Review Article - Interventional Cardiology (2011) Volume 3, Issue 2
Hybrid cardiovascular therapy: interventional (and surgical) procedures in high-risk patients
- Corresponding Author:
- E Murat Tuzcu
Cardiovascular Medicine, Cleveland Clinic
Desk J2-3, 9500 Euclid Avenue, Cleveland, OH, USA
Tel: +1 216 444 8130
Fax: +1 216 445 6186
E-mail: tuzcue@ccf.org
Abstract
Keywords
aortic aneurysm, aortic dissection, hybrid procedures, myocardial revascularization, paravalvular leak, post-infarction ventricular septal rupture, valve replacement
Hybrid cardiovascular care is not a new concept, with cases reported in the literature more than a decade ago [1]. In recent years, however, hybrid therapy for cardiovascular disorders has been thrust further into the spotlight as collaboration between interventional cardiologists and cardiovascular surgeons grows stronger. In addition, a growing population of aging and higher-risk patients means that patients often present with conditions that are not readily treatable with traditional percutaneous or surgical therapies. There has been a merging and blurring of boundaries between interventional cardiology and cardiovascular surgery, with increasingly more aggressive invasive strategies being employed by interventionalists, and a trend towards minimally invasive and robotic surgical technologies. This has facilitated the transition to the arena of hybrid cardiovascular care. While there are many clinical arenas where hybrid cardiovascular care is being used, the most mature and rapidly growing of these include hybrid myocardial revascularization, hybrid therapies for valvular and structural heart diseases, as well as hybrid therapies for vascular diseases, particularly of the aorta. In this article we will discuss paradigms for collaborative cardiovascular care and review hybrid treatment strategies for myocardial revascularization, valvular and structural heart diseases and diseases of the aorta.
Paradigms for collaborative hybrid cardiovascular care
With the increase in the volume of hybrid cardiovascular procedures, there has been considerable interest in developing and optimizing paradigms for collaborative care. Important factors integral to successful delivery of care prior to, during and following hybrid procedures include increased integration and collaboration across disciplines including interventional cardiology, cardiac surgery, vascular surgery, cardiovascular imaging and anesthesia, as well as design, implementation and optimization of a hybrid cardiovascular procedural suite.
Planning for a hybrid cardiovascular procedure must begin well in advance of the procedure itself. Ideally, institutions planning to undertake and develop hybrid cardiovascular programs should encourage and help develop multidisciplinary clinical teams, with members from traditional departments, including interventional cardiology, cardiovascular and vascular surgery, cardiovascular imaging and anesthesia. Regular meetings and conferences should be held by these multidisciplinary teams to discuss and plan cases prior to their performance as well as to debrief and learn from cases after they are complete. Patients scheduled for hybrid procedures should ideally be evaluated by all members of the hybrid multidisciplinary team prior to the planned procedure, such that each practitioner may provide the patient and family with their expert opinion and advice. An atmosphere of collaboration during the procedure should be espoused with established roles for each member of the multidisciplinary team. In addition to collaboration at the physician level, there must also be development of a multidisciplinary team among the nursing and ancillary staff working in the hybrid cardiovascular procedural suite. Identification of individuals with excellent skills and interest in hybrid care from within an institution’s catheterization laboratory and operating rooms followed by training and education of these individuals in important aspects of hybrid care is essential. Postprocedure care should also be of a multidisciplinary nature within a defined clinical care area, such as an intensive care unit where the nursing and support staff have been trained to care for patients having undergone hybrid procedures. The interventional cardiology and surgical members of the team should each provide follow-up care focusing on their area of expertise. Importantly, the credit and blame should be shared equally by all of those participating in the care.
The physical centerpiece of a successful hybrid cardiovascular program is the hybrid cardiovascular suite. The planning, development and operation of this room is essential for a comprehensive hybrid program. The hybrid cardiovascular suite allows for integrated delivery of therapies that are traditionally only available in either an operating room or catheterization laboratory. To achieve this goal, the hybrid suite must combine essential features from both of these procedural areas. First, the hybrid suite must be large enough to accommodate surgical and interventional equipment. It must be ergonomically designed, with ample free space to allow for anesthesia setup and equipment, movement of clinical and ancillary staff and for additional equipment as needed. A high-quality fixed fluoroscopy unit, either floor or ceiling mounted, ideally with biplane capability, is essential. Some centers are employing portable fluoroscopy units and, although less costly, these typically provide inferior image quality that may not be well suited to complex hybrid cases. The fluoroscopy unit must be capable of performing digital subtraction angiography. Additionally, the ability of fixed fluoroscopy units to perform 360° rotational computed tomography angiography is very desirable. Using this technology, a 3D dataset can be acquired, overlayed with fluoroscopy and thus used for real-time guidance during complex hybrid cases that require precise localization and navigation [2]. In addition to traditional imaging with fluoroscopy, the hybrid suite should also allow for integrated multimodality imaging with adequate inputs and well-positioned monitor space to display real-time echocardiography and intravascular ultrasound images. Needless to say, strict surgical sterility, including laminar flow when necessary, should be maintained in a hybrid cardiovascular suite, a concept that is sometimes novel for the interventional cardiology members of the hybrid team.
The physical location of the hybrid cardiovascular suite – whether in the cardiac catheterization laboratory or in the cardiovascular or vascular operating room – has been a topic of debate. We feel that this should be dictated by the institutional needs when the hybrid suite is not in use. Even in the busiest centers, the current volume of hybrid procedures is generally not sufficient for full-time use of a hybrid cardiovascular suite, and thus this suite is likely to be used for traditional nonhybrid procedures on many occasions. For example, a hybrid suite located in the cardiac catheterization laboratory is more likely to be able to perform coronary interventions whereas, one located in the operating room will be able to perform traditional open cardiovascular or vascular procedures. Therefore, the location of the hybrid cardiovascular suite is a practical rather than political decision. In other words, the hybrid ‘suite’ can be a hybrid ‘catheterization laboratory’ or hybrid ‘operating room’. There are advantages and disadvantages to both these models. The hybrid catheterization laboratory is more likely to be used successfully for interventional procedures requiring some surgical aid, whereas hybrid surgical rooms are better suited for procedures requiring major surgery that are facilitated by some interventional techniques.
Hybrid myocardial revascularization
‘Hybrid’ or ‘integrated’ coronary revascularization utilizes both percutaneous coronary intervention (PCI) in combination with traditional surgical methods of coronary artery bypass. This method was first used in patients who had multivessel coronary artery disease (CAD) and presented with an acute coronary syndrome. Interventional cardiologists performed PCI of the culprit lesion, followed by coronary artery bypass grafting (CABG) later during the hospitalization. These procedures were typically performed over the course of a few days to several weeks later. As more patients started requiring both procedures, this led to further collaboration between interventional cardiologists and cardiothoracic surgeons. Cardiac surgeons have continued to explore ways to reduce postoperative healing times and pain, and developed minimally invasive direct coronary artery bypass (MID-CAB) grafting through a small left thoracotomy. Although the MID-CAB procedure is not widely employed in practice at present (a large randomized trial demonstrating its patency rates versus open surgery has still not been completed), its development still led to the first series of integrated revascularization procedures [1,3]. The further development of hybrid cardiac catheterization laboratories and operative suites ultimately led to the ability to perform these cases during one combined procedure.
There have been a number of trials comparing CABG and PCI in left main coronary artery and multivessel PCI [4,5]. While rates of death and myocardial infarction (MI) have been demonstrated to be similar between the two strategies, the PCI group has a higher rate of repeat revascularization [6]. The major advantage conferred to the patient with CABG appears to be related to the use of a left internal mammary arteryleft anterior descending (LIMA-LAD) graft [7]. These grafts have excellent patency rates at 5 and 10 years, respectively [8–11]. This benefit may be emphasized in the diabetic population.
It should be noted that using both mammary arteries as conduits has been demonstrated to be superior to using a single mammary plus a vein graft. This is true both in smaller studies and over long-term follow-up [12]. Patients with full arterial revascularization using the mammary arteries have less incidences of MI and repeat revascularization [13]. However, due to the complexity of the operation, and concerns of increased surgical and recovery times, its use has not been widespread. Early results of the Arterial Revascularisation Trial (ART) suggest similar outcomes between single and bilateral mammary arterial revascularization, with long-term outcomes still being reviewed [14].
The use of grafts other than the mammary arteries, however, does not appear as beneficial as PCI. The most commonly used secondary graft, the saphenous vein graft, has a failure rate of up to 30% at 1 year, and up to 50% at 10 years [9,11,15,16]. Conversely, restenosis rates following PCI in the era of drug-eluting stents (DES) are approximately 8% per year [17–19]. This discrepancy is where the potential benefit of a hybrid revascularization procedure exists. By combining minimally invasive CABG (LIMA-LAD) with PCI (to left circumflex coronary artery and right coronary artery), we may have a superior alternative to traditional CABG or multivessel PCI in select patients.
Specific areas where hybrid revascularization may prove most useful include: the following proximal LAD or bifurcation disease with other coronary lesions suitable for PCI; nongraftable lesions (due to the course of vessel running in atrioventricular groove); repeat operations (where scarring or the course of prior bypass makes full sternotomy undesirable); multisystem organ dysfunction; severe aortic disease; lack of sufficient venous conduits; patients in whom age or frail condition carry excessive risk of traditional CABG; or in patients who refuse median sternotomy. Contraindications to a hybrid revascularization procedure include any contraindication to minimally invasive CABG or PCI (hemodynamically unstable, previously used LIMA, left thoracic surgery, chest wall irradiation, diffusely diseased LAD, severe peripheral arterial disease precluding access, lesions unacceptable for PCI, malignant ventricular arrhythmias, coagulopathy with increased bleeding risk, short life-expectancy due to noncardiac causes, decompensated congestive heart failure with severe left ventricular dysfunction) (Boxes 1 & 2). A significant subclavian artery stenosis would also preclude hybrid revascularization, although it could conceivably be negated if the stenosis is stented prior to the procedure.
To date, there have been several small series of patients treated with hybrid revascularization, which have demonstrated a low mortality rate, with reduced ICU stay and time to discharge [3,20–32]. Patients also have faster recovery times and a favorable cosmetic result. These benefits are offset by the cost of the procedure. Also, minimally invasive CABG requires longer operating time, with late wound complications and longer bouts of rib pain due to retraction. There has also been a rate of restenosis following the procedures between 2 and 23% (average 11%) [25,29,30,33]. This may be related to a higher usage rate of bare-metal stents, which does not reflect current practice and may improve in patients treated with DES. A recently published series by Bonatti et al. demonstrated no increase in bleeding when DES were used [20]. However, long-term follow-up of larger series to evaluate restenosis rates is needed.
