Case Report - Diabetes Management (2017) Volume 7, Issue 2
IDegAsp provides simple intensification without the addition of another insulin injection and simple delivery system
- Corresponding Author:
- Shizuka Kaneko
Takatsuki Red Cross Hospital, Takatsuki, Japan
E-mail: skayamoe@takatsuki.jrc.or.jp
Abstract
Insulin degludec (IDeg) is an ultra-long duration novel basal insulin analogue, with flat and stable glucose-lowering effect. Translating into lower rates of hypoglycemia, especially nocturnal episodes, compared to conventional basal insulin in patients with T2DM, and have the potential for flexible dosing times to accommodate varying lifestyles. A co-formulation of IDeg and the rapid-acting analogue insulin aspart (IAsp) results in the soluble co-formulation insulin degludec/insulin aspart (IDegAsp). This provides both mealtime coverage and full 24-h basal coverage in a single injection. We confirmed the effectiveness of the intensification of therapy by substitution of basal insulin or conventional premix insulin with newly premix insulin IDegAsp in uncontrolled patients by continuous glucose monitoring (CGM). Novel soluble co-formulation insulin degludec/insulin aspart, IDegAsp, provides a simple helpful intensification for poorly controlled T2DM patients on basal insulin and every T2DM patient administrated conventional premix. Using IDegAsp is a patient-friendly intensified insulin therapy from the point of view that it is simple delivery system without need for reconstitution before injection and with no need for additional insulin injections.
Keywords
IDegAsp, conventional premix, CGM, type 2 diabetes, insulin therapy, simple delivery system
Introduction
An ultra-long duration novel basal insulin analogue degludec (IDeg) has a 25 h half-life with flat and stable glucose-lowering effect at steady state [1]. It has four-times less pharmacodynamic variability than insulin glargine (IGlar) [2] Translating into lower rates of hypoglycemia, especially nocturnal episodes, compared to IGlar in patients with T2DM [3], and has the potential for flexible dosing times to accommodate varying lifestyles [4]. Moreover it is possible to change dosing times of IDeg to accommodate varying lifestyles [5].
A co-formulation of IDeg and the rapid-acting analogue insulin aspart (IAsp) results in the soluble co-formulation insulin degludec/insulin aspart (IDegAsp: 70% v/v IDeg as basal insulin and 30% v/v IAsp as prandial insulin). This provides both mealtime coverage and full 24-h basal coverage in a single injection [6]. The most recent ADA/EASD position statement describes when therapy intensification using meal-time bolus insulin should be started. (a) when fasting glucose levels are at target but HbA1c remain above goal after 3–6 months of basal insulin titration; (b) when significant postprandial glucose excursions (more than 180 mg/dL]) occur; (c) when nocturnal or postprandial hypoglycemia occurs by increasing basal insulin dosage [7].
IDegAsp has been newly launched and is already in widespread use in several countries in Asia. We will introduce CGM results which demonstrate the effectiveness of the intensification of therapy by changing basal insulin or conventional premix insulin to IDegAsp. Moreover, we will show CGM results of blood glucose fluctuation in patients undergoing dialysis who were administered IDegAsp.
Results
Informed consents were obtained, and prior insulin therapy was switched to IDegAsp therapy.
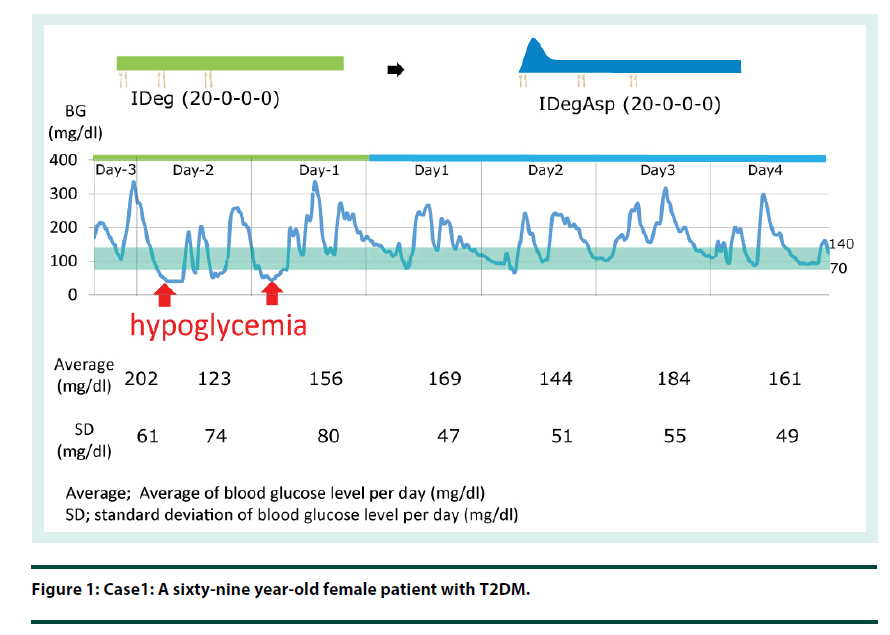
Case 1: A sixty-nine year-old female patient with T2DM (Figure 1).
