Perspective - Interventional Cardiology (2014) Volume 6, Issue 3
How transcatheter aortic valve implantation can revive balloon aortic valvuloplasty
- Corresponding Author:
- Marco De Carlo
Cardiac Catheterization Laboratory, Cardiothoracic Department
Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
Tel: +39 050 995 330
Fax: +39 050 995 325
E-mail: marcodecarlo@gmail.com
Abstract
Percutaneous balloon aortic valvuloplasty (PBAV) was first described as a new therapeutic option in patients with aortic stenosis who could not undergo surgical aortic valve replacement (SAVR) in a pediatric population in 1984 by Lababidi, and in elderly adults in 1986 by Cribier et al. This treatment was found to be an interesting option in high-risk patients with hemodynamic instability, showing good procedural and short-term results, yielding improvement of symptoms, amelioration of global clinical status and improvement of echocardiographic parameters.Keywords
aortic stenosis, balloon valvuloplasty, noncardiac surgery, transcatheter aortic valve implantation
Percutaneous balloon aortic valvuloplasty (PBAV) was first described as a new therapeutic option in patients with aortic stenosis who could not undergo surgical aortic valve replacement (SAVR) in a pediatric population in 1984 by Lababidi [1], and in elderly adults in 1986 by Cribier et al. [2]. This treatment was found to be an interesting option in high-risk patients with hemodynamic instability, showing good procedural and short-term results, yielding improvement of symptoms, amelioration of global clinical status and improvement of echocardiographic parameters [3,4].
However, although favorable initial results were described and procedural and technical advancements were achieved, medium- and long-term results were disappointing due to the high rate of restenosis observed across all kinds of etiology of aortic stenosis [3,5–9]. In particular, in the case of degenerative aortic stenosis, PBAV causes gross fracture of calcific nodules in the cusps of the valve, which allows for increased leaflet motility and excursion, and stretching of the aortic annulus that transiently increases valve area.
However, leaflet recalcification and annulus recoil almost invariably lead to aortic valve restenosis [10–13].
The two largest registries on PBAV described a high rate of acute success, but a poor 1- and 2-year prognosis [3,6]. The National Heart, Lung, and Blood Institute registry included 674 patients, mostly considered inoperable; at 30 days after PBAV, an improvement of at least one New York Heart Association (NYHA) class was observed in 75% of cases, but only 11% of the patients were alive and in NYHA class I or II at 2-year follow-up [6]. The Mansfield registry described a similar clinical outcome: a medium-term follow-up in a similar population, with 65% survival rate at 1 year [3] and 23% at 3 years [7]. Moreover, a dismal prognosis after PBAV was confirmed by Lieberman and coworkers at 6 years follow-up, without differences with respect to the natural history of aortic stenosis, confining PBAV as a palliative treatment [14]. In addition, a high rate of major procedural complications was uniformly reported, especially in the 1990s, further discouraging the use of PBAV [14,15].
Therefore, PBAV had very limited indications in clinical practice, as described in the guidelines on Valvular Heart Disease of the European Society of Cardiology [16], until transcatheter aortic valve implantation (TAVI) entered the clinical arena. In fact, TAVI not only reintroduced PBAV as a preliminary part of the procedure, but also allowed for the re-evaluation of the role of PBAV as a short-term treatment before TAVI.
PBAV as a bridge to SAVR or TAVI
Risk of SAVR increases with patient age and in the presence of left ventricular dysfunction. Temporarily relieving the severity of aortic stenosis may allow for an improvement in left ventricular function, thus reducing the operative risk for SAVR [17,18]. In fact, PBAV as an alternative to SAVR does not improve the clinical outcome of patients, while PBAV before SAVR can positively affect the operative risk, as demonstrated in a study by Lieberman and coworkers [14]. Among 165 patients undergoing PBAV, 42 subsequently underwent SAVR; the great disparity in 1-year survival between patients treated with PBAV and subsequent SAVR versus those treated with PBAV alone (95 vs 52%) demonstrated that PBAV as a bridge to SAVR was a much more effective treatment than isolated PBAV. Importantly, lower left ventricular ejection fraction (LVEF) was found to be an independent predictor of adverse prognosis at multivariable analysis. Similar results were described by Kapadia et al. in a series of 90 patients undergoing a total of 99 PBAV procedures [18]; of these, 33 presented with a severe cardiac condition contraindicating SAVR (cardiogenic shock, low LVEF, severe mitral regurgitation and severe pulmonary hypertension) and underwent PBAV as a tool to improve the clinical status and possibly as a bridge to SAVR [18]. In 27 cases, surgery was eventually performed after a successful PBAV. The 1-year survival was 78 versus 44% (p = 0.02) in patients undergoing SAVR versus standalone PBAV, respectively.
