Research Article - Neuropsychiatry (2016) Volume 6, Issue 6
Human neuropsin gene and social cognition in depression
- Corresponding Author:
- Monika Talarowska
Department of Adult Psychiatry
Medical University of Lodz, Lodz, Poland
Aleksandrowska 159, 91-229, Lodz, Poland
Phone: 48 42 77-51-986
Fax: 48 42 640-50-58
Abstract
Depressive disorders are increasingly more often linked with social cognition deficits, mainly in the scope of detecting subtle nonverbal signals. These skills are particularly important because of the fact that adequate identification of nonverbal emotional expression is a fundamental condition for effective interpersonal communication. The aim of this study is to evaluate expression on the mRNA level for the human neuropsin gene (hNP) in the group of patients with diagnosed depression, treated for the first time and treated for many years due to recurrent episodes of the disease.
Keywords
Recurrent depressive disorder, Human neuropsin, Kallikrein, Social cognition
Background
Depressive disorders are increasingly more often linked with social cognition deficits, mainly in the scope of detecting subtle nonverbal signals [1,2]. These skills are particularly important because of the fact that adequate identification of nonverbal for effective interpersonal communication [3]. Social cognition can be defined as the mental operations that underlie social interactions, including perceiving, interpreting and generating responses to the intentions, dispositions and behaviours of others [4]. It is hypothesised that social cognition acts as a mediator between neurocognition and functional outcome [5].
Symptoms of depressive disorders are associated with dysfunctions in the region of frontal lobes and in temporal lobes (the amygdala, the hippocampus, the cingulate gyrus), which are engaged in cognitive control and affective regulation [6,7]. Social cognition deficits in the group of people suffering from depression have been described in scientific papers published in recent years [8,9]; they are considered the core features of major depressive disorders. They are apparent during acute episodes of the disorders, endure when patients are in remission and have a significant negative impact on patients’ psychosocial outcomes [3].
Neuropsin (NP, kallikrein 8, KLK8), a kallikrein gene-related (KLK) endoprotease, belongs to serine proteases that are involved in extracellular proteolysis [10] and plays an important role in neuroplasticity processes [11]. Enzymatically active neuropsin is necessary to establish the early phase of long-term potentiation (E-LTP, early LTP), while neuropsin deficiency completely impairs the early phase of LTP, leading to the absence of late associativity [12]. Neuropsin is mainly engaged in the early stage of LTP and in the process of synaptogenesis [11], while its deficiency reduces the seizure threshold. Decreased expression of hNP has been confirmed not only in association with deteriorated cognitive functioning, but also in the people affected by brain dysfunctions (among others suffering from multiple sclerosis, SM) [13,14]. Increased expression of human neuropsin (hNP) in the brain is observed in the regions that are engaged in the course of rDD: the hippocampus, the amygdala, the olfactory bulb and the prefrontal cortex [15].
The aim of this study is to evaluate expression on the mRNA level for the human neuropsin gene (hNP) in the group of patients with diagnosed depression, treated for the first time and treated for many years due to recurrent episodes of the disease (i). An attempt has also been made to link hNP expression with the ability of the examined subjects to use nonverbal communication in social interactions (ii) as well as with disease exacerbation.
Material and Methods
▪ Material
208 individuals, aged 18-67 (M=47.64, SD=11.16), meeting the diagnostic criteria for a depressive episode and recurrent depressive disorders (F32.0-7.32.2, F33.0-F33.8), were qualified to participate in the study [16,17]. All the subjects were examined during their hospitalization and no symptoms of concurrent somatic diseases or axis I and II disorders other than depressive episodes were diagnosed. Inflammatory or autoimmune disorders, central nervous system traumas and unwillingness to give informed consent were additional exclusion criteria. Prior to the start of the experiment, a case history was obtained from each patient using the standardized Composite International Diagnostic Interview (CIDI) [18].