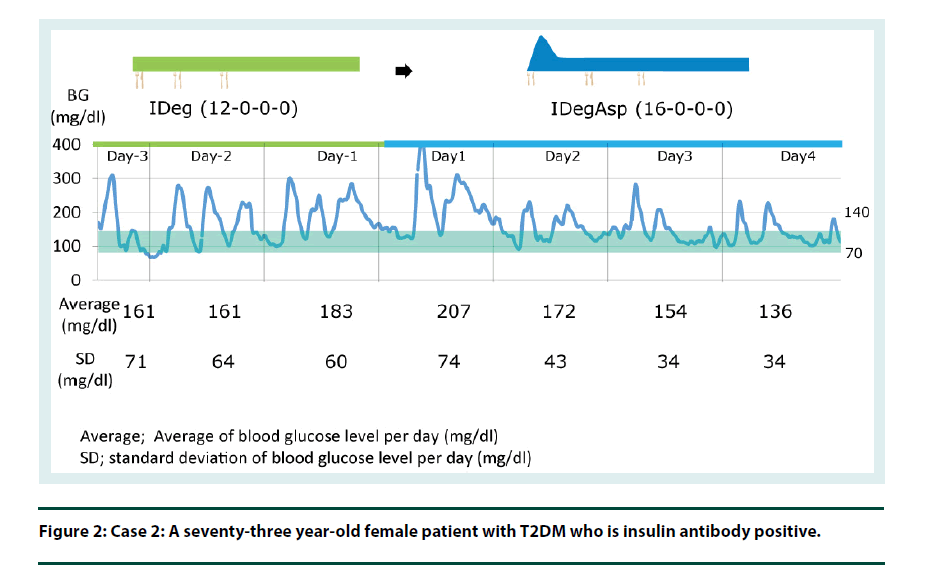
Case 2: A seventy-three year-old female patient with T2DM, who is insulin antibody positive (Figure 2).
By switching from IDeg to IDegAsp, the bolus insulin component can improve the postprandial glucose level whilst maintaining the fasting glucose target (Figure 1 and 2).
Case 3: A seventy-eight year-old male patient with T2DM was administered simplified therapy employing co-formulated IDegAsp. Although IDeg plus insulin aspart therapy was simplified changing to IDegAsp, his CGM result shows blood glucose fluctuation was completely same as before and after changing (Figure 3).
Although both patients were administered with basal insulin for type 2 diabetes, the 2-h postprandial glucose level remained over 200- 250 mg/dl even when achieving the fasting glucose target.
