Perspective - Interventional Cardiology (2010) Volume 2, Issue 1
How to select patients for endovascular balloon-expandable aortic bioprosthesis
- Corresponding Author:
- Raymond Cartier
Department of Cardiac Surgery, Montreal Heart Institute
5000 Belanger Street, Montreal
Quebec H1T 1C8, Canada.
Tel: +1 514 376 3330 ext. 3715
Fax: +1 514 593 2157
E-mail: rc2910@aol.com
Abstract
Keywords
elderly population, risk assessment, transcatheter aortic valve replacement
Percutaneous aortic valve implantation (PAVI) is the result of close to a century of constant struggle by physicians and scientists to correct aortic valve disease. In 1912, Tuffier reported the first indirect intervention for aortic valve disease [1]. With his finger, he attempted to invaginate the aortic wall through the stenotic aortic valve orifice in a 26‑year-old patient in the hope of enlarging the aortic valve area (AVA). Although the patient recovered after the attempt, no one knows if it was truly successful. Almost 30 years later, Russell Brock attempted to dilate a calcified aortic valve by introducing an instrument through the brachio-cephalic artery [2]. Lack of precision and residual aortic insufficiency eventually precluded wide acceptance of the procedure. The first animal experiments on direct valvulotomy of the aortic valve were undertaken by Smithy et al. in 1947 at the University of South Carolina in Charleston, SC, USA [3]. For technical reasons and lack of reliability, this procedure was also abandoned. In the early 1950s, Hufnagel and Campbell in Washington, DC, USA, developed an artificial valve that could be implanted in the descending aorta in cases of aortic insufficiency [4,5]. The caged-ball valve they developed was first implanted in a dog model, and soon thereafter, Hufnagel brought the concept to the clinical setting with some success. In 1954, he described a series of 23 patients and six operative deaths [6]. The valve could be implanted rapidly owing to two external rings with multiple fixation points. As the native dysfunctional valve was left in place, only aortic insufficiency could be addressed by this approach. Due to the remote site of valve implantation, correction was partial. The procedure was rapidly disregarded.
It was only after the advent of cardiopulmonary bypass (CPB) that aortic valve replacement (AVR) at the aortic annulus level became feasible. Bahnsen and Hufnagel independently developed a single leaflet aortic valve that was used intensively in the early days [7]. However, it was mainly the ball valve prosthesis of Harken and Starr in the early 1960s that launched the modern era of AVR worldwide [8,9]. In 2001, Alain Cribier shocked the surgical community when he reported the first human implantation of a transcatheter-delivered aortic prosthesis [10]. Since then, the procedure has continually gained popularity [11–13].
For close to 40 years, direct AVR on CPB has been the gold-standard treatment for aortic valve stenosis. More than 200,000 procedures are performed annually worldwide [14]. In 2006, according to the Society of Thoracic Surgeons (STS) database, the average age of patients undergoing isolated AVR was 68 years, and operative mortality was 2.5%. Over the last decade, despite an increased prevalence of co-morbidities, operative mortality and stroke rate decreased by 24 and 27%, respectively, indicating improvement of the current surgical technique and patient care. Despite these advances, the recent European Heart Survey revealed that close to 30% of patients over 75 years of age, diagnosed with severe aortic stenosis (AS), were denied surgical AVR owing to associated severe comorbidities, leaving room for alternative therapy [15].
The presence of significant AS has been known to compromise patient survival, but surgical management has been shown to improve it. The placement of transcatheter-delivered valves in daily practice is not yet well defined. The following questions will be discussed in this article: What are the indications for PAVI and transapical aortic valve implantation (TAVI)? What are the procedural risks? How do PAVI and TAVI compare with conventional AVR?
Life expectancy and aortic stenosis
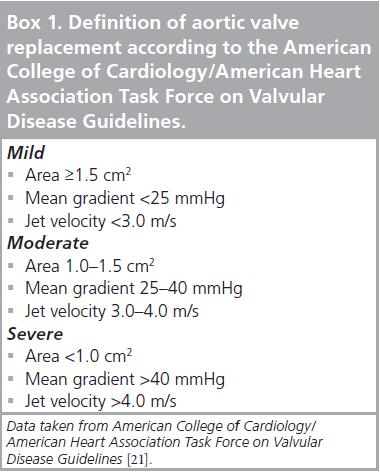
Aortic stenosis is generally caused by calcification of a tri-leaflet or a congenital bi-leaflet valve that usually starts from the annulus to the leaflet itself, imposing movement restriction. Calcification is the result of lipid accumulation, inflammation that resembles the atherosclerotic process, and could also be initiated by rheumatic disease [16–20]. According to the 2008 revised American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the management of patients with valvular heart disease, AS severity is determined by more than one criterion. As listed in Box 1, valve surface area, mean transvalvular gradient and jet velocity are all specific elements that should be considered [21]. Surgical indication for aortic valve correction is mainly based on clinical symptoms. Some patients have no symptoms, even though their stenosis is considered to be severe, whereas others are symptomatic with moderate stenosis. Symptoms rely on myocardial wall adaptation to intraventricular pressure generated by increased after-load. Lack of ventricular wall hypertrophy, previous ventricular dysfunction and loss of atrial contraction are factors that contribute to symptom deterioration [22–26]. Speed of progression varies according to the etiology of AS; senile stenosis progresses at a faster rate than congenital and rheumatic stenosis [27]. Recent prospective studies have demonstrated that once moderate AS (jet velocity 3 m/s) is diagnosed, the average annual progression is 0.3 m/s for velocity, 7 mmHg for the transvalvular gradient and a drop of 0.1 cm2 for surface valvular area [28]. Once patients develop symptoms, such as angina, cardiac failure or syncope, life expectancy is drastically reduced. The average survival of these patients is less than 3 years, with a high risk of sudden death justifying surgical correction [29–31]. However, the outcome is harder to predict in asymptomatic patients.