The examined individuals were divided into two groups: the patients with a diagnosis of the first depressive episode (ED-I, N=72) and the patients treated due to recurrent episodes of the disease (rDE, N=136). No statistical differences were observed in terms of age (Z= 0.117, p=0.91), level of education (X2=2.44, p=0.71) or gender (X2=3.26, p=0.48) in the examined group.
▪ Methods
Depression severity was evaluated and classified using the 21-item Hamilton Depression Rating Scale (HDRS) [19]. Intensity levels of depressive symptoms were measured with the use of the scores presented in the study conducted by Demyttenaere and De Fruyt [20]. Each patient was examined by the same specialist (the same psychiatrist performed the HDRS test, while the psychological tests were conducted by a clinical psychologist).
The following two scales were used to assess and evaluate the interpersonal competences of the examined individuals:
The Faces task [21] to examine the ability to recognize emotions from facial expressions.
The Emotional Intelligence Scale – Faces task is a task validated on the Polish population which taps the ability to recognise emotion from facial expressions [21]. The task consists of 18 pictures of emotional faces, including 8 positive and 10 negative emotional states (9 female faces and 9 male faces). Each emotional face expresses from one to four emotions. Each picture is associated with six emotional labels and participants are required to indicate- for each emotion – whether it is expressed by the face or not. For instance, a picture of a smiling man is presented with the following labels: “troubled” (no), “excited” (yes), “agitated” (no), “euphoric” (no), “happy” (yes), “smug” (yes). Each stimulus receives a score from 0 to 6 (1 point for every emotional label which is correctly attributed – or non-attributed – to the face), for a global score ranging from 0 to 108. No time limit was given for response selection.
Assessment of emotional and linguistic prosody
Two subtests from The Right Hemisphere Language Battery (RHLB-PL), as adapted by E. Ã…Âojek, were used to examine emotional and linguistic prosody: the emotional prosody test and the linguistic prosody test.
The first test is used to assess the ability to understand emotional intonation with which meaninglessness sentences are uttered. The task is composed of 16 sentences: six expressing joy, five-sorrow, and five-anger. The linguistic prosody test makes it possible to assess the ability to understand the manner in which a meaninglessness sentence is uttered. It also consists of 16 sentences: six questions, five statements and five commands [22].
Total RNA isolation
Peripheral blood was used as material in the genotype study (in volumes of 5 ml on EDTA). Total RNA isolation from the patients’ blood samples using an RNA extraction reagent – TRIZOL (Invitrogen Life Technologies) – according to the standard acid-guanidiniumphenol- chlorophorm method was performed based on the modified Chomczyński method [23]. Absorbance of isolated RNA was measured using a spectrophotometer (Picodrop) at λ=260 nm in order to determine total RNA concentration. Isolated RNA was stored at a temperature of -70°C.
Quality analysis of isolated RNA
The quality of total RNA was checked with Agilent RNA 6000 Nano Kit (Agilent Technologies) in accordance with the manufacturer’s recommendations. 1 μl of RNA 6000 Nano dye was added to a test tube containing 65 μl of Agilent RNA 6000 Nano gel matrix and then centrifuged (10 minutes, 13000 xg). The gel-fluorescent dye mixture was applied on the surface of a Nano chip placed in a workstation. Then, 5 μl of RNA Nano marker was added to selected pits. Isolated samples of RNA and RNA size marker were subject to denaturation (2 minutes, 70°C), and then 1 μl of the sample was pipetted to selected pits of the Nano chip and mixed (1 minute, 2400 rpm). The quality of isolated RNA was checked using 2100 Bioanalyzer (Agilent Technologies). The level of degradation of total RNA was determined with the use of an electrophoretogram and RIN values recorded. Only the samples with RIN value >7 were subject to a further analysis.