Over the last decade, the development of TAVI has opened a new therapeutic perspective for patients unsuitable or at high risk for SAVR, who could only be offered PBAV beforehand. Moreover, the need to perform PBAV during TAVI to allow an easier crossing of the aortic valve with the delivery catheter of the bioprosthesis has led to improvement of the materials used for PBAV and of the technical skill of the operators [19]. Nevertheless, a significant proportion of patients cannot undergo TAVI directly for three main reasons: hemodynamic instability (cardiogenic shock, acute pulmonary edema); temporary contraindication for TAVI or SAVR; and presence of a low-flow low-gradient aortic stenosis, with doubtful hemodynamic response to the relief of aortic stenosis. In the first group of patients, performing PBAV as a bridge to TAVI may improve the hemodynamic and clinical status and reduce the procedural risks of TAVI. In the second group of patients, PBAV may reduce the operative risk of an urgent noncardiac surgical intervention. In the third group of patients, PBAV may reveal which patients benefit from a relief of aortic stenosis severity and could undergo subsequent TAVI.
Recently, several authors have described interesting experiences of PBAV as a bridge to TAVI [20–25], leading to the inclusion of PBAV as a bridge to TAVI in the recommendations of the latest guidelines on the management of valvular heart disease of the European Society of Cardiology [26].
Ussia et al. described a single-center experience with 83 patients screened for TAVI, who underwent either direct TAVI (n = 40) or PBAV as a bridge to TAVI (n = 43), because of a high risk of procedural complications [20]. Although patients undergoing PBAV had worse baseline characteristics, including NYHA class, mean pressure gradient and aortic valve area, clinical, hemodynamic and echocardiographic results were similar between groups after TAVI was performed. However, the 30-day mortality was higher in the PBAV group, although the difference was not statistically significant (11.6 vs 2.5%; p = 0.20).
More recently, a few reports described retrospective analyses of larger cohorts of patients treated with PBAV, including a proportion of PBAV as a bridge to TAVI or SAVR ranging from 18 to 42% [23,27,28]. Patients undergoing PBAV as a bridge to a definitive treatment showed a baseline clinical profile similar to patients undergoing standalone PBAV; in all studies, PBAV was effective in improving the clinical status of the patients, allowing TAVI to be performed with a lower procedural risk.
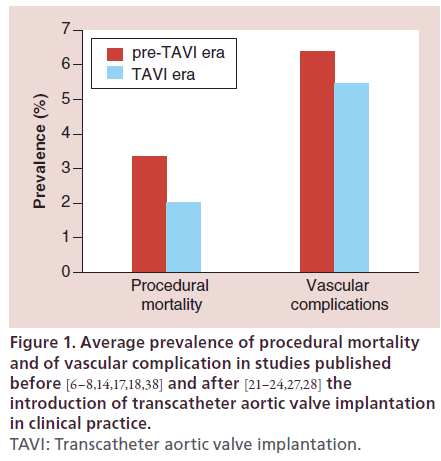
Importantly, the improvements in PBAV devices, operators’ skill and periprocedural care in recent years led to a reduction in the morbidity and mortality of standalone PBAV, so that the benefits of relieving aortic stenosis are not overwhelmed by the procedural risk. In fact, in the PARTNER trial patients randomized to the conservative arm, who underwent PBAV in over 80% of the cases, showed a similar 30-day outcome compared with the TAVI group, while the outcome significantly diverged at 1 year [29].
