to identifying emerging technologies and interventions that need better evidence of effectiveness, there is a need for better methods and study designs to accomplish this in a pragmatic and timely manner. This is particularly true of methods used to evaluate diagnostic tests – both imaging and other forms. Many cite concerns that it is unrealistic to demand that researchers generate data directly linking diagnostic tests to impact on health outcomes, as these kinds of studies are costly and burdensome [3]. Those who work with diagnostic technologies stress that there is a great need for more research on alternative surrogate outcomes and markers that can be reliably used in CER studies to predict improved outcomes. If validated, these alternatives would act as appropriate end points while requiring less time and money. However, there is considerable risk that if surrogate outcomes are not properly validated, they may prove to be inaccurate and, ultimately, harmful in terms of health outcome and economic impact [10].
Of the 100 priorities identified in the IOM report, 11 relate to medical imaging. They are spread across four quartiles used to indicate their priority ranking within the report, with three topics in each of the first, second and third quartiles, and two topics in the fourth quartile. These include studies to compare the effectiveness of imaging technologies in diagnosing, staging and monitoring cancer (first quartile), studies to compare film-screen or digital mammography alone versus mammography plus MRI in community practice-based screening of breast cancer (second quartile), studies to compare the effectiveness of care with and without obstetric ultrasounds in normal pregnancies (second quartile), and studies to compare the effectiveness of diagnostic imaging performed by nonradiologists versus radiologists (fourth quartile), among other topics [11]. A number of these are very broad questions that will have to be addressed by multiple studies, and a few comparative studies have already been initiated through the American College of Radiology Imaging Network (ACRIN), including studies of digital versus film-screen mammography and chest radiography versus CT for lung cancer screening [9].
Probable impact of the report
While it will take significant time and effort to initiate many of the proposed studies, the IOM report serves a number of important purposes; in the short term, it will serve as guidance for both private and public sector funding, most notably for the Request for Applications issued to distribute NIH, AHRQ and DHHS money for CER. Topics and technologies that have been identified as high priority for additional research may now meet with some increase in decision-maker skepticism regarding their effectiveness, at least until the studies are completed and published. Additionally, an unprecedented number of collaborations between talented investigators and academic institutions are forming in preparation for priority CER projects, creating the infrastructure for innovative and collaborative research teams.
These high-functioning teams will also pave the way for changing conventional study designs for medical imaging research, which will shape imaging CER studies for years to come. Primarily, this will include more head-to-head comparative studies, which have not been frequently performed before for a number of reasons, including the high costs of such studies, the burden posed by the complex infrastructure needed to coordinate and carry them out and the lack of financial incentives for product developers given that many imaging tests have historically gained rapid and wide coverage, reimbursement and adoption without the need to produce solid evidence of improved health outcomes [12]. Additionally, research questions for postapproval studies will be less driven by the traditional model of investigator-initiated research, which has often focused on questions of interest to researchers, but bore little relevance to decision-making required in everyday clinical practice. Instead, as with other CER studies, medical imaging research will now be more frequently conducted to answer priority questions relevant to the end users of the evidence. Finally, the improvements in study designs and the advent of innovative collaborations will allow medical imaging research to begin to sort out the thorny question of appropriate outcomes to use in researching diagnostic imaging tests. It is increasingly apparent that different methods will be required in the future to assess the effectiveness of diagnostic tests as compared with medical treatments or therapies.
In addition to the short-term impacts and influences on study design, methods and outcomes research, new evidence generated from research on the topics recommended by the IOM committee has potential to affect imaging use in clinical practice. As previously cited, these types of studies are intended to aid decision-makers, who will be looking to the first and subsequent rounds of results from CER with anticipation. Payers will increasingly consider this information while making coverage and reimbursement decisions, thereby helping to set new standards for providers based on evidence. Relevant professional societies, such as the American College of Radiology, the American College of Cardiology and the American College of Physicians, will also be able to incorporate the best results from CER into clinical practice guidelines and appropriateness criteria, which will aid evidence-based coverage decisions and quality measures, as well as clinical practice. This refined guidance holds potential to eliminate waste, overuse or inappropriate use of medical imaging technologies, with the potential to decrease unnecessary costs in the healthcare system. Another important aspect of CER is that it also emphasizes the use of subpopulation analysis, which will better enable these professional organizations to make recommendations for specific patient characteristics, with the hope of ensuring that the right technology is used by the right provider for the right patient. Since dissemination of results to patients in a friendly format has been stressed as an important aspect of any new CER, patients may be able to directly use it to help them make treatment choices.