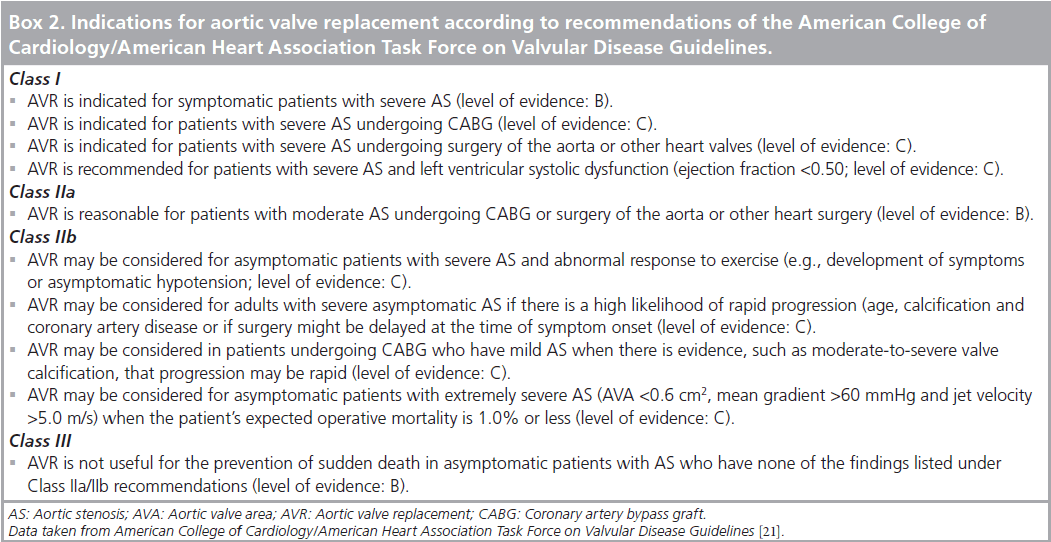
The outcome of asymptomatic patients with AS is comparable with age-matched, normal adults without AS. However, when symptoms appear, the prognosis changes considerably, particularly in the presence of severe AS. In a prospective study, Otto et al. observed a 2‑year event-free survival rate of 84% in patients with mild AS (jet velocity <3 m/s) compared with 21% in those with severe AS (jet velocity >4 m/s) [32]. Others have also reported low event-free survival at 5 years in severe AS patients [33,34]. Monitoring of asymptomatic status is a key point in the management of these patients. The ACC/AHA 2008 Task Force on Practice Guidelines recommended annual echocardiography for asymptomatic patients with severe AS, every 1–2 years for those with mild AS, and every 3–5 years for mild AS (Class I; level of evidence: B). As symptom description is subjective, and elderly patients tend to progressively adapt their physical activity to their limited exercise tolerance, exercise testing (ET) may be used for objective assessment. ET is contra-indicated in symptomatic patients with AS (Class III; level of evidence: C); however, in asymptomatic patients, it may elicit exerciseinduced symptoms and abnormal blood pressure responses (Class IIb; level of evidence: B). Manifestation of symptoms, decreased systolic pressure or low blood pressure responses (<20 mmHg) during exercise may help identify patients at risk of complications related to AS in the following 2 years [35]. Recommendations of the ACC/AHA Task Force on Valvular Disease Guidelines are listed in Box 2.
In summary, AVR is definitely recommended (Class I; level of evidence: B) for symptomatic patients with severe AS, patients with severe AS and left ventricular ejection fraction (LVEF) <50% (Class I; level of evidence: C), asymptomatic patients with good ventricular function and abnormal response to ET (Class II; level of evidence: B) and patients with rapid AS progression. These recommendations were originally defined for conventional AVR but should definitely be used as guidelines for PAVI as well.
What is the current risk of surgical AVR?