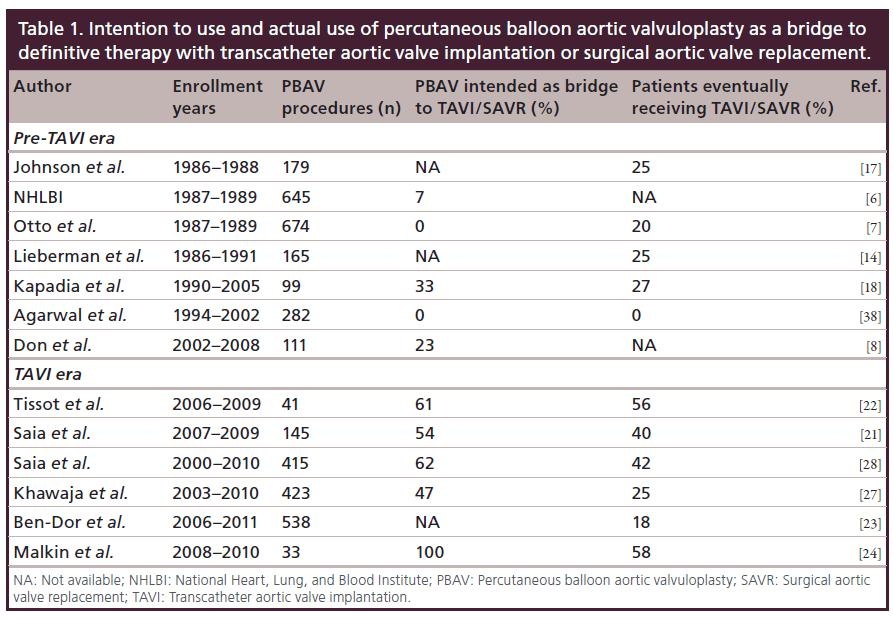
In Table 1, we report the percentage of patients undergoing PBAV as a bridge to SAVR or TAVI as initial intention to treat, and the percentage of patients who actually underwent SAVR or TAVI following PBAV in various papers published before and after the TAVI revolution (Table 1). Interestingly, at the very beginning of PBAV history, PBAV was seldom conceived as a bridge to a definitive treatment, as PBAV itself carried a relevant procedural mortality and morbidity, which would have been added to those of SAVR in case of a staged approach. Nevertheless, approximately a quarter of the patients treated with PBAV eventually underwent SAVR, demonstrating that PBAV was effective in improving the clinical status of patients enough to make the risk of SAVR acceptable. In the 2000s, the procedural risks of PBAV were progressively reduced, allowing for a wider use of PBAV as a tool to improve the clinical status of patients before performing SAVR (Table 1 & Figure 1). With the advent of TAVI, the majority of patients undergoing PBAV (50 to 100%) were treated in view of a possible subsequent TAVI, to be performed if PBAV entailed an improvement in the clinical status. As the procedural morbidity of TAVI is lower than that of SAVR in high-risk patients, the proportion of patients who can eventually undergo a definitive treatment has risen from 25 to over 50% in the TAVI era (Table 1). Nevertheless, a not negligible proportion of patients undergoing PBAV with the intention to perform a subsequent TAVI, still do no undergo TAVI because of the lack of clinical benefit after PBAV. Importantly, the prevalent use of PBAV as a bridge to TAVI currently allows for a less aggressive and less risky approach during PBAV. In fact, as PBAV is not regarded as a standalone treatment, it is reasonable to accept a suboptimal reduction in valvular gradient in order to minimize the risk of moderate or severe aortic insufficiency, with the entailed ominous shortterm outcome. Therefore, we usually perform PBAV as a bridge to TAVI with largely undersized balloons (2–3 mm smaller than the average aortic annulus diameter), preferring multiple inflations with the same balloon over progressive increase in balloon diameter. Halving the transaortic pressure gradient and/or reducing it below 20 mmHg is considered an acceptable result, as it can be achieved without disrupting the native valve in almost all patients.