One of the most important considerations during hybrid revascularization is the dosage and timing of anticoagulation. CABG can be performed either with heparin (which is then usually reversed with protamine prior to PCI) or with bivalirudin [34,35]. If heparin is used, then adjunctive bivalirudin, a direct thrombin inhibitor, can be given as a bolus and infusion per standard practice. Bivalirudin is preferable, as it has been associated with reduced bleeding complications in several large randomized controlled trials [36–39].
Many sites have adopted a strategy of clopidogrel loading just prior to CABG, after induction of anesthesia. This is then followed by standard dosing of clopidogrel following the procedure. A recent report of hybrid PCI procedures used a 300-mg loading dose of clopidogrel (lower than the current standard of 600 mg in most patients), which was found to exert sufficient platelet inhibition without an increase in postprocedural events [40].
An additional usage of the hybrid procedural room involves routine completion angiogram following CABG. Due to the established reports of early graft failure following CABG [9,15,16,41], some centers perform angiogram following the procedure to ensure graft patency. A recent study has demonstrated that, immediately following surgery, 12% of grafts have important angiographic defects. Half of these defects were then successfully improved by performing open-chest PCI [42].
Hybrid coronary revascularization has proven to be safe and feasible in the current era of practice. Selected patients with multivessel CAD may best be served by this type of approach. As both the procedural techniques and hybrid procedure rooms evolve, the use of these procedures will continue to rise, and may, in fact, become the optimal method of coronary revascularization. Due to the small number of patients in the published reports, further studies are necessary to determine the clinical implications of hybrid revascularization procedures. The main question that needs answering is: how is this better than staged procedures? Minimally invasive LIMA to LAD followed in a few days by stenting of left circumflex coronary artery and right coronary artery may be the competing strategy to the previously described simultaneous hybrid approach. Further data on safety and effectiveness of these strategies are necessary prior to making any firm recommendations on patient selection.
Hybrid valve procedures
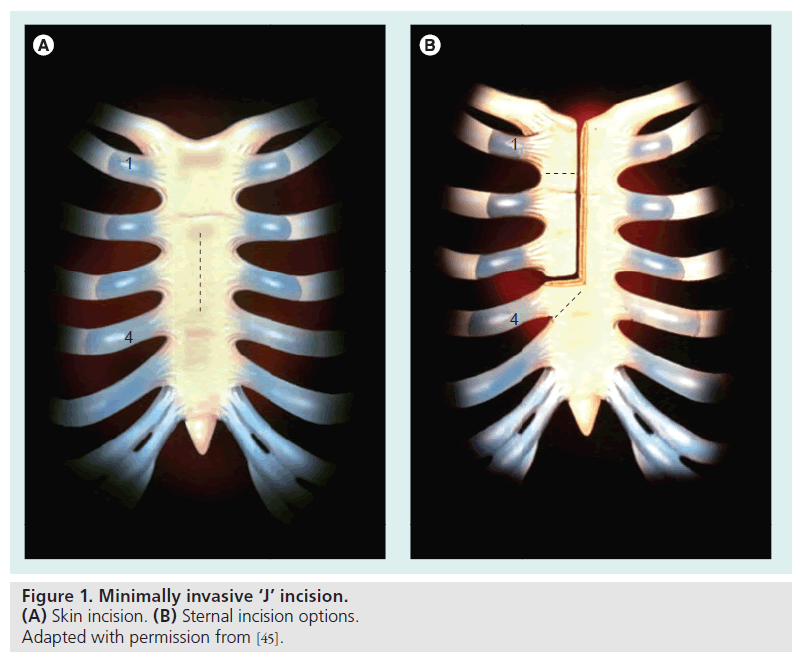
Over the past 15 years, the evolution of minimally invasive surgical procedures has been quite astounding. Modern surgical valve replacement and repair procedures have very low morbidity and mortality risks in the general population; however, the aging population presents a new cohort of patients (octogenarians and nonagenarians, multiple redo surgeries and multiple comorbidities) who are at substantially higher risk for traditional open procedures [43]. The impetus for novel less-invasive surgical procedures is to improve patient outcomes, patient experience and minimize complications, which translates into a reduction in healthcare cost. Pioneering efforts by Cosgrove and colleagues laid the groundwork for the evolution of minimally invasive valve/hybrid procedures when they demonstrated that modifications of the traditional surgical approach could decrease surgical morbidity while providing safe and effective valve replacements through smaller incisions [44]. These types of procedures are obviously attractive to patients and physicians alike. Patients benefit from smaller incisions, less pain, faster recovery and fewer complications/ shorter lengths of stay (Figure 1) [45]. Physicians also share these benefits and, additionally, often benefit from shorter case times and enhanced patient satisfaction.
Figure 1. Minimally invasive ‘J’ incision. (A) Skin incision. (B) Sternal incision options. Adapted with permission from [45].
Percutaneous coronary intervention & traditional valve replacement
Throughout the years, various minimally invasive valvular surgery techniques have been developed and have proven to be successful. The success of minimally invasive surgery has led to the development of hybrid procedures that require close collaboration of the cardiac surgeon and interventional cardiologist. Simultaneous or staged PCIs with minimally invasive valve replacements /repairs and catheter-based transapical aortic valve implantation (TAVI) are two examples of such procedures.
Data for these procedures are somewhat sparse. Byrne and colleagues have reported the largest ‘hybrid’ experience to date [46]. Originally, they reported the results of 26 consecutive patients retrospectively analyzed after undergoing PCI followed by valve surgery during the same hospitalization (within a median 5 days) in patients with complex CAD and concomitant valve disease [46]. Comorbidities and acute presentation made these patients highrisk for traditional open valve CABG; the median age was 72 years, 42% had prior open surgery, 90% were either urgent or emergent (acute coronary syndrome) and nearly half had an acute MI. Preoperative mortality was calculated using the STS algorithm for traditional CABG plus aortic valve replacement (AVR) or CABG plus mitral valve replacement (MVR). The median predicted operative mortality for the group was 22% (range: 3.5–63.5%) based on the Society of Thoracic Surgery (STS) prediction model, whereas the observed operatively mortality was 3.8% with 1-, 3- and 5-year survival rates of 78, 56 and 44% respectively.
All patients were treated with aspirin and 69% received clopidogrel, which likely contributed to approximately 85% of patients requiring at least one unit of blood transfusion. Despite a large number of patients requiring blood products, repeat operations were required in only 8% of patients. Balloon angioplasty was performed in all patients and 85% of patients received a stent. DES were utilized in 12% of patients and no patients suffered acute or subacute stent thrombosis as the result of cessation of antiplatelet therapy.
The vast majority (81%) of patients had mitral valve replacement, 15% had an aortic valve replacement and one patient had double valve surgery (mitral and aortic). Of the four aortic valve replacements, two were performed with a minimally invasive approach. A right thoracotomy was performed in six of the eight mitral valve replacements. Nearly half (42%) of the patients were reoperative valve procedures underscoring the high-risk nature of these patients. The median length of time spent in the ICU was 4 days and median length of stay in the hospital was 17 days. Given that operative mortality was much lower than predicted by the STS model. the authors concluded that a ‘hybrid’ approach for patients with complex coronary and valvular disease is an excellent alternative to conventional surgery in select high-risk patients, albeit at the cost of increased bleeding.
Percutaneous coronary intervention & minimally invasive valve replacement
Logically, the next step in allowing patients to experience the full benefit of a ‘hybrid’ procedure is to use minimally invasive surgical techniques combined with PCI. One of the largest experiences in minimally invasive valve replacements reported in the literature comes from Brigham and Women’s hospital; they describe 1553 patients who underwent minimally invasive aortic and mitral valve replacements. A total of 890 patients underwent minimally invasive aortic valve surgery (15 patients with aortic valve repair) and the remaining 663 had minimally invasive mitral valve surgery (90% received a mitral valve repair) between July 1996 and July 2003 [43]. For the minimally invasive aortic valve cohort, the mean age was 65 years, approximately a third of the patients were NYHA class III or IV and a minority (13%) of patients had prior AVR. Overall, the operative mortality for this group was 2%, compared with the unadjusted mortality risk from the STS database of 3.4–4.4% during the same time span.
Comparatively, the cohort that underwent minimally invasive mitral valve surgery was younger (mean age 57 years), slightly healthier (27% were NYHA class III–IV) and less than 1% had prior mitral valve surgery. Expectedly, the 30-day operative mortality was lower than the aortic group at 0.7% (2% for mitral valve replacement and 0.5% for mitral valve repair). Regardless of the valve procedure performed, patients seem to fare better with a minimally invasive approach even when only considering ‘hard outcomes’. More extensive data are available on the first 1000 patients of this cohort, and it appears that patients also benefit from shorter lengths of stay and are less likely to need rehabilitation services postoperatively than would be expected for conventional surgical procedures [47].
Another large study, from the Cleveland Clinic, later confirmed the benefits of minimally invasive surgery. Propensity matching was used retrospectively to compare 2124 patients who underwent minimally invasive mitral valve repair from January 1995 to January 2004, to a similar cohort of patients who underwent isolated conventional mitral valve surgery. Mean age of the group was 56 years and the majority of patients were NYHA functional class I–II (86%). Patients were excluded if concomitant aortic valve surgery, coronary bypass grafting or redo surgery was needed. Patients with endocarditis were also excluded. In-hospital mortality was very low in the two groups: 0.85% for conventional surgery and 0.17% for the minimally invasive surgery (p = 0.2). Other important outcomes such as MI (p = 0.7), stroke (p = 0.8), renal failure (p > 0.9) or infection (p = 0.8) were also similar between the groups. Patients receiving minimally invasive surgery fared better in several respects, including less mediastinal drainage (mean 250 vs 350 ml; p < 0.0001) and blood transfusions (30 vs 37%; p = 0.01). Patients who underwent minimally invasive surgery were more likely to be extubated earlier and have more favorable pain scores compared with their conventional surgery counterparts [48].