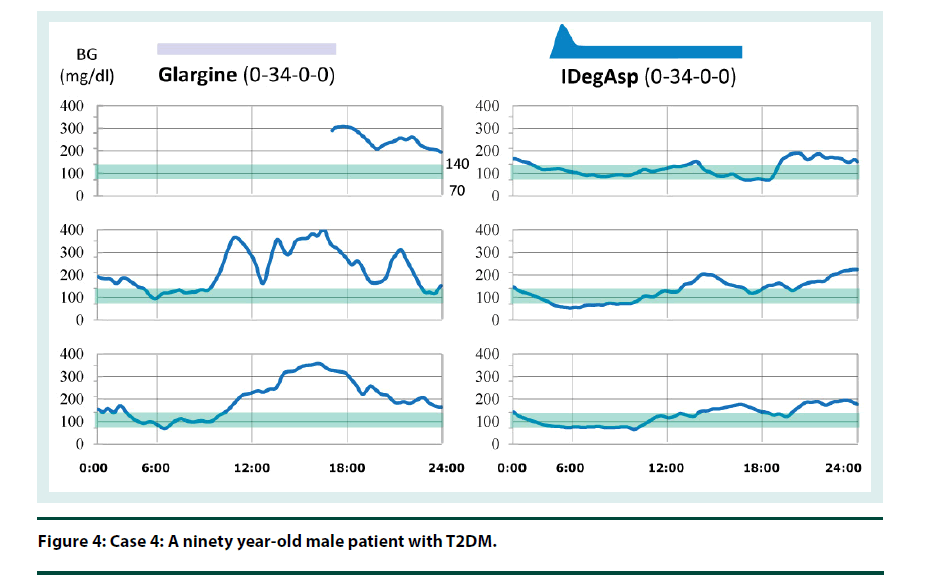
Case 4: CGM result shows that escalation of blood glucose levels largely increases due to lunch meal intake, resulting in hyperglycemia before dinner. Hyperglycemia before dinner causes hyperglycemia after dinner, although escalation due to dinner intake is small. However, once the optimal timing of IDegAsp injection has been found, overall blood glucose control would be expected to improve. His CGM result suggests that it is needed to decrease of the dosage of IDegAsp when changed from glargine (Figure 4).
The insulin therapy can be intensified without the addition of another insulin injection before meals. Therefore, this intensifies insulin therapy with the added benefit of a simplified drug delivery.
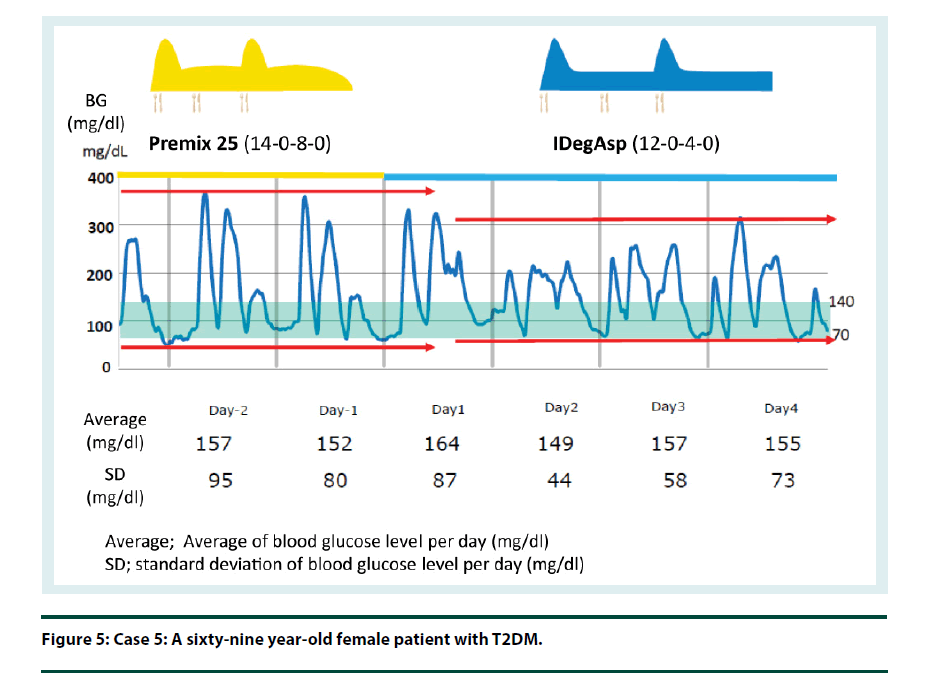
Case 5: Premix insulin such as IDegAsp fulfils the long-held expectation that for patients with T2DM, premix insulin would be developed to change the basal component of intermediate insulin to longer acting basal insulin in order to achieve better blood glucose control and decrease hypoglycemia. Higher percentages of patients using IDegAsp are achieving their personal Hb1Ac targets with a lower risk for hypoglycemia compared with those patients using conventional premix. The total daily dose of insulin is expected to decrease when changed from conventional premix (Figure 5). There is also no need for reconstitution.