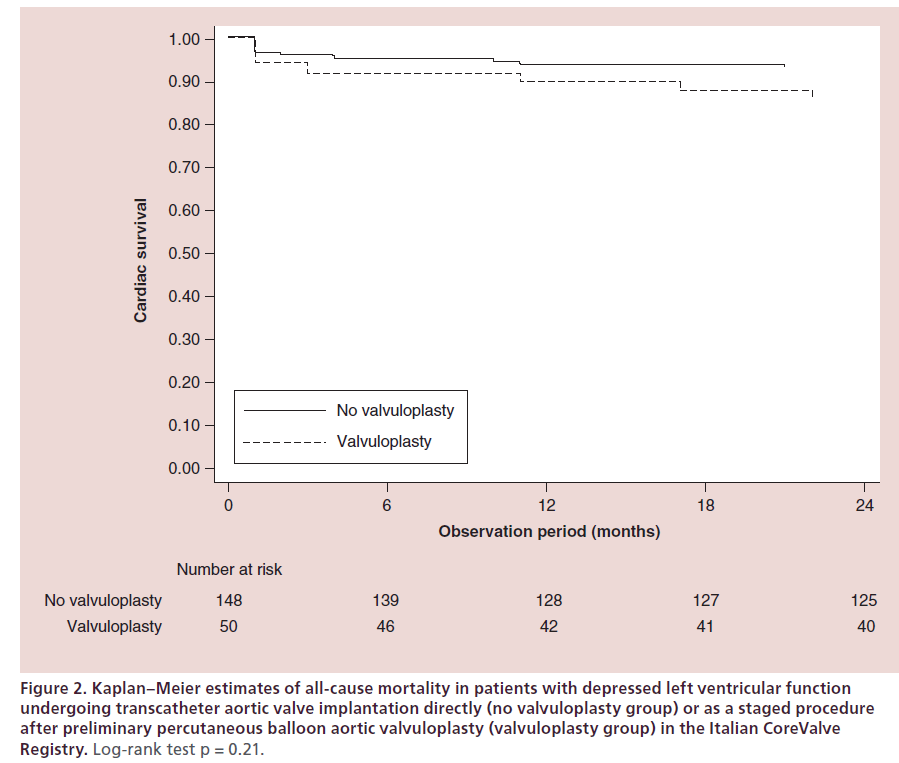
Patients with a very depressed LVEF are those who may benefit most from PBAV, but they are often denied this treatment because of the higher procedural risk and because of their dismal prognosis. However, Pedersen and coworkers recently demonstrated in a small population of 16 patients with LVEF ≤20%, that PBAV can be performed with no procedural mortality and with an improvement in LVEF in 47% of the cases [30]. The feasibility of PBAV as a bridge to TAVI in patients with left ventricular dysfunction has been confirmed in a subgroup analysis of the multicenter Italian CoreValve registry, presented at the EuroPCR 2013 congress [31]. The 50 patients with LVEF <35% who underwent staged PBAV before TAVI showed similar procedural 30-day and 2-year outcomes when compared with 148 patients with LVEF <35% who underwent TAVI directly. In particular, the actuarial freedom from cardiac death was 83.7 ± 6.0% versus 87.4 ± 5.1% (p = 0.21) (Figure 2). Actually, patients undergoing staged PBAV had a numerically higher incidence of events compared with patients undergoing direct TAVI (in-hospital mortality: 10 vs 6%, p = 0.36; 30-day safety composite end point: 70 vs 59%, p = 0.19; 2-year clinical efficacy composite end point: 26 vs 19%, p = 0.31; 2-year all-cause mortality: 24 vs 15%, p = 0.20), although they presented a significantly worse baseline clinical profile, in terms of older age, lower LVEF, worse mitral regurgitation and higher logistic Euroscore.
Figure 2: Kaplan–Meier estimates of all-cause mortality in patients with depressed left ventricular function undergoing transcatheter aortic valve implantation directly (no valvuloplasty group) or as a staged procedure after preliminary percutaneous balloon aortic valvuloplasty (valvuloplasty group) in the Italian CoreValve Registry. Log-rank test p = 0.21.
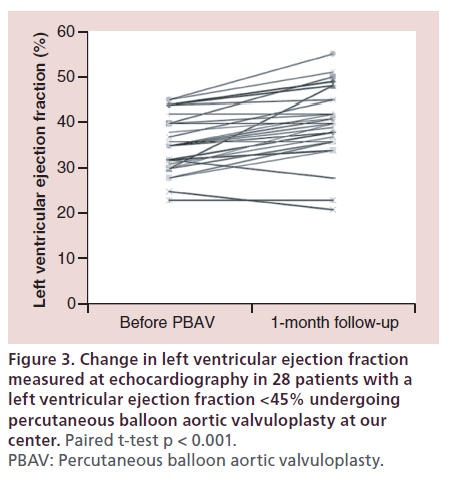
In our center, in the last 3 years we performed PBAV in 30 patients with a LVEF <45% who had dubious indication or contraindications to TAVI; the 28 patients who were discharged alive showed a significant improvement in LVEF from 35 ± 6 to 40 ± 8% (p < 0.001) (Figure 3) [De Carlo M et al.; Unpublished Data]. Importantly, 54% of the patients improved their LVEF of at least 5% after successful PBAV. Similar results were recently described in 16 patients with LVEF <45% by Dworakowski et al., who described an increase in LVEF from 27 ± 2 to 30 ± 3% (p < 0.05) associated with symptomatic improvement [32].