Auspiciously, the Brigham group decided to expand upon their initial observation that highrisk acute coronary syndrome patients with concomitant severe valvular heart disease may derive a mortality benefit from a hybrid approach; they prospectively evaluated 18 high-risk elderly patients (mean age 76 years) with severe aortic stenosis and severe one- or two-vessel CAD in a nonrandomized fashion. All patients received PCI prior to their minimally invasive aortic valve replacement. Six patients received PCI the night prior to the surgery and the other 12 patients received PCI with DES performed the morning prior to surgery. Aspirin 325 mg was administered prior to the procedure (and daily afterwards) and clopidogrel 300 mg bolus was given immediately after the procedure followed by 75 mg daily. Heparin (70 units/kg) or bivalirudin (0.75 mg/kg/h) was used for anticoagulation with eptifibatide bolus (180 μg/kg) and intraprocedural drip (2 μg/kg/min) as needed. In this group, there was one (5.5%) operative death (gastrointestinal perforation) and no late mortality with a mean follow-up of 19 months. Only seven patients received blood products postoperatively, with a mean transfusion requirement of less than one unit of packed red blood cells. No acute or subacute stent thrombosis events were noted [49]. In high-risk patients, a hybrid minimally invasive valve/PCI approach may indeed reduce adverse events in patients with severe CAD and concomitant valvular heart disease; however, it remains unknown if this strategy provides long-term benefit over traditional surgical valve replacement and CABG, especially in those with proximal LAD PCI. This promising hybrid strategy should be tested in larger patient cohorts and against other novel approaches focusing on long-term outcomes, timing of revascularization as well as protocols for various anticoagulation and antiplatelet regimens.
Transapical aortic valve implantation
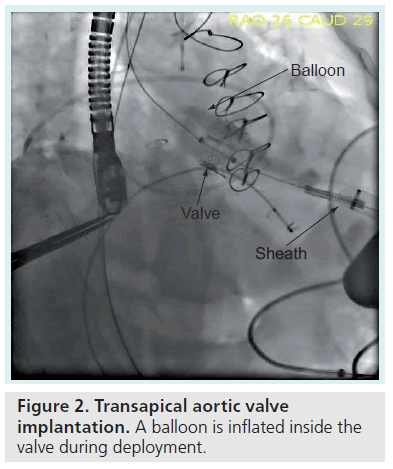
One of the more intriguing additions to the hybrid arena is that of the percutaneous TAVIs. TAVI requires a transcending collaborative effort, utilizing the skill sets of cardiac surgeons, interventional cardiologists and cardiac imaging specialists. Hybrid catheterization laboratory/operating rooms are now available at many institutions worldwide and are the typical venue for these procedures. This novel technique involves placement of a bioprosthetic tissue xenograft valve mounted on a balloonexpandable stent (Figure 2) with placement via a left lateral mini-thoracotomy (Figure 3). TAVI is currently under investigational use only in the USA, but has been approved for commercial use in Europe since 2007 for high-risk patients with severe symptomatic aortic stenosis [50].
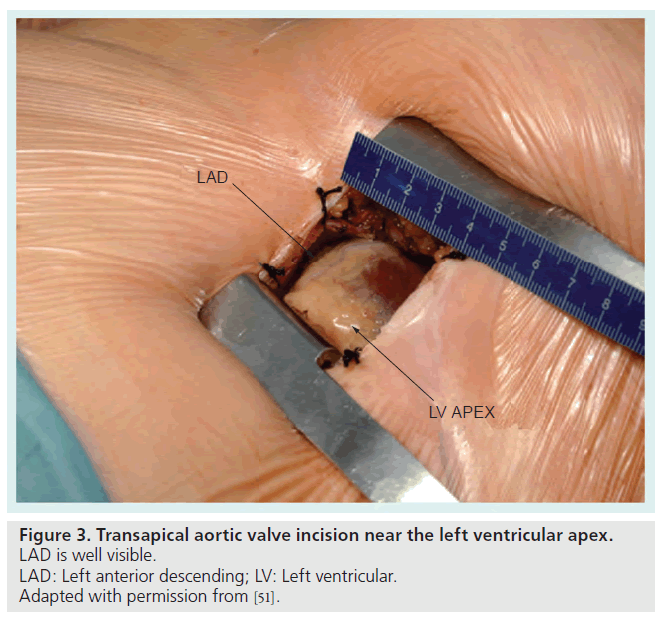
Figure 3. Transapical aortic valve incision near the left ventricular apex. LAD is well visible.
LAD: Left anterior descending; LV: Left ventricular.
Adapted with permission from [51].
Outcomes with transcatheter apical aortic valve implantation have been reliably reproducible. A single-center experience from Berlin, Germany, with 175 high-risk patients diagnosed with severe aortic stenosis demonstrated very favorable results. The average age of the patients was 79 years, 98% had NYHA class III–IV symptoms and ten patients were in cardiogenic shock. No patients required conversion to conventional surgery, although 4.6% required cardiopulmonary bypass. The 30-day mortality rate for the entire cohort was 5.1%, and 3.6% if the ten patients with cardiogenic shock were excluded. Patients with cardiogenic shock as expected, had a higher mortality (30%). At 1 year, the overall survival was 82.6%, which is very encouraging considering the high-risk nature of the group. These results were similar to those of the large multicenter SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) Registry, which was designed to evaluate the Edwards SAPIEN balloon-expandable aortic valve since commercialization [51].
The SOURCE registry enrolled 1123 patients (only 1038 patients were analyzed due to the inability of two centers to provide data on 85 patients) between November 2007 and 31 January 2009, a total of 575 patients (55%) received the valve replacement by the transapical approach and the remainder via the transfemoral approach. EuroScores were 29.1% and 25.7% for the transapical and transfemoral groups, respectively, again suggesting the highrisk nature of these groups; procedural success rate for the entire cohort was very good (93.8%). The 30-day mortality rate was 10.3% for the transapical cohort and 6.3% for the transfemoral cohort; although, as the authors note, a direct comparison cannot be made, because the baseline characteristics of these groups are significantly different in a number of important characteristics including prior surgery, coronary and peripheral arterial disease, carotid artery occlusive disease and aortic calcification (porcelain aorta).
The longest follow-up to date comes from Ye and colleagues, who performed the very first beating heart TAVI in October 2005 [52]. From October 2005 to February 2009, 71 patients underwent transcatheter apical aortic valve implantation with the Edwards Lifesciences bioprosthetic aortic valve without cardiopulmonary bypass support. All patients were prohibitively highrisk for conventional aortic valve surgery and were not candidates for a transfemoral approach, secondary to the lack of adequate vascular access. The mean age of the cohort was 80 years, with a predicted operative mortality of 12.1% by STS and 34.5% by EuroSCORE. The vast majority (86%) of patients were NYHA functional class III–IV at baseline. Nearly a quarter of the patients were not operative candidates because of other comorbidities, such as severe lung disease, porcelain aorta or severe liver disease, although their STS predicted mortality was less than 10%. Although there was a significant ‘learning curve’ with this procedure (30-day mortality was 33% in the first 15 patients and 12.5% in the remaining 56 patients), patients fared better than what was predicated by EuroSCORE. Functional class improved significantly with 75% being NYHA class I or II at 24‑month follow-up. Aortic valve area increased from 0.6 to 1.4 cm2, with the mean transvalvular gradient decreasing from 46 mmHg preoperatively to 10 mmHg postoperatively and remaining stable for up to 3 years. Left ventricular function also increased slightly, from 55% prior to aortic valve implantation to 61% at 2 years. Overall, the 1-, 2- and 3-year survival rates were 72, 68 and 58%, respectively. Although these results are encouraging, they are expected to improve as the operators gain more experience and the procedures become more refined.
In centers outside of the USA, TAVI has become the treatment of choice in these very high-risk populations [53]. Although technically feasible with very promising short-term results, the durability of the balloon-expandable percutaneous aortic valves will need to be compared with minimally invasive and conventional valve replacements. Further refinements, including smaller delivery sheaths, may make this technology more appealing for lower-risk patients if the long-term results are comparable [54].
Hybrid vascular procedures
Endovascular treatment strategies for atherosclerotic vascular diseases of the aorta, mesentery and lower extremities have proliferated and largely supplanted open surgical procedures in recent years. However, despite excellent results with endovascular therapies, subsets of patients who are unsuitable for endovascular treatment and for whom the risks of open surgical therapy would be prohibitive due to their comorbid risk profile still remain. Hybrid endovascular surgical therapy has emerged as a promising approach for such patients. In the following section, we will review hybrid endovascular surgical approaches for treatment of disease of the aortic arch and thoracoabdominal aorta.
In addition to hybrid treatment strategies for aortic disease, hybrid revascularization techniques have also been employed for treatment of coexisting carotid and CAD, coexisting carotid disease and aortic stenosis, as well as peripheral arterial disease of the lower extremities. We present a brief review of these areas of hybrid therapies in the following section.
Aortic arch pathology
Surgical therapy for large (5–6 cm), symptomatic or rapidly enlarging aortic aneurysms involving the aortic arch is recommended to reduce the risk of rupture [55]. In addition, surgical therapy is recommended for aortic dissection involving the aortic arch (Stanford type A) [56,57].
Traditional open surgical repair of aortic arch aneurysm and dissection necessitates aortic crossclamping, cardiopulmonary bypass and deep hypothermic circulatory arrest and is associated with significant morbidity and mortality [58–63]. Patients presenting with aortic arch pathologies tend to be relatively advanced in age and often have significant comorbidities, including cardiac disease, cerebrovascular disease, lung disease and chronic kidney disease, which serve to compound operative morbidity and mortality. Additionally, surgical exposure of the aortic arch can be challenging due to its complex 3D geometry with significant angulation.