RT-PCR reverse transcription
An RT reaction was carried out using TaqMan® RNA Reverse Transcription Kit (Applied Biosystems) based on the manufacturer’s recommendations. The samples were incubated (30 minutes, 16°C and 30 minutes, 42°C) in a thermocycler (Biometra). Reverse transcriptase was inactivated (5 minutes, 85°C) and the obtained cDNA was stored at a temperature of -20°C.
Real-Time PCR reaction
Real-Time PCR reaction was conducted using TaqMan® Universal PCR Master Mix, No UNG (Applied Biosystems), according to the protocol provided by the manufacturer, using specific Hs01012737_m1 and Hs04194366_g1 probes, respectively for hNP (human neuropsin gene) and RPL13A genes, delivered by Applied Biosystems. The reaction mixture ratio was presented in the table. The Ct comparative method was used to calculate relative expression of miRNA genes [24]. The level of hNP gene expression in blood was normalized in relation to the RPL13A reference gene.
Each target probe was amplified in a separate 96- well plate. All samples were incubated at 50°C for 2 minutes and at 95°C for 10 minutes, and then cycled at 95°C for 30 seconds, at 60°C for 30 seconds and at 72°C for 1 minute; 40 cycles were performed in total. Fluorescence emission data were captured and mRNA levels were quantified using the critical threshold (Ct) value. Analyses were performed with ABI Prism 7000 (SDS Software). Controls without RT and with no template cDNA were performed with each assay. Relative gene expression levels were obtained using the ΔΔCt standard 2-ΔΔct calculations and expressed as a fold change of the control sample [25]. Amplification specific transcripts were further confirmed by obtaining melting curve profiles.
▪ Statistical analysis
A statistical analysis of the material was performed with the use of both descriptive and inferential statistics. A two-tailed critical region was employed in the testing of the statistical hypothesis. The qualitative characteristics of the affected subjects and healthy controls were expressed as frequencies and shown as percentages. An arithmetical mean (M) was calculated in order to characterize the average values of quantitative features. Statistical dispersion measures included the values between the minimum and the maximum, and standard deviation (SD).
Distributions were measured with the use of the Shapiro-Wilk test. The following tests were applied in the comparison of nonparametric variables in the test groups: the Pearson χ2 for qualitative variables, the Wilcoxon signed-rank test for two related groups for quantitative variables, and the Mann-Whitney U test for two independent groups to determine the coincidence of distributions. Spearman’s R rank order correlation coefficients were estimated, the aim of which was to evaluate the relationships between the analysed variables. Statistical significance was defined as p <0.05 [26] in all the analyses. All the analyses were conducted using STATISTICA PL, version 10.
▪ Ethics
The individuals taking part in the experiment were native Poles from central Poland (not related). They were chosen for the study group at random without replacement sampling. Participation in the study was voluntary; any personal data and results were kept confidential. Before making a decision to participate in the study, the subjects were informed of the purpose, assured of the voluntary character of the experiment and guaranteed that their personal data would be kept in secret. Written informed consent was given in accordance with the study protocol, approved by the Bioethical Committee of the Medical University of Lodz (No. RNN/272/15/KE).
Results
The social and demographic characteristics of the examined individuals and the information regarding the course of the disease are presented in Table 1.
| ED-I M(SD) | rDE M(SD) | ED-I vsrDE | |
|---|---|---|---|
| Age (years) | 45.35 (12.71) | 48.86 (10.09) | - |
| Male / Female (%) | 33 / 39 (45.83 / 54.17) | 45 / 91 (33.09 / 66.91) | - |
| HDRS-I | 22.61 (6.83) | 22.71 (6.74) | -0.091 |
| HDRS-II | 11.09 (7.76) | 13.45 (7.62) | 0.679 |
| Number of depression episodes | - | 4.55 (2.21) | - |
ED-I – first episode of depression; rDE – recurrent depression episodes; HDRS-I – Hamilton Depression Rating Scale at the onset of therapy; HDRS-II – Hamilton Depression Rating Scale after pharmacological treatment; M – mean; SD – standard deviation; * – p statistically significant.