Figure 3: Change in left ventricular ejection fraction measured at echocardiography in 28 patients with a left ventricular ejection fraction <45% undergoing percutaneous balloon aortic valvuloplasty at our center. Paired t-test p < 0.001. PBAV: Percutaneous balloon aortic valvuloplasty.
PBAV as a diagnostic tool
Following the success of TAVI as an alternative to SAVR in high-risk and inoperable patients, an increasing number of patients are currently referred for TAVI without a clear indication because of the presence of confounding factors including: uncertainty regarding the severity of aortic stenosis; very depressed LVEF with mixed response to dobutamine test (presence of hibernating myocardium); severe comorbidities limiting life expectancy; severe pulmonary hypertension, or chronic obstructive pulmonary disease, or severe mitral regurgitation contributing to the patient’s symptoms; and hemodynamic instability, or acute coronary syndrome requiring emergent or urgent intervention. All these circumstances make the clinical benefit of TAVI unpredictable and represent a relative contraindication to TAVI.
The reduction in the procedural risks of PBAV makes it possible to perform this palliative intervention as a ‘diagnostic tool’ in order to verify what is the effect of relieving the severity of aortic stenosis on the patient’s symptoms. Therefore, a definitive treatment with TAVI (or SAVR) can be offered to those patients who can really benefit from the intervention, while sparing the risks of TAVI to the others. In our experience, most patients who respond to PBAV usually manifest a clinical benefit within 1 month after PBAV, allowing for a quick decision regarding TAVI. However, it must be emphasized that PBAV sometimes provides only a marginal increase in valve area; therefore, while a positive clinical response to PBAV is encouraging, a lack of response does not necessarily mean that aortic stenosis is not a contributor to the patient’s functional decline.
In the retrospective study by Saia and coworkers on 415 patients, PBAV was not associated with a clinical improvement at 30 days after the procedure in 21% of the cases, and these patients were assigned to medical therapy [28]. Similarly, Malkin et al. recently reported a 36% rate of lack of benefit in a smaller population of 33 patients undergoing PBAV as a bridge to TAVI [24]. Interestingly, procedural success was 100% and procedural mortality was zero, demonstrating the safety of this approach to PBAV as a ‘screening’ tool before TAVI.
The presence of severe mitral regurgitation in patients with severe aortic stenosis is not infrequent and complicates the decision-making process. In fact, severe mitral regurgitation per se is associated with poor short-term prognosis if not treated, and is generally regarded as a relative contraindication to TAVI. However, mitral regurgitation in patients with severe aortic stenosis may be secondary to progressive dilatation of the left ventricle and may be partially reversible if a favorable left ventricular remodeling is obtained by correcting aortic stenosis. It is therefore crucial to distinguish functional mitral regurgitation from degenerative mitral regurgitation, as the first may not contraindicate TAVI and, on the contrary, may improve to some extent after TAVI. Interestingly, PBAV may be useful to ascertain the origin of mitral regurgitation in these patients, as it has been proven to reduce functional mitral regurgitation and pulmonary hypertension [33].
In the Italian CoreValve Registry, we demonstrated that the degree of mitral regurgitation improved at 1 year follow-up in 47% of patients with severe regurgitation before TAVI and in 35% of patients with moderate regurgitation, and that the functional etiology was an independent predictor of improvement [34]. Therefore, the use of ‘diagnostic’ PBAV can help to identify those patients with combined aortic stenosis and mitral regurgitation who are more likely to benefit from TAVI.