Browns’ publication in 2009, reporting the STS database experience of single AVR in 928 STS-participating hospitals during the period between 1997 and 2006, is quite representative of current daily practice in North America. More than 108,000 patients were followed up. Patients with endocarditis and incomplete charts were excluded. Remarkably, during this period, mortality declined by 24%, and risk-adjusted mortality fell by 33%, indicating that even though patient comorbidity increased significantly, surgical mortality decreased dramatically [14]. The percentage of bioprosthetic valve implantations went from 50% in 1997 to 78% in 2006, although mean age rose by only 2 years (65.9–67.9 years), showing a strong overall trend toward tissue valves. The risk factors that increased operative mortality were age over 70 years, obesity, hypertension, chronic obstructive pulmonary disease, previous stroke and chronic renal insufficiency. Significant risk factors for mortality during the follow-up period were age over 70 years, diabetes, peripheral vascular disease, low LVEF (<30%), and low BMI. In 2006, overall mortality among patients aged 80–85 years or over 85 years old was 5 and 6.5%, respectively. Stroke prevalence for the same age groups was 1.9 and 2.0%, respectively. The data confirm that even in such a cohort of elderly patients, modern-day surgical results are outstanding. This has been further confirmed by others. Thourani et al. from Emory University, GA, USA, recently reported their experience with octogenarians undergoing isolated AVR [36]. Among the patients aged 80 years or older, more than 94% survived the operation with stroke incidence of 3.4%, and median survival of 7.4 years, confirming that once patients have been accepted for surgical treatment, success in the geriatric population is amazingly good. Studies targeting assessments in populations over 75 years have documented comparable quality of life (QOL) in those who underwent conventional AVR compared with the general population, with significant improvement in their New York Heart Association (NYHA) functional class [37]. Others have disclosed that after undergoing AVR surgery, up to 93% of octogenarians were symptom-free or significantly less symptomatic, 83% were living in their own home and 74% considered themselves as being in excellent or good health [38,39].
The geriatric population is going to be the fastest growing cohort of patients for cardiac surgeons of tomorrow. It is estimated that there will be over 20 million individuals in the USA aged over 80 years by 2010 [101]. Patient selection and preoperative risk assessment will be key issues for procedural success.
How do we define surgical risk?
The development of catheter-based aortic valve therapies has been driven by the premise that many patients, even the most affected, were not receiving proper surgical treatment [40]. The EuroHeart Survey demonstrated that one in three elderly and symptomatic patients with severe AS was left untreated. Ross and Braunwald noted in 1968 that the survival of such patients was very limited [41]. Patients are generally rejected for conventional surgery because they are considered bad surgical candidates. Physicians are reluctant to refer these patients for open heart surgery, while consulting surgeons prefer not to operate on patients suffering from multiple comorbidities [42,43]. Operative risk estimation is known to be a complex process. How do we determine if a patient is ‘too sick to be operated on’? Statistical models have been developed to assist physicians in making these decisions. Scores are based on logistic regressions performed on data comprising thousands of patients. The result is an estimation of operative mortality considering global preoperative patient condition and the anticipated physical stress from surgery. Most of these tools have the benefit of being user-friendly and give an objective assessment of operative risk. Scores are based on large populations of cardiac surgical patients. They include the experience of several centers, tend to represent the operative risk of daily surgical practice and do not focus on experts in specific fields. The Parsonnet score and EuroScore are among the most common tools that guide surgeons in the evaluation of surgical candidates and prediction of operative mortality [44,45].
These scores give an objective evaluation of surgical risk and are more accurate than clinical judgement alone. Patients known for extraaortic pathology with poor short-term prognosis and unsuitable for surgical treatment can become candidates for emerging options. This is the reasoning applied to the selection of patients for catheter-based AVR.
The EuroScore has been frequently used in experimental protocols weighing catheter-based AVR feasibility [46]. Recently, its value has been seriously questioned in the assessment of risk reduction in percutaneous valve replacement. According to Osswald et al., the EuroScore was mainly based on patients undergoing coronary artery bypass graft (CABG) in 1995 and seemed to overestimate the surgical mortality of current patients [47]. It fails to predict the surgical mortality of a given patient in part because catheter-based valve technology is currently limited to highly-specialized centers [48]. Dewey et al. confronted the efficacy of the logistic and additive EuroScore, the Society of Thoracic Surgeons Predictive Risk of Mortality (STSPROM) score, and the Ambler risk score [49]. In their analysis, STS-PROM was found to be the most accurate scoring system for predicting perioperative and long-term mortality in high-risk patients operated for isolated AVR. STS-PROM analyzes 30% more variables than the three other risk estimation models. The authors highlighted the importance of variable choices in these models. For example, STSPROM is the only tool that considers NYHA functional class, the need for counterpulsation intra-aortic balloon pump as well as the presence or absence of cardiogenic shock at the time of operation. On the other hand, STS-PROM does not assess critical preoperative status or left ventricular dysfunction. The Ambler score does not take into account the neurological function of patients in the evaluation of their risk, but includes LVEF in the calculation. Globally, each tool focuses on specific variables but deliberately omits others.
For the reasons stated above, these tools cannot substitute for clinical evaluation, but should serve as an adjunct to the subjective judgement of surgeons. Many important surgical variables are simply not considered by any single model. Nutritional status, previous chest irradiation or the presence of neoplasia are examples of such variables. Although STSPROM was more accurate than the others, it still overestimated the factual operative risk of patients. This obviously could fallaciously promote percutaneous experimental procedures in patients who would otherwise be suitable surgical candidates. Consequently, clinical judgement still remains the most important consideration when offering novel therapy to a given patient.
What is the current risk with PAVI & TAVI?