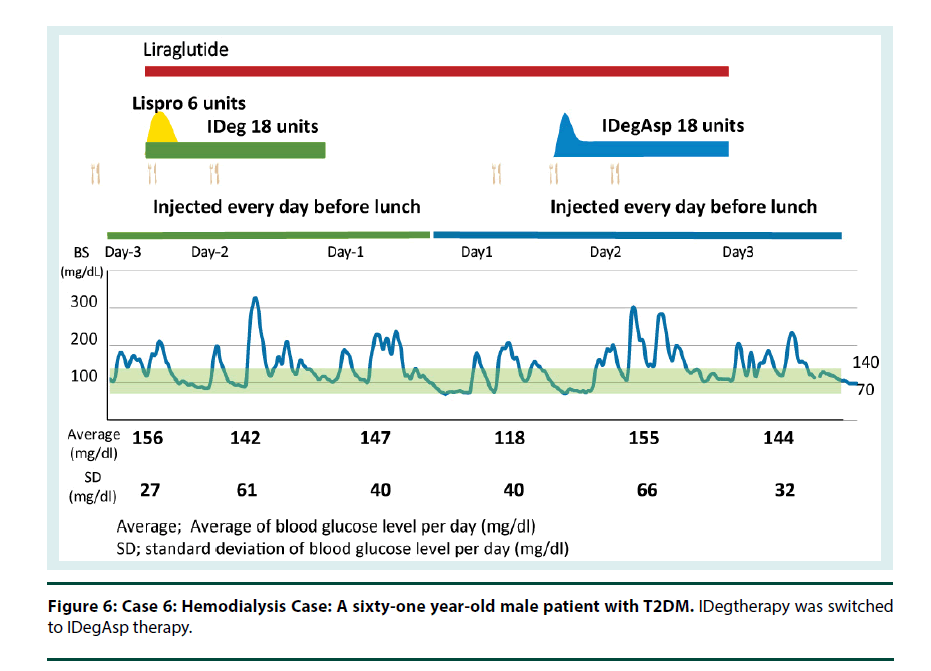
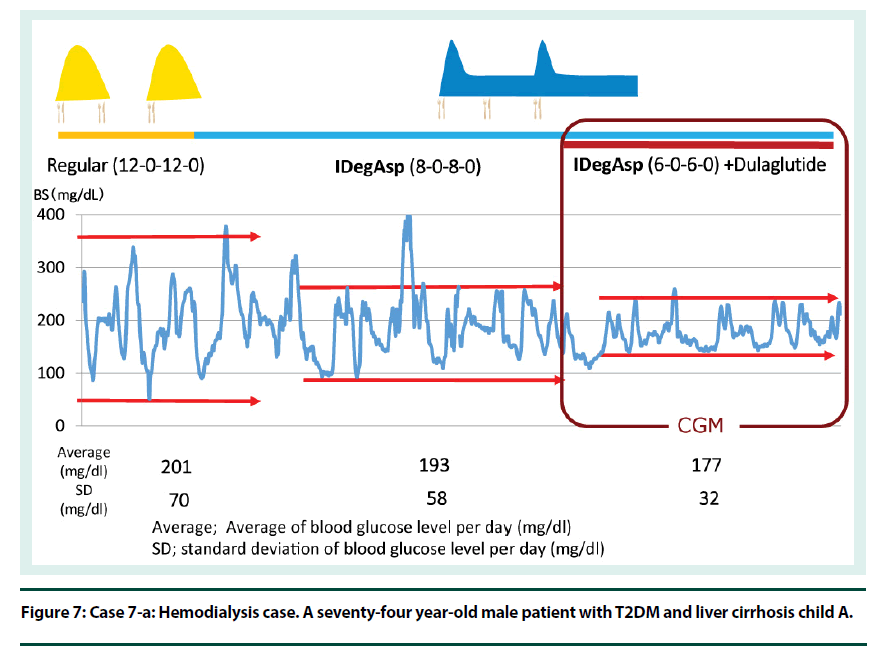
Cases 6 and 7: IDeg is a useful option for patients with renal impairment, including patients undergoing dialysis. I therefore conclude that IDegAsp also can provide a stable control of blood glucose in T2D patients on dialysis (Figures 6 and 7).
Figure 6: Case 6: Hemodialysis Case: A sixty-one year-old male patient with T2DM. IDegtherapy was switched to IDegAsp therapy.
Figure 7: Case 7-a: Hemodialysis case. A seventy-four year-old male patient with T2DM and liver cirrhosis child A.