Table 1: Participants’ demographic and clinical features.
Course of the disease.
Statistically significant differences were observed in both examined groups in terms of the intensity of depression symptoms measured at the onset of the pharmacotherapy and after 8 weeks. A significant improvement of the patients’ mental status was achieved (for the ED-I group: Z=5.627, p<0.001; for the rDE group: Z=7.111, p<000.1).Table 1 shows that disease intensification on the day of admission and after treatment completion was similar in the two groups compared.
Human neuropsin gene
Significant differences were confirmed in the expression of hNP on the mRNA level between the individuals from the ED-I group and the affected diagnosed with rDE (Z=2.029, p<0.05); for the ED-I group: M=0.177 (SD=0.07) and for the rDE group: M=0.197 (SD=0.07). Expression of hNP on the mRNA level for the entire group reached the following value: M=0.191 (SD=0.07).
Faces task and emotional / linguistic prosody task
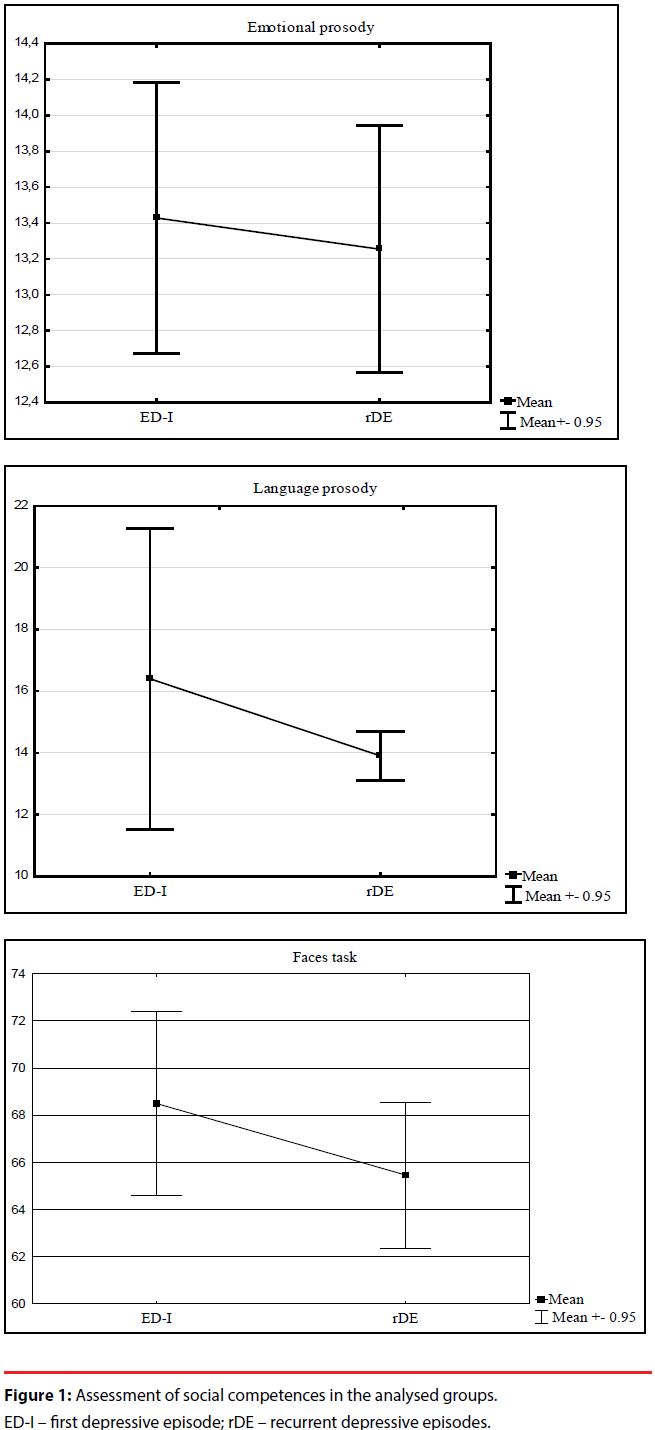
No statistically significant differences in the results of the tests used to assess interpersonal competences were observed in the analysed groups: faces task (p=0.184), emotional prosody (p=0.967), linguistic prosody (p=0.869). The affected with the first episode of depression did better in every task as compared to the patients treated again. The obtained results can be found in Figure 1.