Catheter-based valve replacement is not devoid of complications. Procedural risks depend on patient condition, the technical approach (transfemoral vs transapical) taken and intrinsic prosthesis properties. The transfemoral approach is the less invasive of the two procedures and can be carried out through a short hospitalization stay. However, it has been particularly related to technical complications. Injury to aorto–femoral vessels, failure to deliver the valve, embolic stroke from aortic atheromatous plaque dislodgement and from loose endarterectomized iliac artery tissues have been reported [46,50–52]. On the other hand, the transapical approach requires general anesthesia and surgical transgression of chest integrity. The most frequent complications are pleural effusion and bleeding from the apical implantation site [13,53]. Prosthesis positioning follows the same principles, regardless of the approach taken. Problems of aortic insufficiency after improper placement or over-stretching of the annulus and even delayed prosthesis embolization have been reported [53–55].
Clinical results with catheter-based aortic valve delivery
At present, two transcatheter prostheses have been studied in clinical trials and are now available commercially.
▪ REVIVE II and REVIVAL II
A recent update on the first two feasibility trials (Registry of Endovascular Implantation of Valves in Europe [REVIVE II] and Transcatheter Endovascular Implantation of Valves II trial [REVIVAL II]) with the Edwards Sapien valve (23 mm) was presented at the 2008 Transcatheter Cardiovascular Therapeutic meeting in Washington, USA [56]. These trials were designed to include patients over 70 years of age, with severe symptomatic AS, whose expected surgical risks were high (EuroScore >20% predicted operative mortality). Combined, the two studies included 161 patients (REVIVE II: 106 patients, REVIVAL II: 55 patients). Major end points were mortality, myocardial infarction (MI), NYHA functional class and paravalvular leak at 1, 3, 6 and 12 months. Patients in REVIVE II were followed for 12 months and those in REVIVAL II for 2 years. All subjects were closely monitored.
On average, patients were 83 years old with an equal distribution of genders. Mean AVA was 0.57 cm2, and transvalvular gradient was 43 mmHg. More frequently associated comorbidities were previous cardiac surgery (29%), chronic obstructive pulmonary disease (27%), diabetes (26%), chronic renal disease (22%), peripheral vascular disease (21%) and history of cerebrovascular accident (CVA) or transient ischemic attack (TIA; 16%). Predicted operative mortality (EuroScore) was 30%. Implantation was successful in 88%. Among the 19 failures, nine were due to unsuccessful access, three to inability to cross the valve, three to cardiac perforations, two to prosthesis misplacement and two to anesthetic problems. A total of 44 patients died during the study (27%) and, among them, 18 (11%) succumbed in the first 30 days (operative mortality). A total of seven (4.3%) patients sustained stroke, and six (3.1%), MI. Only two patients needed urgent cardiac surgery, which took place in the first 30 days. Vascular complications occurred in 15.5% of patients and they had a worse prognosis than those without vascular complications. The procedure was very efficient in decreasing aortic transvalvular gradient and in improving aortic orifice area (AOA). Residual aortic insufficiency was less frequent with the 26‑mm valve but remained significantly present (2+) in 36% of the cohort. The majority of patients (89%) improved their functional state by at least one NYHA class (preprocedure: 86% III/IV; postprocedure: 87% I/II), and this amelioration was sustained for up to 12 months. Gains in QOL were also maintained at 12 months. Actuarial survival at 2 years was 62%. Multivariate analysis revealed that initial NYHA class and history of prior CABG were major causes of death. The authors concluded that retrograde transcatheter AVR could be performed in the majority of patients with acceptable risk (11 vs 30% expected), providing sustained improvement of functional class and QOL. Residual paravalvular leak did not affect ventricular volume or LVEF over time. However, the authors cautioned against vascular complications and residual mitral insufficiency, as well as poor functional class and prior CABG as causes of long-term mortality. Unfortunately, from the surgical standpoint, peripheral vascular disease, mitral insufficiency and re-do surgery are definitely frequent comorbidities that significantly heighten the risk of open surgery in the elderly.
▪ CoreValve Safety and Efficacy Study
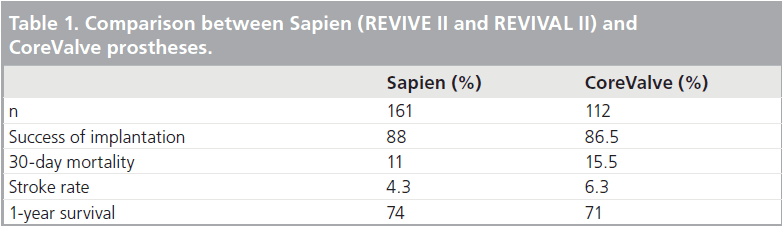
The 18 Fr CoreValve Safety and Efficacy Study, performed for CE marking, enrolled 112 patients [57]. It was a prospective, multicenter, nonrandomized, single-arm, observational trial undertaken between May 2006 and June 2007. Inclusion criteria were severe AS (AVA <0.6 cm2), age of 75 years or over, logistic EuroScore 15% or over or high-risk comorbidities. Average age was 82 years, women accounted for 57% of the cohort, the majority of patients were classes III (54%) or IV (21%) prior to the procedure, and average predicted mortality (logistic EuroScore) was 23%. Among pre-existing comorbidities, the most frequent were history of congestive heart failure (55%), chronic renal failure (44%), prior CABG (27%), peripheral vascular disease (18%) and prior MI (17%). All patients were closely monitored. At the procedure, three patients had their valve explanted for technical reasons, and five patients died. Implantation was successful in 86.5% of the cohort; 30‑day mortality was 15.5%, with cardiac-related mortality in two-thirds of them (10.7%). Thromboembolic events affected 12.5% of patients equally distributed between TIAs and CVAs. Periprocedural MI occurred in 3.6%, and 27% required a permanent pacemaker. The 1-year overall and cardiac year survival was 71 and 82%, respectively. Major adverse cardiac event (MACE)- free and stroke-free survival was 62 and 92%, respectively. The procedure was successful in improving AOA with an average of 1.72 cm2 at 1‑year follow-up. Patient functional status was also dramatically improved. A total of 76% of the cohort were in NYHA classes III and IV prior to the procedure, whereas 88% were in classes I and II after confirming the efficiency of prosthesis implantation. Table 1 compares the experience of the two types of prosthesis. Broadly, clinical experience with these two prosthesis types appears to be comparable, with over 85% success, 10–15% procedural mortality and 4–6% stroke risk.