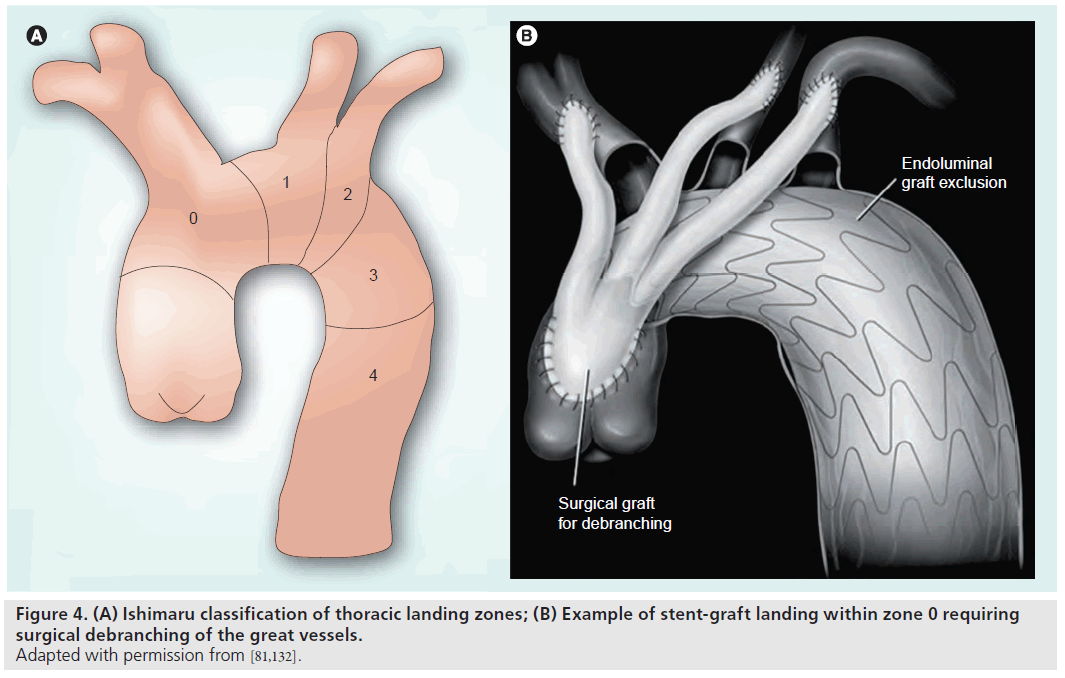
Endovascular therapies of the aortic arch are limited by the need to preserve the great vessels, as well as the availability of adequate diseasefree proximal and distal landing zones. For this reason, hybrid techniques have evolved that involve open surgical debranching of the aortic arch vessels followed by endovascular exclusion with stent graft placement. A variety of surgical techniques have been described for arch vessel debranching and repair/replacement of proximal or distal landing zones [64,65]. While a full description of the various operative techniques is beyond the scope of this article, in general, these techniques involve initial surgical debranching of the aortic arch vessels followed by antegrade or retrograde placement of a stent graft to exclude the diseased arch segment. The Ishimaru classification is commonly used to categorize thoracic aortic landing zones for thoracic stent grafts (Figure 4) [66]. Typically, landing in zones 0–1 requires debranching of the brachiocephalic vessels to allow for cerebral perfusion. Landing in zone 2 will result in coverage of the left subclavian artery and debranching may be required according to clinical circumstances. Open debranching may involve creation of bypasses or transposition of arch branch vessels to allow coverage of the ostia of these vessels by the stent graft without vascular compromise or end-organ injury. The two-step process of debranching and stent graft placement can be performed in one procedure or in a staged fashion.
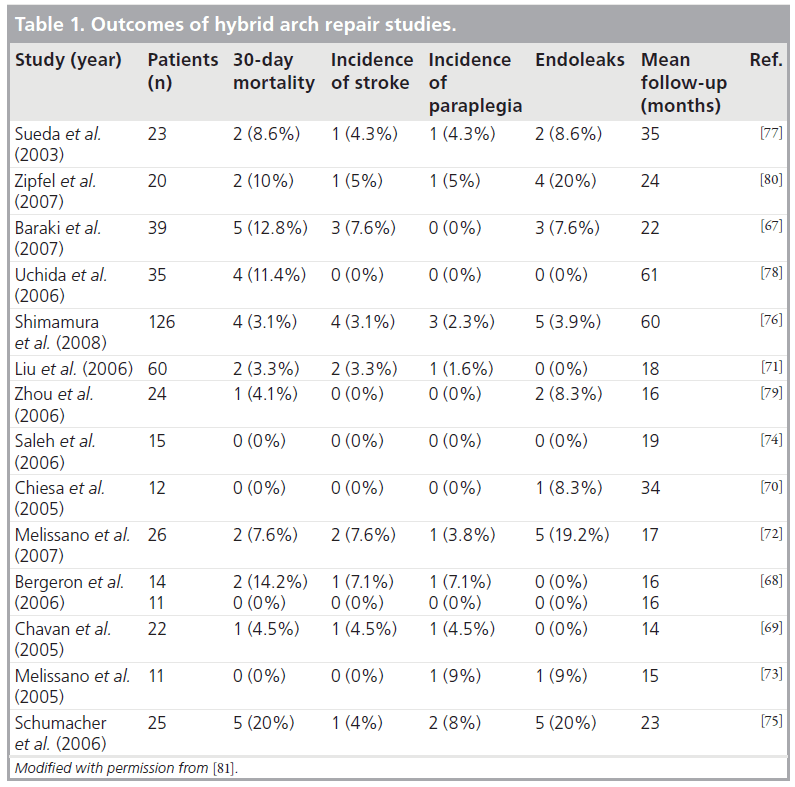
Results of hybrid surgical endovascular aortic arch procedures have generally been promising. Table 1 summarizes the outcomes of hybrid arch procedures reported by several groups [67–80]. As noted, short-term mortality and stroke rates are generally low, but long-term follow-up data are needed. A recent meta-analysis of these 15 studies, including 463 patients undergoing hybrid procedures for aortic arch disease, was published, demonstrating an overall 30-day mortality of 8.3%, stroke rate of 4.2% and endoleak rate of 9.2% [81]. It should be highlighted that these generally low mortality rates are being achieved in otherwise high-risk patients who are often deemed poor surgical candidates.
Endoleaks are a complication unique to endovascular repair and represents persistent vascular communication into the excluded segment. Reported rates of endovascular leaks vary by proximal stent graft landing zone, ranging from 7 to 33% [72]. Specific therapies tailored to the type of endoleak have been devised and are commonly employed.
Thoracoabdominal aortic aneurysm
Surgical therapy is recommended for large (6–7 cm), symptomatic or rapidly enlarging aneurysms to reduce the risk of rupture [55]. However, traditional open surgical therapy for thoracoabdominal aortic aneurysms (TAAAs) is associated with high rates of mortality (10–20%), as well as significant morbidity [82,83]. Aortic cross-clamping is mandatory in open repair and often a source of significant morbidity, including paraplegia due to spinal cord ischemia as well as mesenteric and renal ischemia. Additionally, patients with atherosclerotic aneurysmal disease of the thoracoabdominal aorta are typically older with significant comorbidities, further magnifying their operative risk.
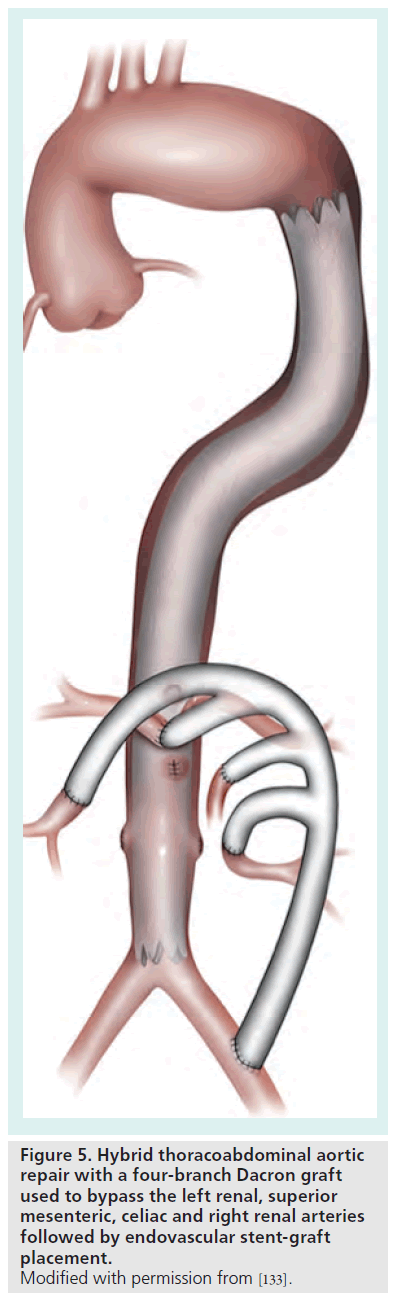
For these reasons, endovascular approaches have attracted significant attention. Although purely endovascular approaches have been described utilizing fenestrated grafts or grafts with formal branches, such techniques are limited to highly specialized centers and select patients in the context of ongoing clinical trials [84,85]. Outside of such settings, purely endovascular techniques are generally not feasible given the need to preserve major visceral and renal branches. Hybrid procedures involving laparoscopic or open surgical bypass of visceral and renal vessels followed by endovascular stent graft placement for aneurysm exclusion have thus been developed (Figure 5). The two steps of this hybrid procedure may be performed in one setting or as a staged procedure [86].
Figure 5. Hybrid thoracoabdominal aortic repair with a four-branch Dacron graft used to bypass the left renal, superior mesenteric, celiac and right renal arteries followed by endovascular stent-graft placement. Modified with permission from [133].
Results of hybrid therapy for TAAA have been encouraging. In a report of collaborative data on 107 patients from three major European vascular units, the 30-day mortality rate was 15% [87]. The mean age in this cohort was 67 years, with almost a fifth being over 75 years of age. Additionally, there was a high frequency of comorbidities in the cohort resulting in many patients being ineligible for open surgery. A pooled analysis of 108 patients undergoing hybrid therapy for TAAA reported a 30-day mortality of 10% in elective cases, which increased to 14.8% when emergency cases were included [88]. Over a mean follow-up of 10.6 months, graft patency rate was excellent at 97%. Once again, this was an older (mean age 68 years) cohort of patients with significant comorbidities.
An analysis of studies focusing on hybrid treatment of acute TAAA (ruptured or symptomatic) demonstrated a 30-day mortality of 32% [89]. This is encouraging given that these patients were treated in hybrid fashion as they were deemed too high risk for traditional open repair. Left untreated, the mortality risk for these patients with acute TAAA approaches 100%. Endoleak rates were 32.2% in the Drinkwater et al. cohort and 16.6% in the pooled analysis by Bakoyiannis et al. [87,88]. Specific therapies and treatments exist and are employed to treat endoleaks as appropriate.
Co-existing carotid & CAD
Coexisting significant atherosclerotic disease of the carotid and coronary arteries is a relatively frequent finding, with up to 50% of carotid endarterectomy (CEA) patients having significant CAD and up to 14% of patients undergoing CABG having significant carotid artery disease [90,91]. Clinically, this manifests as MI in patients undergoing CEA, with rates of MI as high as 17% in patients with severe CAD, or stroke in patients undergoing CABG, with rates as high as 20% in the setting of severe bilateral carotid disease [92,93].
Many studies of combined CEA and CABG have been performed, with widely varying results, and same reported rates of cumulative adverse events (stroke, MI and death) as high as 27% [92,94–99]. With the advent of carotid artery stenting (CAS) with distal embolic protection devices, there has been interest in combined CAS and CABG procedures to mitigate the risks associated with a combined surgical approach. Two basic strategies of combined CAS–CABG have been studied, the f irst involving CAS followed by staged CABG, and the other employing CAS followed by immediate CABG.