Correlations
Spearman’s rank correlation analysis did not demonstrate any statistically significant relationship between disease intensification measured based on HDRS and expression on the mRNA level for the hNP gene both on the day of admission (p=0.464) and on the day of discharge (p=0.416) (N=208 for the entire group analysed).
In case of an analysis of the interrelation between disease intensification and interpersonal abilities a significant correlation (of a negative nature) was confirmed for disease intensification on the day of admission and for the level of performance of the test assessing linguistic prosody (R=-0.278, p=009). The correlations had a negative character in case of the emotional prosody and the faces task; however, the level of statistical significance was not achieved (N=208 for the entire group analysed).
Expression for the hNP gene on the mRNA level decreased with age in the entire group analysed (R=-0.072, p<0.311, N=208). However, no statistically significant differences in this scope were observed.
Significant interrelations between expression on the mRNA level for the hNP gene and the variables used to assess social competences were not confirmed, either. Results of the statistical analysis make it possible to confirm an inversely proportional correlation between the analysed variables (Table 2).
| R Spearman | p | |
|---|---|---|
| Faces task & hNP mRNA 2-ΔΔct | -0.069 | 0.487 |
| Emotional prosody & hNP mRNA 2-ΔΔct | -0.163 | 0.149 |
| Linguistic prosody & hNP mRNA 2-ΔΔct | -0.144 | 0.202 |
* – p statistically significant
Table 2: Results of Spearman’s rank correlation for the variables analysed (N = 208).
Discussion
The presented results confirmed the significant differences in expression on the mRNA level for the hNP gene in the group of patients with diagnosed depression, treated for the first time and treated for many years due to recurrent episodes of the disease. Expression turned out to be significantly higher among the patients affected by rDE. These results are in conformity with our prior studies [27], where we demonstrated increased expression of hNP among patients with depression symptoms as compared to a control group of healthy subjects. Similarly as in the case of the cited work, this paper is the first one where hNP gene expression was analysed in the patients with the first and successive episodes of depression. Therefore, we can only refer to the studies by Viel et al. [28]. The said authors associated the Kallikrein- Kinin System (KKS) to inflammatory and immunogenic responses in the peripheral and central nervous system by the activation of two receptors, namely B1 receptor and B2 receptor. The B1 receptor is absent or under-expressed in physiological conditions, being up-regulated during tissue injury or in the presence of cytokines. The B2 receptor is constitutive and mediates most of the biological effects of kinins [28]. Increased expression of hNP in people with depression, as a defense reaction of the organism against disease recurrence, is only a hypothesis which can be verified in subsequent studies.
In our studies we did not confirm the significant correlation between expression on the mRNA level for the hNP gene and the level of performance of the tests used to assess social competences of the examined individuals. The results of the statistical analysis make it possible to confirm an inversely proportional correlation between the analysed variables. This matter still requires further research.
No significant differences were observed in the performance of the tests used to assess interpersonal competences in the analysed groups. Ladegaard et al. [29] obtained results similar to ours. They compared a group of first-episode depressed patients with a group of chronically depressed patients (duration>2 years) on a broad array of higher-order social cognitive measures, including the abbreviated metacognition assessment scale. Deficits in social cognition were roughly equivalent between the two groups. Despite the absence of differences in social competences between the patients with the first and successive episodes of depression, the differences in the analysed scope between the individuals with depressive disorders and the healthy subjects were confirmed in previous studies [30,31].