PBAV before noncardiac surgery
Patients with valvular heart disease are at higher risk of perioperative cardiovascular complications during noncardiac surgery [16,35]. Aortic stenosis is the most common valvular heart disease in Europe, and with the increase in life expectancy, a growing number of elderly patients develop severe aortic stenosis associated with various noncardiac comorbidities, which may require noncardiac surgery. The presence of aortic stenosis represents a well-established risk factor for perioperative mortality and nonfatal myocardial infarction, and its severity is predictive of these complications [36]. Therefore, according to the guidelines on the management of valvular heart disease of the European Society of Cardiology, preoperative PBAV may be considered for patients with severe aortic stenosis who need major noncardiac surgery, although the level of evidence for this recommendation is low (class IIb recommendation, level of evidence C) [26]. In fact, very little evidence is available regarding the comparison of different therapeutic options in these patients [37]. In our opinion, PBAV may represent a reasonable option in elderly patients with aortic stenosis requiring major noncardiac surgery because of cancer disease, when their life expectancy is not defined and TAVI is not indicated. In order to verify the potential benefits of this strategy, we began to prospectively collect the data on the patients undergoing PBAV at our center before major noncardiac surgery (oncologic surgery in 70% of the cases, major vascular surgery in 30%). The 13 patients treated in 2013 had a mean age of 82 years and an average logistic Euroscore of 34%, procedural success (reduction of mean aortic gradient by at least 50%) was 100% and all patients underwent noncardiac surgery without cardiac complications. We re-assessed the indication to definitive intervention by TAVI or SAVR, 1 or 2 months after noncardiac surgery, when the overall prognosis could be redefined.
Conclusion
Until recently, percutaneous PBAV was conceived as a palliative treatment for patients with severe aortic stenosis with contraindications to SAVR, and its use was quite limited and declining, because its procedural risks were not balanced by a lasting improvement in clinical conditions. The advent of TAVI has had a deep impact on PBAV: on one hand, the need for PBAV during TAVI has led to improvements in devices and operators’ skill; on the other, the lower invasiveness and procedural risks of TAVI have made it possible to perform TAVI in very high-risk patients if they show a clinical benefit from transient relief of aortic stenosis with PBAV. This is how TAVI revived its ‘grandmother’, plain old balloon valvuloplasty.
Future perspective
The future of PBAV is strictly related to the future of TAVI, as TAVI indications will be extended and standalone PBAV will become less and less frequent. The most important indication to PBAV will be the need to ascertain the potential benefit of relief of aortic stenosis in patients with low-gradient aortic stenosis and in patients with comorbidities that make the contribution of aortic stenosis to the functional decline unclear, as the progressive reduction in the procedural risks of TAVI will make it feasible directly even in patients with unstable hemodynamic conditions. Further improvements in device technology will reduce the morbidity and mortality of PBAV.
Executive summary
Background
• Until transcatheter aortic valve implantation (TAVI) entered the clinical arena, percutaneous balloon aortic valvuloplasty (PBAV) had very limited indications because of its procedural complications and high rate of short-term restenosis. TAVI led to improvements in PBAV devices, operators’ skill and periprocedural care.
PBAV as bridge to TAVI or surgical aortic valve replacement
• In the past, PBAV was seldom used as a bridge to surgical aortic valve replacement in elderly patients with depressed left ventricular function. In the TAVI era, high-surgical risk patients are usually referred for TAVI; however, a significant proportion of patients have contraindications to perform TAVI directly.
• Patients presenting with hemodynamic instability may undergo PBAV as a bridge to TAVI, in order to improve the hemodynamic and clinical status and reduce the procedural risks of TAVI.
• Patients with a low-flow low-gradient aortic stenosis, which has a doubtful hemodynamic response to the relief of aortic stenosis, may also undergo PBAV before TAVI in order to reveal who may actually benefit from a stable decrease in the aortic gradient.
• Finally, patients needing urgent major noncardiac surgery may undergo PBAV as a bridge to TAVI or surgical aortic valve replacement in order to reduce the risk of cardiac complications during noncardiac surgery.
• PBAV as a bridge to TAVI has been included in the recommendations of the latest Guidelines on the management of valvular heart disease of the European Society of Cardiology.
PBAV as a diagnostic tool
• Following the success of TAVI, an increasing number of patients are currently referred for TAVI without a clear indication because of the presence of confounding factors.
• The reduction in the procedural risks of PBAV makes it possible to perform this palliative intervention as a ‘diagnostic tool’ in order to verify what is the effect of relieving the severity of aortic stenosis on the patient’s symptoms.
• It is crucial to distinguish functional mitral regurgitation from degenerative mitral regurgitation, as the first may not contraindicate TAVI and, on the contrary, may improve to some extent after TAVI. Interestingly, PBAV may be useful to ascertain the origin of mitral regurgitation in these patients, as it has been proven to reduce functional mitral regurgitation and pulmonary hypertension.