Ziada et al. reported the results from our institution of patients undergoing CAS followed by staged CABG, compared with patients undergoing combined CEA–CABG [99]. In this important analysis, the combined risk of MI or stroke was significantly lower in patients undergoing CAS followed by staged CABG (5.4%) compared with patients undergoing combined CEA–CABG (18.9%). The observation of a lower incidence of adverse events with CAS followed by CABG occurred despite a higher baseline risk profile in this group compared with the CEA–CABG group. Other investigators have reported lower rates of adverse outcomes with combined CEA– CABG, although still higher than with CEA alone [100]. More recently, Versaci et al. reported results on combined CAS–CABG for coexisting severe carotid and CAD [101]. In this cohort of 101 patients who underwent CAS followed immediately by on-pump CABG, the combined 30-day incidence of stroke, MI or death was 4%. Although the two approaches of combined CAS–CABG and CAS, followed by staged CABG have not been compared, the rates of major adverse outcomes associated with these hybrid techniques is favorable when compared with combined CEA–CABG.
Co-existing carotid artery & aortic valvular stenosis
As with coexisting carotid artery and CAD, coexisting carotid stenosis and aortic valvular stenosis often occur in the same milieu of advancing age and traditional risk factors, including hypertension and hyperlipidemia. It is estimated that up to 13% of patients with degenerative calcific aortic stenosis have coexisting carotid stenosis [102,103]. The presence of carotid stenosis significantly increases the risk of perioperative stroke in patients undergoing cardiac surgery [104].
As discussed previously, traditional strategies of combined or staged CEA and cardiac surgery have been associated with high rates of adverse outcomes, a finding in line with the often high comorbid risk profile of patients with coexistent disease. To mitigate this risk, a hybrid strategy of CAS followed by surgical management of aortic stenosis has been proposed. CAS in patients with severe aortic stenosis is particularly challenging, given that patients with severe aortic stenosis typically do not tolerate hypotension and reductions in preload – events that often occur with carotid bulb stimulation during carotid stenting.
Kar et al. recently analyzed the results of CAS in patients with severe AS from our institution [105]. In this study, 52 patients with severe coexisting carotid and aortic valvular stenosis underwent CAS. There was one transient ischemic attack and one case of cardiac arrest with successful resuscitation during the CAS procedure. Ionotropic agents were required in seven patients (13%) and another seven patients (13%) had Swan-Ganz catheters placed for hemodynamic monitoring during CAS. No MI post-CAS was observed. A total of 29 patients (56%) underwent eventual aortic valve replacement (AVR) with no postoperative strokes occurring in this group. Of the remaining 23 patients (44%) that did not undergo AVR, five died prior to AVR, seven were deemed not to be surgical candidates, eight had asymptomatic aortic stenosis and three refused AVR. Mortality for the entire cohort was 37% at a median follow-up of 3.8 years. Overall, this study demonstrated that hybrid CAS–AVR is feasible in this high-risk population and appears to reduce the risk of stroke following AVR. Further larger studies are needed to confirm and explore these findings.
Peripheral arterial disease of the lower extremities
A variety of hybrid procedures for the revascularization of lower extremity arterial disease have been reported [106–110]. A comprehensive discussion is beyond the scope of this article, however, we will briefly highlight this area. In general, hybrid techniques in this area involve the use of an endovascular stenting procedure in conjunction with an open surgical component to achieve complete revascularization. Such hybrid techniques offer the advantage of complete revascularization of often complex, diffuse disease in patients that are often of advanced age, with multiple comorbidities. Hybrid revascularization can often be performed in this difficult population with a less-extensive operative procedure and associated lower risk of perioperative morbidity and complications [107]. The endovascular portion of the procedure may be used for proximal/ inflow or distal/outflow revascularization or a combination of these during the hybrid revascularization procedure [106,109,111]. For example, a patient with multifocal disease involving the iliofemoral and femoropopliteal system may be treated in hybrid fashion with endovascular stenting of the iliac artery, followed by surgical bypass grafting of the femoropopliteal disease. Additionally, hybrid techniques are well suited for disease that extends through the common femoral artery – an area that is difficult to treat in a purely endovascular manner [112,113]. In such cases, the common femoral artery disease is treated with open endarterectomy and patch angioplasty, followed by endovascular therapy of the more proximal or distal disease in the iliac or superficial femoral artery segments. Studies of hybrid lower extremity revascularization techniques have demonstrated good outcomes with low perioperative mortality and morbidity, reasonable rates of primary and secondary patency and excellent limb-salvage rates [106,109,111–113].
Hybrid cardiovascular procedures related to access
Percutaneous therapies are favored to open surgical procedures in patients at high risk, including those who are critically ill, and those that have been previously operated. For these reasons, percutaneous therapies have been reported for disorders traditionally treated with surgical therapies, including postinfarction ventricular septal ruptures (PI-VSR) as well paravalvular leaks (PVLs) of previously placed prosthetic valves [114–120]. Despite the success observed with these techniques, there remains a subset of patients with these lesions that cannot be treated effectively from a purely percutaneous approach. The barriers to successful percutaneous treatment of these lesions are primarily related to difficulty in adequate lesion access. In the following section, we will briefly describe hybrid techniques for treatment of prosthetic valve PVL and PI-VSR.
Hybrid closure of PVL
Development of a PVL is a rare but often serious and difficult-to-treat complication. PVLs typically manifest within 6 months of valvular replacement, with a reported incidence of 3–12.5% [121]. Such lesions, if severe, may lead to congestive heart failure or significant hemolysis. The traditional therapy for a symptomatic PVL has been reoperation. Such redo surgeries carry an increased risk, with mortality rates as high as 18% [122]. Furthermore, the patients presenting with these lesions are often of advanced age, with multiple comorbidities, often making the risk of reoperation prohibitive. Percutaneous transcatheter closure of PVLs have been developed and have become increasingly popular with reported success rates of 60–90% [114,117–119,123]. Percutaneous closure of a PVL requires traversal of the leak with a guidewire, followed by successful deployment of a device to reduce or ideally obliterate the leak. However, in rare cases, it is not possible to close a PVL from a percutaneous approach, due to difficulty accessing the lesion. Examples include medial and posteromedial mitral PVLs and other mitral PVLs that are directly adjacent to the interatrial septum and aortic valve. PVLs in these locations are difficult to access from the antegrade route, due to acute angulation once the interatrial septum is crossed. Similarly, retrograde approaches may not be feasible. In these circumstances, a percutaneous transapical approach may be preferably allowing easy crossing of the defect by the wire. This wire is then externalized via the transseptal sheath and the occluder device is delivered over the externalized wire transseptally.
In rare cases, transapical delivery of the occluder device may be required. For these patients, hybrid techniques for PVL closure have been reported [124,125]. In these cases of mitral PVL closure, the cardiac surgeon typically performs a limited lateral thoracotomy to provide access to the left ventricle or left atrium. In the former, a sheath is introduced into the left ventricle via the apex and, under fluoroscopic and transesophageal echo guidance, the PVL is crossed with a guidewire over which the sheath is advanced into the left atrium. Subsequently, the occluder device of choice is deployed. This technique does not require cardiopulmonary bypass. Other potential hybrid approaches include limited thoracotomy followed by opening of the left atrium and deployment of an occluder device across the PVL under direct visualization. In contrast to the previous technique, this procedure does require cardiopulmonary bypass.
Hybrid closure of post-infarction ventricular septal rupture
Postinfarction ventricular septal rupture is a rare but serious complication of MI. The incidence has been reported as 0.2% with a 30-day mortality of 47% in medically treated patients and 94% in surgically treated patients [126]. Surgical repair of PI-VSR is often difficult, due to friable tissue at the margins of the defect, and carries a significant risk of dehiscence, which is associated with increased mortality rates [126–130]. In addition, surgical repair of PI-VSR is particularly challenging when the defect is posterobasal in location. Furthermore, in patients who are critically ill in the setting of recent MI complicated by PI-VSR or cardiogenic shock, the surgical risk is often quite high. As a result, percutaneous closure techniques for closure of PI-VSR have been developed and are increasingly frequently employed in these settings [115,116,120].
Despite the less invasive and adaptive nature of percutaneous techniques for PI-VSR closure, such techniques are limited in certain circumstances, particularly in the acute phase of acute MI. In certain cases, delivery of the occluder device cannot be accomplished due to interference by or entrapment of valvular leaflets or apparatus. Additionally, challenging PI-VSR location and geometry can sometimes limit adequate device closure via purely percutaneous techniques. As a result, hybrid techniques for closure of PI-VSR have been employed and reported. At our institution, we have performed hybrid PI-VSR closure from the left ventricular side with femoral artery transcatheter deployment of an Amplatzer® (St Jude Medical Inc., MN, USA) occluder device, followed by surgical opening of the right atrium and further stabilization of the right ventricular disc with patching and/or plegeted sutures. Others have reported hybrid techniques where, under cardiopulmonary bypass, the right atrium is opened and the delivery sheath is delivered across the PI-VSR from the right ventricular side and the device is deployed under direct visualization [131]. Such techniques have the advantage of direct device manipulation, reinforcement and augmentation where needed – maneuvers that are not possible from a purely percutaneous approach. In addition, such hybrid techniques do not require ventriculotomy as an advantage over a traditional surgical approach.
Future perspective
With the continued evolution of interventional and surgical techniques, the landscape of hybrid cardiovascular therapies will undoubtedly continue to evolve. As interventional cardiologists become more invasive and aggressive with new devices and techniques, and as cardiac and vascular surgeons move towards increasingly less-invasive techniques with frequent use of robotic technologies, the gap between these fields will continue to narrow. This will likely lead to increased hybridization and continued growth of hybrid cardiovascular techniques. In addition, there will always remain a population of patients that are not candidates for traditional interventional or surgical therapies for clinical or technical reasons. Specific areas of further potential research include further elucidation of optimal timing strategies between stages of hybrid procedures, as well as timing strategies for administration of antiplatelet agents, as they pertain to hybrid myocardial revascularization procedures. In addition, there will be continued improvements, growth and integration in the multimodality imaging technologies employed for hybrid cardiovascular procedures, including computed tomography and echocardiography. Finally, new hybrid cardiovascular procedures and techniques will undoubtedly be developed, with perhaps the greatest potential of growth in the realm of valvular heart disease. For these reasons, we look to the future of hybrid cardiovascular care with great enthusiasm and excitement.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Hybrid myocardial revascularization
▪ A viable option for multivessel coronary artery disease, particularly in patients poorly suited for coronary artery bypass grafting using saphenous vein grafts.
▪ Hybrid myocardial revascularization may have increasing interest due to improved long-term patency of drug-eluting stents in comparison with saphenous vein grafts.
▪ Further studies are required to determine optimal anticoagulant strategies during these procedures.
Hybrid valve procedures
▪ The impetus for novel less-invasive surgical valvular procedures is to improve outcomes, patient experience and minimize complications.