Conclusion
It is important to acknowledge that even with any new evidence that may be generated by CER, the way that stakeholders view, demand and cover medical imaging technologies is complex. In May 2009, the Centers for Medicare and Medicaid Services (CMS) issued a national coverage decision memo denying coverage for CT colonography (virtual colonoscopy) for colon cancer screening. This was cited as an unprecedented decision based on evidence-based medicine presented at a November 2008 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MedCAC), and CMS asserted that the current evidence base did not apply, nor could be generalized, to the Medicare population. Although a higher level of evidence is needed for CT colonography as a screening test, this decision has been met with a great deal of pressure from several stakeholders, including the imaging community, patients demanding the technology, radiologist groups and others with a financial stake in the technology. Despite pressure from a number of congressional representatives to reconsider, it remains to be seen if CMS will remain firm in their final decision [13]. It is worth noting the similarities of this situation to the CMS decision on CT angiography (CTA) 2 years earlier. In 2007, CMS tried to withdraw broad local coverage of CTA by Medicare Part B carriers through a national coverage decision that would require “coverage with evidence development” (CED) study participation because there was no evidence of benefit in the Medicare population. Similarly, this was met with congressional pressure and a great deal of protest, and CMS eventually decided to continue to leave the decision to contractor discretion with almost all Part B carriers approving coverage. However, most private insurers do not cover CTA [11]. In both cases, the lesson is that even when there is evidence demonstrating that an imaging technology has no proven benefit to a population, other nonscientific considerations, primarily industry and patient demand, are additionally used to shape coverage decisions. Even with an increasing amount of data that demonstrate higher risks caused by radiation exposure from imaging technology than originally estimated without comparable evidence of greater benefits, CMS has demonstrated little ability to reflect this in a change in their coverage policies. It remains to be seen whether evidence generated from any of the IOM-recommended studies will be used to better shape coverage decisions based on evidence or whether secondary factors remain the primary consideration.
In addition, when technologies are already in common clinical practice and are covered by third-party payers, it has proven difficult to motivate clinical researchers to generate additional evidence for technologies, even when substantial questions exist regarding their optimal use. One potential policy tool that may facilitate participation in CER is CED. Piloted by CMS in 2005, CED provides insurance coverage for promising but unproven new medical technologies that would otherwise not be covered, under the condition that patients participate in a registry or clinical trial that generates evidence regarding effectiveness, which can be used for later coverage decisions [14]. This would allow further CER in Medicare-relevant populations and more relevant decision-making. As noted previously, this approach was proposed by CMS for CTA but ultimately withdrawn after the notice and comment period, leaving coverage decisions at the local rather than the national level.
There is little experience with CED in the private sector. One notable exception is the National Cancer Institute’s trials of high-dose chemotherapy with autologous bone marrow transplantation for breast cancer. A pilot effort is being led by the Center for Medical Technology Policy (CMTP) [103], a Baltimorebased, private, nonprofit organization that provides a neutral forum in which patients, clinicians, payers, manufacturers and researchers can work together to improve the quality and efficiency of CER to benefit decision-making in clinical and health policy. CMTP is currently working with private payers and a range of other stakeholders to develop a model for CED in the private sector [104]. The goal is to establish a routine process by which important emerging technologies can be identified for CED and adequately designed studies can be developed. Individual health plans can then make a decision to participate in a given CED initiative and the actual research will be subcontracted to an independent and credible research organization. Programs like CED offer promise for aligning incentives across all stakeholder groups to generate better evidence for decision-making. Better evidence of comparative effectiveness for imaging technologies identified in the IOM committee report, as well as new technologies in general, ultimately benefits multiple stakeholders in the healthcare system, but especially patients themselves.