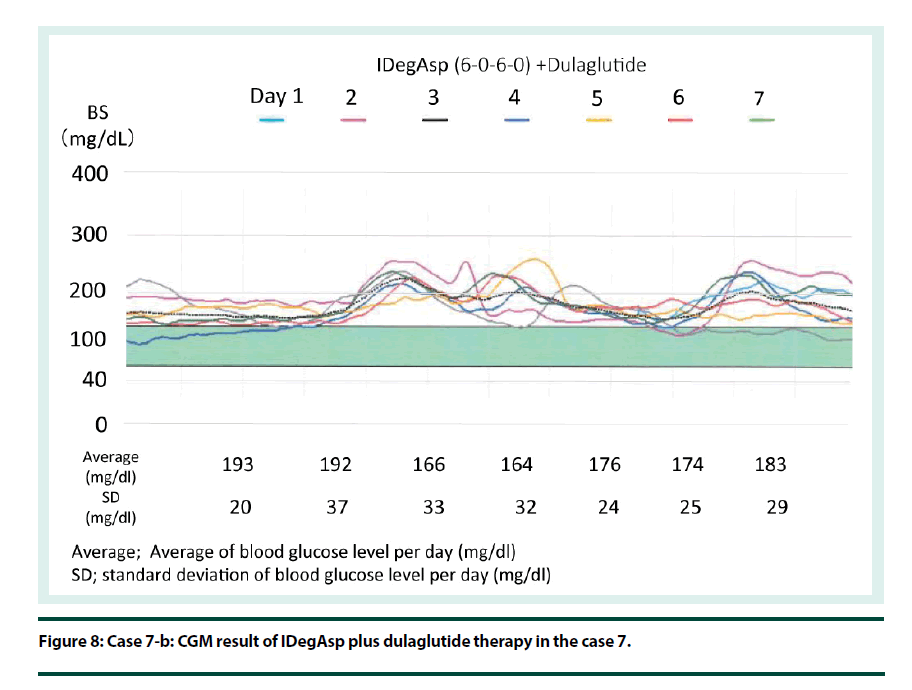
Case 7: Case is of a seventy-four year-old male patient with T2DM and liver cirrhosis Child A. Regular insulin therapy was switched to IDegAsp therapy. Next, GLP1RAg therapy was added (Figure 7). His CGM result of IDegAsp plus dulaglutide therapy shows stable blood glucose control (Figure 8).
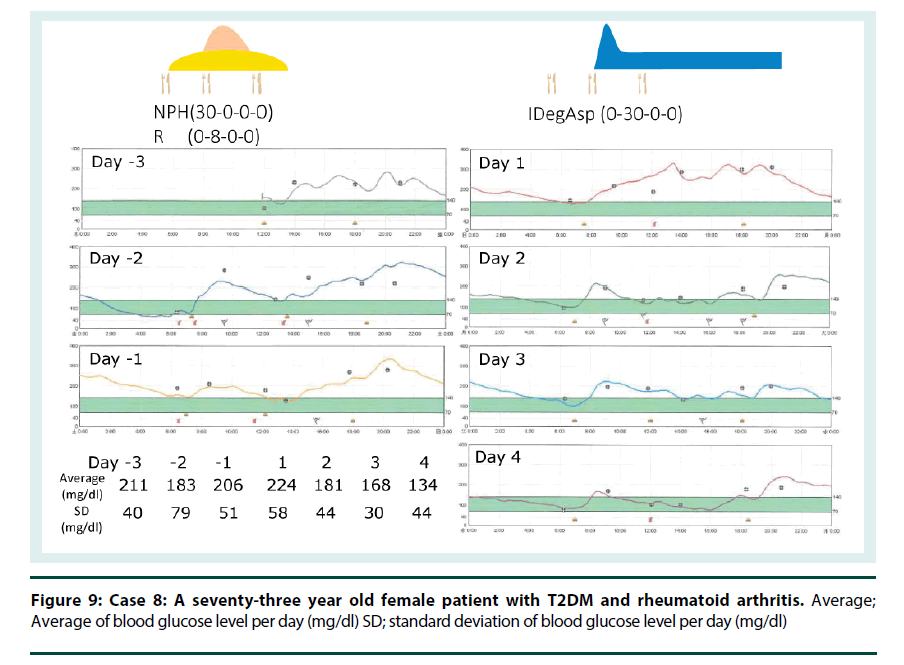
Case 8: Steroid induced hyperglycemia caused escalation of blood glucose levels after lunch. Therefore, injection of IDegAsp before lunch can be helpful and effective for a patient who is suffering from steroid induced hyperglycemia. A seventy-three year old female patient with T2DM and Rheumatoid Arthritis was suffering from steroid induced hyperglycemia and swan-neck deformity in her fingers. Change from NPH plus regular insulin therapy to IDegAsp simplified insulin injection and helped her (Figure 9).