Recent experience
With time and experience, better procedural efficiency and lower morbidity/mortality are expected. In the most recent publication of the Vancouver group, significant improvement has been reported [58]. Between January 2005 and October 2008, a total of 168 transcatheter aortic valves were implanted, 113 transarterial and 55 transapical. From the initial to the second half of the study, procedural success went from 89.3 to 98.8%, and 30‑day mortality decreased from 12.3 to 3.6% for transfemoral and from 25 to 11.1% for transapical valves. STS-predicted mortality was 10.3 and 8.7% for TAVI and PAVI, respectively. Procedure-unrelated deaths were more frequent in the TAVI group. Observed 30‑day mortality remained higher than predicted by STS score, suggesting suboptimal risk-evaluation by the conventional model in TAVI patients who were rejected for conventional surgery anyway. Although nonsignificant, postprocedural stroke was less common with the transapical procedure (1.8 vs 5.3%), indicating that less manipulation of the aortic arch could benefit elderly patients with atheromatous aorta. The 1‑year survival was 74%. These data definitely confirm that once beyond the learning curve, excellent results can be obtained, even in high-risk patients. Besides skills improvement, technical device refinement is and will remain a major player in further optimization of the results [59].
Upcoming trial
The cardiovascular community is waiting for the results of the Placement of Aortic Transcatheter Valve (PARTNER) trial designed to evaluate the Sapien prosthesis in two different clinical settings. The trial comprises two arms and is intended to treat patients judged to be at high-risk for conventional open-heart surgery or to be nonoperable. In the clinical trial, it was important to be able to account for the entire high-risk patient population and to ensure that patients could be randomized to their most appropriate control group. The solution was a two‑arm trial. The surgical arm of the trial will focus on patients who are candidates for conventional open-heart surgery and will include approximately 350 subjects. These patients will be evenly randomized to receive either the Edwards Sapien transcatheter heart valve or an Edwards surgical valve. Among the inclusion criteria, the most pertinent are: surgical risk estimated to be above 15% or STS score more than 10, NYHA functional class II or above, aortic transvalvular velocity more than 4 m/s, indexed efficient orifice area (EOA) less than 0.5 cm2, transvalvular gradient more than 40 mmHg and LVEF more than 20%. There is no age restriction in the trial. The nonsurgical arm will focus on patients who do not qualify for conventional openheart surgery, as determined by clinical judgement and a statistical model that accounts for the impact of patient risk factors on operative mortality and morbidity. An important inclusion criterion is assessment by one cardiologist and two surgeons estimating the surgical risk to be above 50%. These patients (approximately 250) will be evenly randomized to receive either the Edwards Sapien transcatheter heart valve or appropriate medical therapy. The primary study end points are freedom from death at 30 days and 6 months. They will be compared against fixed targets based on historical data for high-risk AVR surgery. The secondary end points of this study are freedom from death or emergent cardiac or vascular surgery from the index procedure to 1 year. The trial results will be primordial because they will define the value of the technology in symptomatic patients unsuitable for conventional surgery. They will also define the capacity of this less invasive procedure in reducing mortality and morbidity related to conventional surgery in high-risk patients. An exhaustive listing of inclusion and exclusion criteria is available at [102].
Future perspective
Currently, there are 12 different percutaneous/ transapical prostheses in development [60]. All of them are not retrievable once released in the aortic annulus, but some can be repositioned before final delivery. This technical aspect should decrease morbidity related to prosthesis malpositioning during release. The ultimate prosthesis will be the one that can be deployed and retrieved endlessly until perfect positioning is achieved without harming the ascending aorta and left ventricular outflow tract. A promising aspect of transcatheter AVR is its hemodynamic profile. In a recent publication from Laval Hospital, the investigators case-matched 50 patients who underwent PAVI with the Sapien prosthesis to 50 patients who submitted to AVR with a stented Edward Magna aortic valve and 50 patients who received a stentless Frestyle Medtronic aortic valve [61]. Patients were matched for sex, aortic annulus, left ventricular function, body surface area and BMI. Postprocedural PAVI patients had the lowest transvalvular gradient. At 6- and 12‑month follow-up, the PAVI gradient remained significantly lower than the stented valve, but was equivalent to stentless prostheses. Such findings seem to confirm that avoiding a rigid supporting ring can definitely contribute to improved AVR hemodynamics. Considering that stentless valve implantation carries a higher risk in small annulus [62,63], this could eventually be an argument in favor of PAVI in patients with small annulus.