▪ Percutaneous coronary intervention coupled with traditional valve replacement or minimally invasive valve replacement may provide an attractive alternative strategy to traditional full-sternotomy cardiac surgical procedures in select high-risk populations with combined coronary and valvular heart disease.
▪ Transcatheter aortic valve replacement is emerging as the treatment of choice in very high-risk patients with aortic stenosis.
Hybrid vascular procedures
▪ Hybrid techniques are increasingly employed for aneurysmal disease and dissections of the aortic arch and thoracoabdominal aorta.
▪ The Ishimaru classification is often used to classify aortic arch landing zones for stent grafts and determine the need for debranching/ bypass of aortic arch branch vessels.
▪ The interval of staging between debranching/bypass and stent graft placement is variable in the published literature, and the ‘optimal’ interval remains undefined.
▪ Combined carotid artery stenting–coronary artery bypass grafting in an immediate or staged fashion appear to be effective strategies with lower risks of major adverse cardiac events compared with combined carotid endarterectomy-coronary artery bypass grafting in patients with combined carotid and coronary artery disease.
▪ Hybrid procedures for revascularization of lower extremity peripheral arterial disease have been widely reported with good outcomes and typically involve endovascular stenting in conjunction with surgical endarterectomy and/or bypass to treat complex, diffuse/ multifocal disease.
Hybrid cardiovascular procedures related to access
▪ Some complex lesions such as periprosthetic valvular leaks and postinfarction ventricular rupture may be difficult to access from standard surgical or percutaneous approaches. Hybrid cardiovascular techniques for treatment of these lesions have been developed and reported.
References
Papers of special note have been highlighted as:
▪ of interest
- Angelini GD, Wilde P, Salerno TA, Bosco G, Calafiore AM: Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularisation. Lancet 347(9003), 757–758 (1996).
- Krishnaswamy A, Tuzcu EM, Kapadia SR: Three-dimensional computed tomography in the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. DOI: 10.1002/ ccd.22740 (2010) (Epub ahead of print).
- Lloyd CT, Calafiore AM, Wilde P et al.: Integrated left anterior small thoracotomy and angioplasty for coronary artery revascularization. Ann. Thorac. Surg. 68(3), 908–911 (1999).
- Bravata DM, Gienger AL, Mcdonald KM et al.: Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Ann. Intern. Med. 147(10), 703–716 (2007).
- Hoffman SN, Tenbrook JA, Wolf MP, Pauker SG, Salem DN, Wong JB: A meta-analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: one- to eight-year outcomes. J. Am. Coll. Cardiol. 41(8), 1293–1304 (2003).
- Serruys PW, Morice MC, Kappetein AP et al.: Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360(10), 961–972 (2009).
- Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA: ACCF/SCAI/STS/AATS/ AHA/ASNC 2009 appropriateness criteria for coronary revascularization: a report by the American College of Cardiology Foundation appropriateness criteria task force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J. Am. Coll. Cardiol. 53(6), 530–553 (2009).
- BARI Investigators: The final 10‑year follow-up results from the BARI randomized trial. J. Am. Coll. Cardiol. 49(15), 1600–1606 (2007).
- Hayward PA, Buxton BF: Contemporary coronary graft patency: 5‑year observational data from a randomized trial of conduits. Ann. Thorac. Surg. 84(3), 795–799 (2007).
- Kim KB, Cho KR, Jeong DS: Midterm angiographic follow-up after off-pump coronary artery bypass: serial comparison using early, 1‑year, and 5‑year postoperative angiograms. J. Thorac. Cardiovasc. Surg. 135(2), 300–307 (2008).
- Tatoulis J, Buxton BF, Fuller JA: Patencies of 2127 arterial to coronary conduits over 15 years. Ann. Thorac. Surg. 77(1), 93–101 (2004).
- Taggart DP, D’Amico R, Altman DG: Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 358(9285), 870–875 (2001).
- Rankin JS, Tuttle RH, Wechsler AS, Teichmann TL, Glower DD, Califf RM: Techniques and benefits of multiple internal mammary artery bypass at 20 years of follow-up. Ann. Thorac. Surg. 83(3), 1008–1014 (2007).
- Taggart DP, Altman DG, Gray AM et al.: Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1‑year results of the Arterial Revascularisation Trial (ART). Eur. Heart J. 31(20), 2470–2481
- Fukui T, Tabata M, Manabe S, Shimokawa T, Takanashi S: Graft selection and one-year patency rates in patients undergoing coronary artery bypass grafting. Ann. Thorac. Surg. 89(6), 1901–1905 (2010).
- Glineur D, D’hoore W, El Khoury G et al.: Angiographic predictors of 6‑month patency of bypass grafts implanted to the right coronary artery a prospective randomized comparison of gastroepiploic artery and saphenous vein grafts. J. Am. Coll. Cardiol. 51(2), 120–125 (2008).
- Dangas G, Ellis SG, Shlofmitz R et al.: Outcomes of paclitaxel-eluting stent implantation in patients with stenosis of the left anterior descending coronary artery. J. Am. Coll. Cardiol. 45(8), 1186–1192 (2005).
- Kim YH, Dangas GD, Solinas E et al.: Effectiveness of drug-eluting stent implantation for patients with unprotected left main coronary artery stenosis. Am. J. Cardiol. 101(6), 801–806 (2008).
- Solinas E, Dangas G, Kirtane AJ et al.: Angiographic patterns of drug-eluting stent restenosis and one-year outcomes after treatment with repeated percutaneous coronary intervention. Am. J. Cardiol. 102(3), 311–315 (2008).
- Bonatti J, Schachner T, Bonaros N et al.: Simultaneous hybrid coronary revascularization using totally endoscopic left internal mammary artery bypass grafting and placement of rapamycin eluting stents in the same interventional session. The combination pilot study. Cardiology 110(2), 92–95 (2008).
- Davidavicius G, Van Praet F, Mansour S et al.: Hybrid revascularization strategy: a pilot study on the association of robotically enhanced minimally invasive direct coronary artery bypass surgery and fractional-flowreserve- guided percutaneous coronary intervention. Circulation 112(Suppl. 9), I317–3I22 (2005).
- De Canniere D, Jansens JL, Goldschmidt- Clermont P, Barvais L, Decroly P, Stoupel E: Combination of minimally invasive coronary bypass and percutaneous transluminal coronary angioplasty in the treatment of double-vessel coronary disease: two-year follow-up of a new hybrid procedure compared with “on-pump” double bypass grafting. Am. Heart J. 142(4), 563–570 (2001).
- Gao C, Yang M, Wu Y et al.: Hybrid coronary revascularization by endoscopic robotic coronary artery bypass grafting on beating heart and stent placement. Ann. Thorac. Surg. 87(3), 737–741 (2009).
- Gilard M, Bezon E, Cornily JC et al.: Same-day combined percutaneous coronary intervention and coronary artery surgery. Cardiology 108(4), 363–367 (2007).
- Holzhey DM, Jacobs S, Mochalski M et al.: Minimally invasive hybrid coronary artery revascularization. Ann Thorac. Surg 86(6), 1856–1860 (2008).
- Katz MR, Van Praet F, De Canniere D et al.: Integrated coronary revascularization: Percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation 114(Suppl. 1), I473–I476 (2006).
- Kon ZN, Brown EN, Tran R et al.: Simultaneous hybrid coronary revascularization reduces postoperative morbidity compared with results from conventional off-pump coronary artery bypass. J. Thorac. Cardiovasc. Surg. 135(2), 367–375 (2008).
- Lee MS, Wilentz JR, Makkar RR et al.: Hybrid revascularization using percutaneous coronary intervention and robotically assisted minimally invasive direct coronary artery bypass surgery. J. Invasive Cardiol. 16(8), 419–425 (2004).
- Reicher B, Poston RS, Mehra MR et al.: Simultaneous “hybrid” percutaneous coronary intervention and minimally invasive surgical bypass grafting: feasibility, safety, and clinical outcomes. Am. Heart J. 155(4), 661–667 (2008).
- Riess FC, Bader R, Kremer P et al.: Coronary hybrid revascularization from January 1997 to January 2001: a clinical follow-up. Ann. Thorac. Surg. 73(6), 1849–1855 (2002).
- Stahl KD, Boyd WD, Vassiliades TA, Karamanoukian HL: Hybrid robotic coronary artery surgery and angioplasty in multivessel coronary artery disease. Ann. Thorac. Surg. 74(4), S1358–S1362 (2002).
- Us MH, Basaran M, Yilmaz M et al.: Hybrid coronary revascularization in high-risk patients. Tex. Heart Inst. J. 33(4), 458–462 (2006).
- Kiaii B, McClure RS, Stewart P et al.: Simultaneous integrated coronary artery revascularization with long-term angiographic follow-up. J. Thorac. Cardiovasc. Surg. 136(3), 702–708 (2008).
- Merry AF, Raudkivi PJ, Middleton NG et al.: Bivalirudin versus heparin and protamine in off-pump coronary artery bypass surgery. Ann. Thorac. Surg. 77(3), 925–931 (2004).
- Smedira NG, Dyke CM, Koster A et al.: Anticoagulation with bivalirudin for off-pump coronary artery bypass grafting: the results of the evolution-off study. J. Thorac. Cardiovasc. Surg. 131(3), 686–692 (2006).
- Lincoff AM, Bittl JA, Harrington RA et al.: Bivalirudin and provisional glycoprotein IIB/ IIIA blockade compared with heparin and planned glycoprotein IIB/IIIA blockade during percutaneous coronary intervention: Replace-2 randomized trial. JAMA 289(7), 853–863 (2003).
- Lincoff AM, Steinhubl SR, Manoukian SV et al.: Influence of timing of clopidogrel treatment on the efficacy and safety of bivalirudin in patients with non-ST-segment elevation acute coronary syndromes undergoing percutaneous coronary intervention: an analysis of the acuity (acute catheterization and urgent intervention triage strategy) trial. JACC Cardiovasc. Interv. 1(6), 639–648 (2008).
- Stone GW, Ware JH, Bertrand ME et al.: Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the acuity trial. JAMA 298(21), 2497–2506 (2007).
- Stone GW, White HD, Ohman EM et al.: Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the acute catheterization and urgent intervention triage strategy (acuity) trial. Lancet 369(9565), 907–919 (2007).
- Gao P, Xiong H, Zheng Z, Li L, Gao R, Hu SS: Evaluation of antiplatelet effects of a modified protocol by platelet aggregation in patients undergoing “one-stop” hybrid coronary revascularization. Platelets 21(3), 183–190 (2010).
- Sabik JF 3rd, Blackstone EH: Coronary artery bypass graft patency and competitive flow. J. Am. Coll. Cardiol. 51(2), 126–128 (2008).