PBAV before noncardiac surgery
• The presence of aortic stenosis represents a well-established risk factor for perioperative mortality and nonfatal myocardial infarction, and its severity is predictive of these complications.
• Preoperative PBAV may be considered for patients with severe aortic stenosis who need major noncardiac surgery, as well as for elderly patients requiring major noncardiac surgery because of cancer disease, when their life expectancy is not yet defined.
Conclusion
• The need for PBAV during TAVI has led to improvements in devices and operators’ skill, thus reducing the procedural morbidity and mortality.
• TAVI is currently feasible in extremely high-risk patients if they show a clinical benefit from transient relief of aortic stenosis with PBAV.
• As the progressive reduction in the procedural risks of TAVI will make it feasible directly even in patients with unstable hemodynamic conditions, in the future PBAV will probably be used mainly to ascertain the benefit of relieving aortic stenosis in patients with low-gradient aortic stenosis.
Financial & competing interests disclosure
AS Petronio is clinical proctor for Medtronic Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Lababidi Z, Wu JR, Walls JT. Percutaneous balloon aortic valvuloplasty: results in 23 patients. Am. J. Cardiol. 53(1), 194–197 (1984).
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1(8472), 63–67 (1986).
- McKay RG. The Mansfield Scientific Aortic Valvuloplasty Registry: overview of acute hemodynamic results and procedural complications. J. Am. Coll. Cardiol. 17(2), 485–491 (1991).
- Eltchaninoff H, Cribier A, Tron C et al. Balloon aortic valvuloplasty in elderly patients at high risk for surgery, or inoperable. Immediate and mid-term results. Eur. Heart J. 16(8), 1079–1084 (1995).
- Robicsek F, Harbold NB Jr, Daugherty HK et al. Balloon valvuloplasty in calcified aortic stenosis: a cause for caution and alarm. Ann. Thorac. Surg. 45(5), 515–525 (1988).
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 84(6), 2383–2397 (1991).
- Otto CM, Mickel MC, Kennedy JW et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 89(2), 642–650 (1994).
- Don CW, Witzke C, Cubeddu RJ et al. Comparison of procedural and in-hospital outcomes of percutaneous balloon aortic valvuloplasty in patients >80 years versus patients < or =80 years. Am. J. Cardiol. 105(12), 1815–1820 (2010).
- Ben-Dor I, Pichard AD, Satler LF et al. Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC Cardiovasc. Interv. 3(11), 1150–1156 (2010).
- McKay RG, Safian RD, Lock JE et al. Balloon dilatation of calcific aortic stenosis in elderly patients: postmortem, intraoperative, and percutaneous valvuloplasty studies. Circulation 74(1), 119–125 (1986).
- Safian RD, Mandell VS, Thurer RE et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J. Am. Coll. Cardiol. 9(3), 655–660 (1987).
- Waller BF, McKay C, VanTassel JW, Taliercio C, Howard J, Green F. Catheter balloon valvuloplasty of stenotic aortic valves. Part I: anatomic basis and mechanisms of balloon dilation. Clin. Cardiol. 14(10), 836–846 (1991).
- Waller BF, Dorros G, Lewin RF, King JF, McKay C, van Tassel JW. Catheter balloon valvuloplasty of stenotic aortic valves. Part II: balloon valvuloplasty during life subsequent tissue examination. Clin. Cardiol. 14(11), 924–930 (1991).
- Lieberman EB, Bashore TM, Hermiller JB et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve longterm survival. J. Am. Coll. Cardiol. 26(6), 1522–1528 (1995).
- Isner JM. Acute catastrophic complications of balloon aortic valvuloplasty. The Mansfield Scientific Aortic Valvuloplasty Registry Investigators. J. Am. Coll. Cardiol. 17(6), 1436–1444 (1991).
- Vahanian A, Baumgartner H, Bax J et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur. Heart J. 28(2), 230–268 (2007).
- Johnson RG, Dhillon JS, Thurer RL, Safian RD, Weintraub RM. Aortic valve operation after percutaneous aortic balloon valvuloplasty. Ann. Thorac. Surg. 49(5), 740–744; discussion 744–745 (1990).