▪ How to select patients?
Patient selection remains the ultimate question and should be debated in view of current experience with standard AVR. AVR with either mechanical or bioprosthetic valve is a reliable operation with 5‑year overall survival and MACE-free survival of 85 and 70%, respectively [64]. Octogenarians are expected to have a 5‑year survival of 70% after AVR [65]. Tissue prostheses issued by the latest technology have good longevity. Less than 20% of patients with valve implantation after 65 years of age are expected to need re-intervention to correct structural deterioration [66]. These elements set the bar very high for any less-invasive procedure that wants to claim similar results at a lower morbidity cost.
Percutaneous aortic valve implantation still carries operative and stroke risks of 10–12 and 4–6%, respectively, in highly selected centers with high-level expertise, but with experience and engineering advances, this is expected to decrease. Prosthesis lifespan is not yet known since the earliest were implanted less than 5 years ago. The results of the PARTNER trial will help us to better define the place of this new technology as an alternative to medical treatment for patients rejected for surgery and as an alternative to conventional AVR in highrisk patients. For now, patients with a hostile anatomy, such as radiation-induced chest osteitis or native coronary arteries depending on retro-sternal patent bypass grafts crossing the midline, and elderly patients with an expected operative risk equal to or above 15% with a life expectancy of less than 5 years are the most suitable candidates. Until we learn more about prosthesis longevity, it will not be ethically correct to recommend implantation in younger patients with a life expectancy of 5 years or more. Holding onto the inclusion criteria of the PARTNER study appears to be the most reasonable choice at this point. Level of expertise is obviously the key to success and before making user-friendly prostheses available to future generations, the technology should be concentrated first in centers with a very high level of expertise. The choice of approach – percutaneous versus transapical – has been mainly driven by anatomical issues to date. In view of the high morbidity and poorer outcome encountered in patients with peripheral vascular complications, the transapical approach should be considered whenever such complications are anticipated. Future studies will be necessary to sort out which technique will be the best choice among patients suitable for both options.
Conclusion
Transcatheter aortic valve implantation is a new technology that carries reasonable morbidity and mortality in high-risk patients. The technique has been shown to successfully improve the EOA of the aortic valve, the functional class of patients as well as their QOL. Longterm structural deterioration of these prosthetic valves is not yet known. Until technological improvement and long-term performance of these prostheses are documented, clinical implantation should remain limited to highrisk patients in centers with high-level expertise.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Transcatheter aortic valve replacement is a very new procedure and patient selection is still not well defined.
▪ The procedure itself carries significant risk but offers potential benefits in elderly patients with severe comorbidities.
▪ Selection should be based of pre-existing risk model assessment and clinical judgement.
▪ We present a literature review of the current experience with trans-apical aortic valve replacement and classic surgical aortic valve replacement and focus on recent prospective randomized trials that should shed some light on how clinicians should select the best candidate for this new endovascular procedure.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Tuffier T: Etat actuel de la chirurgie intrathoracique. Trans. Int. Congr. Med. 1913 (London, 1914), 7, Surgery 2, 249 (1914).
- Brock RC: The arterial route to the aortic and pulmonary valves: the mitral route to the aortic valves. Guys Hosp. Rep. 99, 236–246 (1950).
- Smithy HG, Pratt-Thomas HR, Deyerle HP: Aortic valvulotomy: experimental methods and early results. Surg. Gynecol. Obstet. 86(5), 513–523 (1948).
- Hufnagel CA: Aortic plastic valvular prostheses. Bull. Georgetown Med. Cent. 4, 128–130 (1954).
- Campbell JM: Artificial aortic valve. J. Thorac. Cardiovasc. Surg. 19, 312–318 (1954).
- Hufnagel CA, Harvey WP, Rabil PJ et al.: Surgical correction of aortic insufficiency. Surgery 35, 673–683 (1954).
- Hufnagel CA, Conrad PW: The direct approach for the correction of aortic insufficiency. JAMA 178, 275–279 (1961).
- Harken DE, Soroff HS, Taylor WJ, Lefemine AA, Gupta SK, Lunzer S: Partial and complete prosthesis in aortic insufficiency. J. Thorac. Cardiovasc. Surg. 40, 744–762 (1960).
- Starr A, Edwards ML, McCord CW, Griswold HE: Aortic replacement: clinical experience with a semirigid ball-valve prosthesis. Circulation 27, 779–783 (1963).
- Cribier A, Eltchaninoff H, Bash A et al.: Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106, 3006–3008 (2002).
- Grube E, Schuler G, Buellesfeld L et al.: Percutaneous aortic valve replacement for severe aortic stenosis in high risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30‑day clinical outcome. J. Am. Coll. Cardiol. 50, 69–76 (2007).
- Webb JG, Pasupati S, Humphries K et al.: Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116, 755–763 (2007).