- Zhao DX, Leacche M, Balaguer JM et al.: Routine intraoperative completion angiography after coronary artery bypass grafting and 1-stop hybrid revascularization results from a fully integrated hybrid catheterization laboratory/operating room. J. Am. Coll. Cardiol. 53(3), 232–241 (2009).
- Soltesz EG, Cohn LH: Minimally invasive valve surgery. Cardiol. Rev. 15(3), 109–115 (2007).
- Cosgrove DM 3rd, Sabik JF: Minimally invasive approach for aortic valve operations. Ann. Thorac. Surg. 62(2), 596–597 (1996).
- Svensson LG: Minimally invasive surgery with a partial sternotomy “J” approach. Semin. Thorac. Cardiovasc. Surg. 19(4), 299–303 (2007).
- Byrne JG, Leacche M, Unic D et al.: Staged initial percutaneous coronary intervention followed by valve surgery (“hybrid approach”) for patients with complex coronary and valve disease. J. Am. Coll. Cardiol. 45(1), 14–18 (2005).
- Mihaljevic T, Cohn LH, Unic D, Aranki SF, Couper GS, Byrne JG: One thousand minimally invasive valve operations: early and late results. Ann. Surg. 240(3), 529–534 (2004).
- Svensson LG, Atik FA, Cosgrove DM et al.: Minimally invasive versus conventional mitral valve surgery: a propensity-matched comparison. J. Thorac. Cardiovasc. Surg. 139(4), 926–932 E921–E922 (2010).
- Brinster DR, Byrne M, Rogers CD et al.: Effectiveness of same day percutaneous coronary intervention followed by minimally invasive aortic valve replacement for aortic stenosis and moderate coronary disease (“hybrid approach”). Am. J. Cardiol. 98(11), 1501–1503 (2006).
- Vahanian A, Alfieri O, Al-Attar N et al.: Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 29(11), 1463–1470 (2008).
- Thomas M, Schymik G, Walther T et al.: Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (source) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 122(1), 62–69 (2010).
- Ye J, Cheung A, Lichtenstein SV et al.: Transapical transcatheter aortic valve implantation: follow-up to 3 years. J. Thorac. Cardiovasc. Surg. 139(5), 1107–1113, 1113 E1101 (2010).
- Pasic M, Unbehaun A, Dreysse S et al.: Transapical aortic valve implantation in 175 consecutive patients: excellent outcome in very high-risk patients. J. Am. Coll. Cardiol. 56(10), 813–820 (2010).
- Tuzcu EM, Kapadia SR, Svensson LG: “SOURCE” of enthusiasm for transcatheter aortic valve implantation. Circulation 122(1), 8–10 (2010).
- Elefteriades JA: Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann. Thorac. Surg. 74(5), S1877–S1880 (2002).
- Erbel R, Alfonso F, Boileau C et al.: Diagnosis and management of aortic dissection. Eur. Heart J. 22(18), 1642–1681 (2001).
- Hagan PG, Nienaber CA, Isselbacher EM et al.: The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 283(7), 897–903 (2000).
- Appoo JJ, Augoustides JG, Pochettino A et al.: Perioperative outcome in adults undergoing elective deep hypothermic circulatory arrest with retrograde cerebral perfusion in proximal aortic arch repair: evaluation of protocol-based care. J. Cardiothorac. Vasc. Anesth. 20(1), 3–7 (2006).
- Bachet J, Guilmet D, Goudot B et al.: Antegrade cerebral perfusion with cold blood: a 13‑year experience. Ann. Thorac. Surg. 67(6), 1874–1878 (1999).
- Okita Y, Ando M, Minatoya K, Kitamura S, Takamoto S, Nakajima N: Predictive factors for mortality and cerebral complications in arteriosclerotic aneurysm of the aortic arch. Ann. Thorac. Surg. 67(1), 72–78 (1999).
- Safi HJ, Miller CC 3rd, Estrera AL et al.: Optimization of aortic arch replacement: twostage approach. Ann. Thorac. Surg. 83(2), S815–S818 (2007).
- Sundt TM 3rd, Orszulak TA, Cook DJ, Schaff HV: Improving results of open arch replacement. Ann. Thorac. Surg. 86(3), 787–796 (2008).
- Svensson LG, Crawford ES, Hess KR et al.: Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J. Thorac. Cardiovasc. Surg. 106(1), 19–28 (1993).
- Bockler D, Nassar J, Kotelis D et al.: Hybrid approach for arch and thoracoabdominal pathologies. J. Cardiovasc. Surg. (Torino) 50(4), 461–474 (2009).
- Desai ND, Szeto WY: Complex aortic arch aneurysm and dissections: hybrid techniques for surgical and endovascular therapy. Curr. Opin. Cardiol. 24(6), 521–527 (2009).
- Mitchell RS, Ishimaru S, Criado FJ et al.: Third international summit on thoracic aortic endografting: lessons from long-term results of thoracic stent-graft repairs. J. Endovasc. Ther. 12(1), 89–97 (2005).
- Baraki H, Hagl C, Khaladj N et al.: The frozen elephant trunk technique for treatment of thoracic aortic aneurysms. Ann. Thorac. Surg. 83(2), S819–S823 (2007).
- Bergeron P, Mangialardi N, Costa P et al.: Great vessel management for endovascular exclusion of aortic arch aneurysms and dissections. Eur. J. Vasc. Endovasc. Surg. 32(1), 38–45 (2006).
- Chavan A, Karck M, Hagl C et al.: Hybrid endograft for one-step treatment of multisegment disease of the thoracic aorta. J. Vasc. Interv. Radiol. 16(6), 823–829 (2005).
- Chiesa R, Melissano G, Marrocco- Trischitta MM, Civilini E, Setacci F: Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J. Vasc. Surg. 42(1), 11–17 (2005).
- Liu ZG, Sun LZ, Chang Q et al.: Should the “elephant trunk” be skeletonized? Total arch replacement combined with stented elephant trunk implantation for stanford type a aortic dissection. J. Thorac. Cardiovasc. Surg. 131(1), 107–113 (2006).
- Melissano G, Bertoglio L, Civilini E et al.: Results of thoracic endovascular grafting in different aortic segments. J. Endovasc. Ther. 14(2), 150–157 (2007).
- Melissano G, Civilini E, De Moura MR, Calliari F, Chiesa R: Single center experience with a new commercially available thoracic endovascular graft. Eur. J. Vasc. Endovasc. Surg. 29(6), 579–585 (2005).
- Saleh HM, Inglese L: Combined surgical and endovascular treatment of aortic arch aneurysms. J. Vasc. Surg. 44(3), 460–466 (2006).
- Schumacher H, Von Tengg-Kobligk H, Ostovic M et al.: Hybrid aortic procedures for endoluminal arch replacement in thoracic aneurysms and type b dissections. J. Cardiovasc. Surg. (Torino) 47(5), 509–517 (2006).
- Shimamura K, Kuratani T, Matsumiya G et al.: Long-term results of the open stent-grafting technique for extended aortic arch disease. J. Thorac. Cardiovasc. Surg. 135(6), 1261–1269 (2008).
- Sueda T, Orihashi K, Okada K, Sugawara Y, Imai K, Kochi K: Fate of aneurysms of the distal arch and proximal descending thoracic aorta after transaortic endovascular stent-grafting. Ann. Thorac. Surg. 76(1), 84–89 (2003).
- Uchida N, Ishihara H, Shibamura H, Kyo Y, Ozawa M: Midterm results of extensive primary repair of the thoracic aorta by means of total arch replacement with open stent graft placement for an acute type a aortic dissection. J. Thorac. Cardiovasc. Surg. 131(4), 862–867 (2006).
- Zhou W, Reardon M, Peden EK, Lin PH, Lumsden AB: Hybrid approach to complex thoracic aortic aneurysms in high-risk patients: surgical challenges and clinical outcomes. J. Vasc. Surg. 44(4), 688–693 (2006).
- Zipfel B, Hammerschmidt R, Krabatsch T, Buz S, Weng Y, Hetzer R: Stent-grafting of the thoracic aorta by the cardiothoracic surgeon. Ann. Thorac. Surg. 83(2), 441–448 (2007).
- Koullias GJ, Wheatley GH 3rd: State-of-theart of hybrid procedures for the aortic arch: a meta-analysis. Ann. Thorac. Surg. 90(2), 689–697 (2010).
- Davies RR, Goldstein LJ, Coady MA et al.: Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann. Thorac. Surg. 73(1), 17–27 (2002).
- Johansson G, Markstrom U, Swedenborg J: Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J. Vasc. Surg. 21(6), 985–988 (1995).
- Greenberg RK, Lytle B: Endovascular repair of thoracoabdominal aneurysms. Circulation 117(17), 2288–2296 (2008).
- Greenberg RK, Sternbergh WC 3rd, Makaroun M et al.: Intermediate results of a united states multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J. Vasc. Surg. 50(4), 730–737 E731 (2009).
- Bakoyiannis CN, Economopoulos KP, Kalles VC, Papalambros E: Concurrent versus two-stage hybrid procedures in the treatment of thoracoabdominal aortic aneurysms. J. Endovasc. Ther. 16(6), 757–759 (2009).
- Drinkwater SL, Bockler D, Eckstein H et al.: The visceral hybrid repair of thoracoabdominal aortic aneurysms – a collaborative approach. Eur. J. Vasc. Endovasc. Surg. 38(5), 578–585 (2009).
- Bakoyiannis C, Kalles V, Economopoulos K, Georgopoulos S, Tsigris C, Papalambros E: Hybrid procedures in the treatment of thoracoabdominal aortic aneurysms: a systematic review. J. Endovasc. Ther. 16(4), 443–450 (2009).
- Von Meyenfeldt EM, Schnater JM, Reekers JA, Balm R: An emergency visceral hybrid procedure for ruptured thoraco-abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 38(2), 162–168 (2009).
- Borger MA, Fremes SE, Weisel RD et al.: Coronary bypass and carotid endarterectomy: does a combined approach increase risk? A metaanalysis. Ann. Thorac. Surg. 68(1), 14–20 (1999).
- Salasidis GC, Latter DA, Steinmetz OK, Blair JF, Graham AM: Carotid artery duplex scanning in preoperative assessment for coronary artery revascularization: the association between peripheral vascular disease, carotid artery stenosis, and stroke. J. Vasc. Surg. 21(1), 154–160 (1995).
- Trachiotis GD, Pfister AJ: Management strategy for simultaneous carotid endarterectomy and coronary revascularization. Ann. Thorac. Surg. 64(4), 1013–1018 (1997).