It was possible to confirm the interrelation between disease intensification and reduced interpersonal efficiency in the scope of soft social competences among the examined individuals. Air et al. [32], using the Wechsler Advanced Clinical Solutions, examined the following: Social Perception Subtest, measuring facial affect recognition in isolation and in combination with prosody and body language interpretation. No statistically significant differences in the level of performance of the tests used were found between healthy subjects from the control groups and the remitted depression and currently depressed groups. However, an inversely proportional correlation between the intensification of depression symptoms, anxiety and somatic ailments, and the efficiency in identification of emotional expression was confirmed. Lee at al. [33] presented similar research results to the ones quoted above, i.e. affective depression symptoms, such as anhedonia, had a significant inverse relationship with performance in a theory of mind task. Moreover, according to Weightman et al. [34], social cognitive performance appears to be inversely associated with the severity of depression, whilst the bias towards negative emotions persists even in remission. Additionally, it turns out that the social cognitive and metacognitive ability may improve with symptom remission in major depression, although it may not reach a level equal to that of the people who have never experienced depression [35].
Further research is required in this area to better understand the functional impact of these findings and the way in which targeted therapy could aid depressed individuals with social interactions [34]. To sum up, we may take a risk and claim that disease intensification, and not the number of episodes, is of importance for the deficits in terms of soft social competences. This means that intensive pharmacological and therapeutic action targeted at “cold” (attention, memory, executive functions) and “hot” (emotional bias) cognitive impairments, as well as at social cognition domains (empathy, theory of mind), may have key importance in the prevention of disease recurrence.
Conclusions
a) Expression of the hNP gene on the mRNA level, evaluated based on peripheral blood, is significantly higher in the patients with rDE than in ED-I.
b) Increased hNP expression is associated with a reduction of interpersonal abilities in the people affected by depression.
c) Disease intensification, not the number of episodes, is of significant importance for the deficits in terms of soft social competences.
Acknowledgements
All authors have approved the final article.
This study was supported with scientific research grants from the National Science Centre, No. 2012/07/B/NZ7/04212, and from the Medical University of Lodz, No. 503/5-062-02/503-51- 006.
Limitations
Fairly young age of the examined individuals in both groups, which may be the reason for the differences in hNP expression.
Conflict of interest
None to declare.
References
- Bayliss AP, Tipper SP, Wakeley J, et al.Vulnerability to depression is associated with a failure to acquire implicit social appraisals. Cogn. Emot6(1), 1-9(2016).
- Domes G, Normann C, Heinrichs M. The effect of oxytocin on attention to angry and happy faces in chronic depression. BMC. Psychiatry16(1), 92.
- Hörtnagl CM, Oberheinricher S, Hofer A. Social cognition in patients with mood disorders: part I: major depressive disorder: a comprehensive review of the literature. Neuropsychiatr28(2), 74-83(2014).
- Green MF, Penn DL, Bentall R, et al.Social cognition in schizophrenia: an NIMH workshop on definitions, assessment and research opportunities. Schizophr.Bull34(6), 1211-1220(2008).
- Bartholomeusz CF, Allott K. Neurocognitive and social cognitive approaches for improving functional outcome in early psychosis: theoretical considerations and current state of evidence. Schizophr. Res. Treat815315(2012).
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain. Mapp30(8), 2313-2335(2009).
- Lan CC, Tsai SJ, Huang CC, et al.Functional Connectivity Density Mapping of Depressive Symptoms and Loneliness in Non-Demented Elderly Male. Front. Aging. Neurosci7(1), 251(2016).
- Cusi AM, Nazarov A, Holshausen K, et al. Systematic review of the neural basis of social cognition in patients with mood disorders. J. Psychiatry. Neurosci 37(3), 154-169(2012).
- Loi F, Vaidya JG, Paradiso S. Recognition of emotion from body language among patients with unipolar depression. Psychiatry. Res209(1), 40-49(2013).