- Ye J, Cheung A, Lichtenstein SV et al.: Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur. J. Cardiothorac. Surg. 31, 16–21 (2007).
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS: Isolated aortic valve replacement in North America comprising 108,687 patients: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 137, 82–90 (2009).
- Iung B, Cachier A, Baron G et al.: Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur. Heart J. 26, 2714–2720 (2005).
- Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J: Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J. Am. Coll. Cardiol. 23, 1162–1170 (1994).
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD: Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis: histological and immunohistochemical studies. Circulation 90, 844–853 (1994).
- O’Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM: Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 16, 523–532 (1996).
- O’Brien KD, Shavelle DM, Caulfield MT et al.: Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 106, 2224–2230 (2002).
- Wallby L, Janerot-Sjoberg B, Steffensen T, Broqvist M: T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart 88, 348–351 (2002).
- Bonow RO, Carabello BA, Chatterjee K et al.: American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. Circulation 118, E523–E661 (2008).
- Krayenbuehl HP, Hess OM, Ritter M, Monrad ES, Hoppeler H: Left ventricular systolic function in aortic stenosis. Eur. Heart J. 9 (Suppl. E), 19–23 (1988).
- Ross J Jr: Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog. Cardiovasc. Dis. 18, 255–264 (1976).
- Gunther S, Grossman W: Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation 59, 679–688 (1979).
- Huber D, Grimm J, Koch R, Krayenbuehl HP: Determinants of ejection performance in aortic stenosis. Circulation 64, 126–134 (1981).
- Stott DK, Marpole DG, Bristow JD, Kloster FE, Griswold HE: The role of left atrial transport in aortic and mitral stenosis. Circulation 41, 1031–1041 (1970).
- Rosenhek R, Klaar U, Schemper M et al.: Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur. Heart J. 25, 199–205 (2004).
- Faggiano P, Aurigemma GP, Rusconi C, Gaasch WH: Progression of valvular aortic stenosis in adults: literature review and clinical implications. Am. Heart J. 132, 408–417 (1996).
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP: Spontaneous course of aortic valve disease. Eur. Heart J. 8, 471–483 (1987).
- Horstkotte D, Loogen F: The natural history of aortic valve stenosis. Eur. Heart J. 9(Suppl. E), 57–64 (1988).
- Iivanainen AM, Lindroos M, Tilvis R, Heikkila J, Kupari M: Natural history of aortic valve stenosis of varying severity in the elderly. Am. J. Cardiol. 78, 97–101 (1996).
- Otto CM, Burwash IG, Legget ME et al.: Prospective study of asymptomatic valvular aortic stenosis: clinical, echocardiographic, and exercise predictors of outcome. Circulation 95, 2262–2270 (1997).
- Rosenhek R, Binder T, Porenta G et al.: Predictors of outcome in severe, asymptomatic aortic stenosis. N. Engl. J. Med. 343, 611–617 (2000).
- Pellikka PA, Sarano ME, Nishimura RA et al.: Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 111, 3290–3295 (2005).
- Amato MC, Moffa PJ, Werner KE, Ramires JA: Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 86, 381–386 (2001).
- Thourani VH, Myung R, Kilgo P et al.: Long-term outcomes after isolated aortic valve replacement in octogenarians: a modern perspective. Ann. Thorac. Surg. 86, 1458–1465 (2008).
- Sundt TM, Bailley MS, Moon MR et al.: Quality of life aortic valve replacement at the age of >80 years. Circulation 102(Suppl. 3), III70–III74 (2000).
- Fruitman DS, MacDougall CE, Ross DB: Cardiac surgery in octogenarians: can elderly patients benefit? Quality of life after cardiac surgery. Ann. Thorac. Surg. 68, 2129–2135 (1999).
- Huber CH, Goeber V, Berdat P, Carrel T, Eckstein F: Benefits of cardiac surgery in octogenarians – a post-operative quality of life assessment. Eur. J. Cardiothorac. Surg. 31, 1099–1105 (2007).
- Cleland JG, Swedberg K, Follath F et al.: ‘The EuroHeart Failure Survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 1: Patient characteristics and diagnosis. Eur. Heart. J. 24, 442–463 (2003).
- Ross J Jr, Braunwald E: Aortic stenosis. Circulation 38(Suppl. 1), 61–67 (1968).
- Bramstedt KA: Aortic valve replacement in the elderly: frequently indicated yet frequently denied. Gerontology 49, 46–49 (2003).
- Grossi EA, Schwartz CF, Yu PJ et al.: High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann. Thorac. Surg. 85, 102–107 (2008).
- Nashel SAM, Michel P, Gauducheau E, Lemeshow S, Salamon R; the EuroScore Study Group: European System for Cardiac Operative Risk Evaluation (EuroScore). Eur. J. Cardiothorac. Surg. 16, 9–13 (1999).
- Bernstein AD: Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann. Thorac. Surg. 69, 823–828 (2000).
- Grube E, Schuler G, Buellesfeld L et al.: Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30‑day clinical outcome. J. Am. Coll. Cardiol. 50, 69–76 (2007).