- Van Der Heyden J, Suttorp MJ, Bal ET et al.: Staged carotid angioplasty and stenting followed by cardiac surgery in patients with severe asymptomatic carotid artery stenosis: early and long-term results. Circulation 116(18), 2036–2042 (2007).
- Allie DE, Lirtzman M, Malik AP, Kowalski JM, Barker EA, Walker CM: Rapid-staged strategy for concomitant critical carotid and left main coronary disease with left ventricular dysfunction: IABP use. Ann. Thorac. Surg. 66(4), 1230–1235 (1998).
- Giangola G, Migaly J, Riles TS et al.: Perioperative morbidity and mortality in combined vs. staged approaches to carotid and coronary revascularization. Ann. Vasc. Surg. 10(2), 138–142 (1996).
- Minami K, Fukahara K, Boethig D, Bairaktaris A, Fritzsche D, Koerfer R: Long-term results of simultaneous carotid endarterectomy and myocardial revascularization with cardiopulmonary bypass used for both procedures. J. Thorac.Cardiovasc. Surg. 119(4 Pt 1), 764–773 (2000).
- Plestis KA, Ke S, Jiang ZD, Howell JF: Combined carotid endarterectomy and coronary artery bypass: immediate and long-term results. Ann. Vasc. Surg. 13(1), 84–92 (1999).
- Vassilidze TV, Cernaianu AC, Gaprindashvili T, Gallucci JG, Cilley JH Jr, Delrossi AJ: Simultaneous coronary artery bypass and carotid endarterectomy. Determinants of outcome. Tex. Heart Inst. J. 21(2), 119–124 (1994).
- Ziada KM, Yadav JS, Mukherjee D et al.: Comparison of results of carotid stenting followed by open heart surgery versus combined carotid endarterectomy and open heart surgery (coronary bypass with or without another procedure). Am. J. Cardiol. 96(4), 519–523 (2005).
- Chiti E, Troisi N, Marek J et al.: Combined carotid and cardiac surgery: improving the results. Ann. Vasc. Surg. 24(6), 794–800 (2010).
- Versaci F, Reimers B, Del Giudice C et al.: Simultaneous hybrid revascularization by carotid stenting and coronary artery bypass grafting: the sharp study. JACC Cardiovasc. Interv. 2(5), 393–401 (2009).
- Aronow WS, Kronzon I, Schoenfeld MR: Prevalence of extracranial carotid arterial disease and of valvular aortic stenosis and their association in the elderly. Am. J. Cardiol. 75(4), 304–305 (1995).
- Kablak-Ziembicka A, Przewlocki T, Hlawaty M et al.: Internal carotid artery stenosis in patients with degenerative aortic stenosis. Kardiol. Pol. 66(8), 837–842 (2008).
- Ricotta JJ, Faggioli GL, Castilone A, Hassett JM: Risk factors for stroke after cardiac surgery: Buffalo cardiac–cerebral study group. J. Vasc. Surg. 21(2), 359–363 (1995).
- Kar S, Krishnaswamy A, Shishehbor MH et al.: Safety and efficacy of carotid stenting in individuals with concomitant severe carotid and aortic stenosis. EuroIntervention 6(4), 492–497 (2010).
- Cotroneo AR, Iezzi R, Marano G, Fonio P, Nessi F, Gandini G: Hybrid therapy in patients with complex peripheral multifocal steno-obstructive vascular disease: two-year results. Cardiovasc. Intervent. Radiol. 30(3), 355–361 (2007).
- Dosluoglu HH, O’Brien-Irr MS, Lukan J, Harris LM, Dryjski ML, Cherr GS: Does preferential use of endovascular interventions by vascular surgeons improve limb salvage, control of symptoms, and survival of patients with critical limb ischemia? Am. J. Surg. 192(5), 572–576 (2006).
- Leville CD, Kashyap VS, Clair DG et al.: Endovascular management of iliac artery occlusions: extending treatment to transatlantic inter-society consensus class c and d patients. J. Vasc. Surg. 43(1), 32–39 (2006).
- Miyahara T, Miyata T, Shigematsu H et al.: Long-term results of combined iliac endovascular intervention and infrainguinal surgical revascularization for treatment of multilevel arterial occlusive disease. Int. Angiol. 24(4), 340–348 (2005).
- Nelson PR, Powell RJ, Schermerhorn ML et al.: Early results of external iliac artery stenting combined with common femoral artery endarterectomy. J. Vasc. Surg. 35(6), 1107–1113 (2002).
- Dougherty MJ, Young LP, Calligaro KD: One hundred twenty-five concomitant endovascular and open procedures for lower extremity arterial disease. J. Vasc. Surg. 37(2), 316–322 (2003).
- Gisbertz SS, Ramzan M, Tutein Nolthenius RP et al.: Short-term results of a randomized trial comparing remote endarterectomy and supragenicular bypass surgery for long occlusions of the superficial femoral artery [the REVAS trial]. Eur. J. Vasc. Endovasc. Surg. 37(1), 68–76 (2009).
- Gisbertz SS, Tutein Nolthenius RP, De Borst GJ et al.: Remote endarterectomy versus supragenicular bypass surgery for long occlusions of the superficial femoral artery: medium-term results of a randomized controlled trial (the REVAS trial). Ann. Vasc. Surg. 24(8), 1015–1023 (2010).
- Cortes M, Garcia E, Garcia-Fernandez MA, Gomez JJ, Perez-David E, Fernandez- Aviles F: Usefulness of transesophageal echocardiography in percutaneous transcatheter repairs of paravalvular mitral regurgitation. Am. J. Cardiol. 101(3), 382–386 (2008).
- Eshtehardi P, Garachemani A, Meier B: Percutaneous closure of a postinfarction ventricular septal defect and an iatrogenic left ventricular free-wall perforation using two Amplatzer muscular VSD occluders. Catheter. Cardiovasc. Interv. 74(2), 243–246 (2009).
- Maltais S, Ibrahim R, Basmadjian AJ et al.: Postinfarction ventricular septal defects: Towards a new treatment algorithm? Ann. Thorac. Surg. 87(3), 687–692 (2009).
- Pate GE, Al Zubaidi A, Chandavimol M, Thompson CR, Munt BI, Webb JG: Percutaneous closure of prosthetic paravalvular leaks: case series and review. Catheter. Cardiovasc. Interv. 68(4), 528–533 (2006).
- Shapira Y, Hirsch R, Kornowski R et al.: Percutaneous closure of perivalvular leaks with Amplatzer occluders: feasibility, safety, and shortterm results. J. Heart Valve Dis. 16(3), 305–313 (2007).
- Sorajja P, Cabalka AK, Hagler DJ et al.: Successful percutaneous repair of perivalvular prosthetic regurgitation. Catheter. Cardiovasc. Interv. 70(6), 815–823 (2007).
- Thiele H, Kaulfersch C, Daehnert I et al.: Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur. Heart J. 30(1), 81–88 (2009).
- Genoni M, Franzen D, Vogt P et al.: Paravalvular leakage after mitral valve replacement: improved long-term survival with aggressive surgery? Eur. J. Cardiothorac. Surg. 17(1), 14–19 (2000).
- Echevarria JR, Bernal JM, Rabasa JM, Morales D, Revilla Y, Revuelta JM: Reoperation for bioprosthetic valve dysfunction. A decade of clinical experience. Eur. J. Cardiothorac. Surg. 5(10), 523–526 (1991).
- Kim MS, Casserly IP, Garcia JA, Klein AJ, Salcedo EE, Carroll JD: Percutaneous transcatheter closure of prosthetic mitral paravalvular leaks: are we there yet? JACC Cardiovasc. Interv. 2(2), 81–90 (2009).
- Kulik A, Labinaz M, Beauchesne LM, Nicholson D, Bedard P: Hybrid repair of mitral paravalvular leak: open surgical placement of a percutaneous occluder device. J. Thorac. Cardiovasc. Surg. 132(6), 1469–1470 (2006).
- Lang N, Kozlik-Feldmann R, Dalla Pozza R et al.: Hybrid occlusion of a paravalvular leak with an Amplatzer Septal Occluder after mechanical aortic and mitral valve replacement. J. Thorac. Cardiovasc. Surg. 139(1), 221–222 (2010).
- Crenshaw BS, Granger CB, Birnbaum Y et al.: Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) trial investigators. Circulation 101(1), 27–32 (2000).
- Bouchart F, Bessou JP, Tabley A et al.: Urgent surgical repair of postinfarction ventricular septal rupture: early and late outcome. J. Card. Surg. 13(2), 104–112 (1998).
- Deja MA, Szostek J, Widenka K et al.: Post infarction ventricular septal defect – can we do better? Eur. J. Cardiothorac. Surg. 18(2), 194–201 (2000).
- Goldstein JA, Casserly IP, Balzer DT, Lee R, Lasala JM: Transcatheter closure of recurrent postmyocardial infarction ventricular septal defects utilizing the Amplatzer postinfarction VSD device: a case series. Catheter. Cardiovasc. Interv. 59(2), 238–243 (2003).
- Labrousse L, Choukroun E, Chevalier JM et al.: Surgery for post infarction ventricular septal defect (VSD): risk factors for hospital death and long term results. Eur. J. Cardiothorac. Surg. 21(4), 725–731 (2002).
- Lee MS, Kozitza R, Mudrick D et al.: Intraoperative device closure of postinfarction ventricular septal defects. Ann. Thorac. Surg. 89(6), E48–E50 (2010).
- Wheatley GH, Diethrich EB: How to retrain the cardiothoracic surgeon. Interact. Cardiovasc. Thorac. Surg. 5(3), 236–237 (2006).
- Hughes GC, Nienaber JJ, Bush EL, Daneshmand MA, Mccann RL: Use of custom dacron branch grafts for “hybrid” aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J. Thorac. Cardiovasc. Surg. 136(1), 21–28, 28 E21–E26 (2008).
▪ First report of a hybrid procedure.
▪ Important paper highlighting the potential importance of completion angiography in hybrid revascularization.
▪ One of the first cases series to report the results of a ‘hybrid’ percutaneous revascularization strategy with conventional valve surgery in high-risk patients in an effort to reduce the overall risk to the patient.
▪ Describes one of the largest published series of minimally invasive aortic and mitral valve procedures with long-term (5 year) follow-up.
▪ Recent comprehensive meta-analysis of studies of hybrid procedures for aortic arch disease.
▪ Large consecutive series of hybrid procedures for thoracoabdominal aortic aneurysms from three major European vascular units.
▪ Systematic review of hybrid endovascular repair of thoracoabdominal aortic aneurysmal disease.
▪ Systematic review of hybrid procedures for ruptured thoracoabdominal aortic aneurysms.
▪ Recent good-quality study of simultaneous hybrid carotid and coronary revascularization.