- Hedstrom L. Serine protease mechanism and specificity. Chem. Rev102(12), 4501-4524(2002).
- Ziemiańska K, Konopka A, Wilczyński GM. The role of extracellular proteolysis in synaptic plasticity of the central nervous system. Adv. Hyg. Exp. Med66(1), 959-975(2012).
- Shiosaka S, Ishikawa Y. Neuropsin - a possible modulator of synaptic plasticity. J. Chem. Neuroanat42(1), 24-29(2011).
- Harris VK, Diamanduros A, Good P, et al.Bri2-23 is a potential cerebrospinal fluid biomarker in multiple sclerosis. Neurobiol. Dis40(1), 331-339(2010).
- Dutra RC, Moreira EL, Alberti TB, et al.Spatial reference memory deficits precede motor dysfunction in an experimental autoimmune encephalomyelitis model: the role of kallikrein - kinin system. Brain. Behav. Immun33(1), 90-101(2013).
- Horii Y, Yamasaki N, Miyakawa T, et al.Increased anxiety-like behavior in neuropsin (kallikrein-related peptidase 8) gene-deficient mice. Behav. Neurosci122(3), 498-504(2008).
- International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Genewa: World Health Organization (2015).
- APA (US), Diagnostic and Statistical Manual of Mental Disorders fifth Edition. APA (2013).
- Patten S. Performance of the Composite International Diagnostic Interview Short Form for major depression in community and clinical samples. Chron. Dis. Can18(3), 109-112(1997).
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry23(1), 56-62(1960).
- Demyttenaere K, De Fruyt J. Getting what you ask for: on the selectivity of depression rating scales. Psychothery. Psychosom72(2), 61-70(2003).
- Matczak A, Piekarska J, Studniarek E. Emotional Intelligence Scale-Faces. Warsaw: PTP (2005).
- Bryan KL. The right hemisphere language battery. Second edition. London: Whurr Publishers Ltd, (1995).
- Chomczynski P, Sacchi N. Single -step method of RNA isolation by acid guanidinium thiocyanate- phenol - chloroform extraction. Anal. Biochem162(1), 156-159(1987).
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc 3(6), 1101-1108(2008).
- Livak K, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods25(4), 402-408(2001).
- Kirkwood B, Sterne J. Essential medical statistics, 2nd edition. Wiley-Bleckwell, (2003).
- Bobińska K, Szemraj J, Gałecki P, et al. Human neuropsin gene in depression (2016).
- Viel TA, Buck HS. Kallikrein - kinin system mediated inflammation in Alzheimer's disease in vivo. Curr. Alzheimer. Res8(1), 59-66(2011).
- Ladegaard N, Lysaker PH, Larsen ER, et al.A comparison of capacities for social cognition and metacognition in first episode and prolonged depression. Psychiatry. Res220(3), 883-889(2014).
- Ladegaard N, Larsen ER, Videbech P, et al.Higher-order social cognition in first-episode major depression. Psychiatry. Res216(1), 37-43(2014b).
- Talarowska M, Zajączkowska M, Gałecki P. Emotional and language prosody and working memory in patients with depression. Pol. Merkur. Lekarski38(227), 269-272(2015).
- Air T, Weightman MJ, Baune BT. Symptom severity of depressive symptoms impacts on social cognition performance in current but not remitted major depressive disorder. Front. Psychol6(1), 1118(2015).
- Lee L, Harkness KL, Sabbagh MA, et al.Mental state decoding abilities in clinical depression. J. Affect. Disord 86(2-3), 247-258(2005).
- Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Front. Psychiatry5(1), 179(2014).
- Ladegaard N, Videbech P, Lysaker PH, et al.The course of social cognitive and metacognitive ability in depression: Deficit are only partially normalized after full remission of first episode major depression. Br. J. Clin. Psychol55(3), 269-286(2015).