- Osswald BR, Badowski-Zyla D, Tochtermann U, Thomas G, Hagl S, Blackstone EH: Overestimation of aortic replacement risk by EuroSCORE: implications for percutaneous valve replacement. Eur. Heart. J. 30, 74–80 (2009).
- Jin R: Does the logistic EuroSCORE offer an advantage over the additive model? Interact. Cardiovasc. Thorac. Surg. 5, 15–17 (2006).
- Dewey TD, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ: Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J. Thorac. Cardiovasc. Surg. 135, 180–187 (2008).
- Cribier A, Eltchaninoff H, Tron C et al.: Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J. Am. Coll. Cardiol. 47, 1214–1223 (2006).
- Webb JG, Pasupati S, Humphries K et al.: Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116, 755–763 (2007).
- Berry C, Lamarche Y, Marcheix B et al.: Novel therapeutic aspects of percutaneous aortic valve replacement with the 21F CoreValve Revalving System. Cathet. Cardiovasc. Interv. 70, 610–616 (2007).
- Walther T, Falk V, Borger MA et al.: Minimally invasive transapical beating heart aortic valve implantation – proof of a concept. Eur. J. Cardiothorac. Surg. 31, 9–15 (2007).
- Litzler PY, Cribier A, Zajarias A et al.: Surgical aortic valve replacement after percutaneous aortic valve implantation: what have we learned? J. Cardiovasc. Thorac. Surg. 136, 697–701 (2008).
- Clavel MA, Dumont E, Pibarot P et al.: Severe valvular regurgitation and late prosthesis embolization after percutaneous aortic valve implantation. Ann. Thorac. Surg. 87, 618–621 (2009).
- Susheel Kodali: Pooled analysis with extended follow-up from REVIVE II and RIVIVAL II trans-femoral feasibility Registries. Presented at: The TCT Meeting. Washington DC, USA 12–17 October 2008.
- Jean Claude Laborde: Transcatheter AVR: Self-expanding valve systems. Presented at : ACC-i2 SUMMIT Aortic Stenosis Symposium, The American College of Cardiology American College of Cardiology (ACC) 58th Annual Scientific Session. Orlando, FL, USA, 29–31 March 2009.
- Webb JG, Altwegg L, Boone RH et al.: Transcatheter aortic valve implantation impact on clinical and valve-related outcomes. Circulation 119, 3009–3016 (2009).
- Webb JG, Altwegg L, Masson JB, Al Bugami S, Al Ali A, Boone RA: A new transcatheter aortic valve and percutaneous valve delivery system. J. Am. Coll. Cardiol. 53, 1855–1858 (2009).
- Chiam PTL, Ruiz CE: Percutaneous transcatheter aortic valve implantation: evolution of the technology. Am. Heart. J. 157, 229–242 (2009).
- Clavel MA, Webb JG, Pibarot P et al.: Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J. Am. Coll. Cardiol. 53, 1883–1891 (2009).
- Borger MA, Prasongsukarn K, Armstrong S, Feindel CM, David TE: Stentless aortic valve reoperations: a surgical challenge. Ann. Thorac. Surg. 84(3), 737–743 (2007).
- Van Nooten G, Caes F, Francois K, Van Belleghem Y, Taeymans Y: Stentless or stented aortic valve implants in elderly patients? Eur. J. Cardiothorac. Surg. 15, 31–36 (1999).
- Lytle BW, Cosgrove DM, Taylor PC et al.: Primary isolated aortic valve replacement: early and late results. J. Thorac. Cardiovasc. Surg. 97, 675–694 (1989).
- Chiappini B, Camurri N, Loforte A, Di Marco L, Di Bartolomeo R, Marinelli G: Outcome after aortic valve replacement in octogenarians. Ann. Thorac. Surg. 78, 85–89 (2004).
- Puvimanasinghe JP, Takkenberg JJ, Eijkemans MJ et al.: Comparison of Carpentier- Edwards pericardial and supraannular bioprosthesis in aortic valve replacement. Eur. J. Cardiothorac. Surg. 29, 374–379 (2006).
▪ First report of human implantation of a transcathether balloon-expandable aortic prosthesis.
▪▪ Extensive review of single aortic valve replacement procedure in North America during the current era.
▪ Latest current guidelines used for surgical indication of aortic valve replacement (AVR).
▪ Current experience with the use of the EuroScore scoring system in patient selection for percutaneous aortic valve implantation (PAVI).
▪▪ Use of conventional risk-assessment model for predicting survival after conventional AVR in high-risk patients.
▪ Specific complications related to PAVI using an antegrade approach.
▪ Peripheral vascular complications related to PAVI using a retrograde approach.
▪▪ Recent experience with Sapiens Edwards transcatheter valve showing a decrease in procedural mortality and morbidity.
▪ Survey of the current technologic development in transcatheter AVR.
▪▪ Interesting study comparing hemodynamic performance of conventional and transcatheter AVR showing better performance in small aortic annulus.
▪ Websites
- US Census Bureau: US Interim Projections by Age, Sex, Race and Hispanic Origin: 2000–2050 (2004). www.census.gov/ipc/www/usinterimproj (Accessed October 2007)
- ClinicalTrials.gov. A service of the US NIH. http://clinicaltrials.gov/ct2/show/NCT00530 894?term=transcatheter&rank=7