Future perspective
Evidence generated from CER on topics recommended by the IOM committee on CER will probably impact imaging use and practice by clinical and policy decision-makers. Patients may be able to use CER to make appropriate choices between treatment courses based on evidence that reflects their particular characteristics. Payers may use results to make coverage and reimbursement decisions, thereby helping to set new standards for providers based on evidence. Relevant professional societies will be able to incorporate best evidence from CER into clinical practice guidelines and appropriateness criteria, which will further aid evidence-based coverage decisions and quality measures. This refined guidance holds potential to eliminate waste, overuse or inappropriate use of medical imaging technologies, with a potentially large impact on decreasing unnecessary costs in the healthcare system. However, it is important to note that in the process of moving towards evidence-based decision-making there will be a number of political barriers and stakeholder negotiations that delay implementation of all the uses described in this article.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Iglehart JK: Health insurers and medicalimaging policy – a work in progress. N. Engl. J. Med. 360(10), 1030–1037 (2009).
- Mitka M: Costly surge in diagnostic imaging spurs debate. JAMA 293(6), 665–667 (2005).
- Redberg RF, Walsh J: Pay now, benefits may follow – the case of cardiac computed tomographic angiography. N. Engl. J. Med. 359(22), 2309–2311 (2008).
- Lauer MS: Elements of danger – the case of medical imaging. N. Engl. J. Med. 361(9), 841–843 (2009).
- Redberg RF: Cancer risks and radiation exposure from computed tomographic scans: how can we be sure that the benefits outweigh the risks? Arch. Intern. Med. 169(22), 2049–2050 (2009).
- Berrington de González A, Mahesh M, Kim KP et al.: Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch. Intern. Med. 169(22), 2071–2077 (2009).
- Smith-Bindman R, Lipson J, Marcus R et al.: Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 169(22), 2078–2086 (2009).
- Demmerle C, Glaudemans J: Diagnostic Imaging: Spending Trends and the Increasing Use of Appropriateness Criteria and Accreditation. Avalere Health, DC, USA (2008).
- Sox HC, Greenfield S: Comparative effectiveness research: a report from the Institute of Medicine. Ann. Intern. Med. 151(3), 203–205 (2009).
- Institute of Medicine (IOM): Initial National Priorities for Comparative Effectiveness Research. Ratner R, Eden J, Wolman D, Greenfield S, Sox H (Eds). National Academies Press, DC, USA (2009).
- Redberg RF: Evidence, appropriateness and technology assessment in cardiology: a case study of computed tomography. Health Aff. (Millwood) 26(1), 86–95 (2007).
- Iglehart JK: The new era of medical imaging – progress and pitfalls. N. Engl. J. Med. 354(26), 2822–2828 (2006).
- Dhruva SS, Phurrough SE, Salive ME, Redberg RF: CMS’s landmark decision on CT colonography – examining the relevant data. N. Engl. J. Med. 360(26), 2699–2701 (2009).
- Tunis SR, Pearson SD: Coverage options for promising technologies: Medicare’s ‘coverage with evidence development’. Health Aff. (Millwood) 25(5), 1218–1230 (2006).
- Medicare Part B Imaging Services www.gao.gov/new.items/d08452.pdf
- Brice J: Medical imaging given priority on comparative effectiveness research list. Diagnostic Imaging. 1 July 2009 www.diagnosticimaging.com/news/display/ar ticle/113619/1425785?=cid&verify=0 (Accessed on 16 October 2009).
- Center for Medical Technology Policy www.cmtpnet.org
- Coverage for Evidence Development: a conceptual framework. CMTP Issue Brief, January 2009 http://cmtpnet.org/cmtp-research/ applied-policy-and-methods/coverage-withevidence- development/20090108%20-%20 CMTP%20-%20CED%20Issue%20Brief. pdf/view
• Discussion of the current state of medical imaging technology, public and private coverage policies enacted to influence their use, and new programs being developed to generate additional evidence.
• Summary of recent studies that demonstrate unanticipated excess of radiation exposure from imaging technologies, indicating the need for further research and more cautious use.
• Background, context and summary of the Institute of Medicine’s report on comparative effectiveness research.
• List of the Institute of Medicine’s top 100 research priorities for future comparative effectiveness research studies.
Websites