- Kapadia SR, Goel SS, Yuksel U et al. Lessons learned from balloon aortic valvuloplasty experience from the pretranscatheter aortic valve implantation era. J. Interv. Cardiol. 23(5), 499–508 (2010).
- Sack S, Kahlert P, Khandanpour S et al. Revival of an old method with new techniques: balloon aortic valvuloplasty of the calcified aortic stenosis in the elderly. Clin. Res. Cardiol. 97(5), 288–297 (2008).
- Ussia GP, Capodanno D, Barbanti M et al. Balloon aortic valvuloplasty for severe aortic stenosis as a bridge to high-risk transcatheter aortic valve implantation. J. Invasive Cardiol. 22(4), 161–166 (2010).
- Saia F, Marrozzini C, Moretti C et al. The role of percutaneous balloon aortic valvuloplasty as a bridge for transcatheter aortic valve implantation. EuroIntervention 7(6), 723–729 (2011).
- Tissot CM, Attias D, Himbert D et al. Reappraisal of percutaneous aortic balloon valvuloplasty as a preliminary treatment strategy in the transcatheter aortic valve implantation era. EuroIntervention 7(1), 49–56 (2011).
- Ben-Dor I, Maluenda G, Dvir D et al. Balloon aortic valvuloplasty for severe aortic stenosis as a bridge to transcatheter/surgical aortic valve replacement. Catheter Cardiovasc. Interv. 82(4), 632–637 (2013).
- Malkin CJ, Judd J, Chew DP, Sinhal A. Balloon aortic valvuloplasty to bridge and triage patients in the era of trans-catheter aortic valve implantation. Catheter Cardiovasc. Interv. 81(2), 358–363 (2013).
- Daly MJ, Monaghan M, Hamilton A et al. Short-term efficacy of palliative balloon aortic valvuloplasty in selected patients with high operative risk. J. Invasive Cardiol. 24(2), 58–62 (2012).
- Vahanian A, Alfieri O, Andreotti F et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 42(4), S1–S44 (2012).
- Khawaja MZ, Sohal M, Valli H et al. Standalone balloon aortic valvuloplasty: indications and outcomes from the UK in the transcatheter valve era. Catheter Cardiovasc. Interv. 81(2), 366–373 (2013).
- Saia F, Marrozzini C, Ciuca C et al. Emerging indications, in-hospital and long-term outcome of balloon aortic valvuloplasty in the transcatheter aortic valve implantation era. EuroIntervention 8(12), 1388–1397 (2013).
- Leon MB, Smith CR, Mack M et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Pedersen WR, Goldenberg IF, Pedersen CW et al. Balloon aortic valvuloplasty in high risk aortic stenosis patients with left ventricular ejection fractions <20%. Catheter Cardiovasc. Interv. doi:10.1002/ccd.25328 (2013) (Epub ahead of print).
- De Carlo M, De Caro F, Fiorina C et al. Staged valvuloplasty before TAVI in patients with left ventricular dysfunction: insights from the Italian CoreValve registry. Eurointervention, EuroPCR Abstracts 330 (2013). www.pcronline.com/eurointervention/Abstracts2013_ issue/330
- Dworakowski R, Bhan A, Brickham B, Monaghan M, MacCarthy P. Effectiveness of balloon aortic valvuloplasty is greater in patients with impaired left ventricular function. Int. J. Cardiol. 150(1), 103–105 (2011).
- Maluenda G, Ben-Dor I, Laynez-Carnicero A et al. Changes in mitral regurgitation after balloon aortic valvuloplasty. Am. J. Cardiol. 108(12), 1777–1782 (2011).
- Bedogni F, Latib A, De Marco F et al. Interplay between mitral regurgitation and transcatheter aortic valve replacement with the CoreValve Revalving System: a multicenter registry. Circulation 128(19), 2145–2153 (2013).
- Fleisher LA, Beckman JA, Brown KA et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. Circulation 113(22), 2662–2674 (2006).
- Kertai MD, Bountioukos M, Boersma E et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am. J. Med. 116(1), 8–13 (2004).
- Hamid T, Eichhöfer J, Clarke B, Mahadevan VS. Aortic balloon valvuloplasty: is there still a role in high-risk patients in the era of percutaneous aortic valve replacement? J. Interv. Cardiol. 23(4), 358–361 (2010).
- Agarwal A, Kini AS, Attanti S et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am. J. Cardiol. 95(1), 43–47 (2005).