Discussion
Ralph deFronzo reported that deterioration of β cell function was significant and glucagon secretion in the fasting state from β-cells was upregulated in patients with type 2 diabetes, especially in those patients with a blood glucose level of over 140 mg/dL [8]. Müller et al. [9] and Mitrakou et al. [10] demonstrated that insulin secretion in healthy subjects occurs rapidly by glucose loading, which is the first phase of insulin secretion and results in suppression of glucagon through zinc binding to SUR on α-cells [11].
Glucagon is reported to contribute approximately half of the increases in blood glucose levels after meals. Collectively, gluconeogenesis and glycogenolysis through upregulation of glucagon enhance hepatic glucose production, which causes the increase in fasting and postprandial blood glucose levels. IDeg, ultra-long acting basal insulin, provides uniform insulin coverage throughout the day and night and is prescribed mainly to control blood glucose by suppressing hepatic glucose production in between meals and during sleep [12-14].
This means that exogenous basal insulin treatment with the aim of lowering the fasting blood glucose levels improves endogenous insulin secretion and incretin action, which in turn will act on alpha cells to suppress enhanced glucagon and will result in the decrease in hepatic glucose production and improvement in postprandial glucose levels, effectively, through physiological processes.
It was reported that basal insulin treatment to achieve the fasting blood glucose target of 100 mg/dl, enhances hepatic glucose uptake via improvement of endogenous insulin secretion and decreases postprandial glucose levels [15].
In addition, it was reported that there was a correlation between fasting blood glucose levels and total endogenous insulin secretion in Japanese subjects. Therefore total insulin secretion was lower at fasting blood glucose levels over 110 mg/dL [16]. Taken together, pancreatic beta-cells may no longer maintain total insulin secretion at adequate fasting blood glucose levels, and exogenous basal insulin treatment targeting fasting blood glucose levels lower than 100 mg/dL may improve endogenous postprandial insulin secretion. It is also reported that administration of basal insulin over 8 weeks results in improvements in first- and second-phase insulin secretion [17].
When appropriate and timely introduction of insulin is needed before the loss of the endogenous insulin secreting ability in patients with type 2 diabetes, treatment with long acting basal insulin should be considered as an alternative to in multiple insulin injections.
However, the introduction of long acting basal insulin treatment might not improve the postprandial glucose level, even when achieving the fasting blood glucose target, in this instance, IDegAsp could be useful, because it provides stable fasting blood glucose level and decreases postprandial blood glucose once or twice depending on the number of insulin injections administrated.
▪ Intensification employing Novel insulin analogue combinations
When intensification is needed to decrease post prandial glucose levels after stabilization of fasting blood glucose levels, a short acting insulin analogue before meals is needed. It was reported that glargine plus one insulin injection before a main meal improved HbA1c [18]. Instead of administering another insulin injection, switching from long acting basal analogue insulin to IDegAsp, it makes it possible to easily intensify insulin therapy with only one daily injection instead of 2 daily injections [19].
Since the basal insulin component of the co-formulated, IDegAsp combination, is an ultr-along-acting insulin analogue, injection timing and dosing can be changed.
The therapeutic options of once daily injections, such as: A) Basal insulin, B) The more intensified option, IDegAsp and C) Basal insulin plus the OHAs (DPP4 inhibitor, SGLT2 inhibitor, alpha-glucosidase inhibitor, glinide, or biguanide), all enable us to move toward greater intensification of therapy and patient-centered care for those with T2DM [20].
A more complex treatment regimen with several injections of rapid-acting insulin at meal times is necessary when the patient is not tolerant to a GLP-1 receptor agonist or when the glucose target is not achieved, even with the combination therapy of basal insulin and a GLP-1 receptor agonist.
Treatment can be intensified by prescribing 2 or 3 injections per day before meals with basal insulin treatment, or, alternatively, utilizing IDegAsp therapy to avoid increasing the number of injections.
▪ The intensification of therapy by substitution of IDegAsp for conventional premix insulin
Conventional premix insulin contains intermediate insulin as the basal insulin component, and therefore has a peak effect which might carry the risk of hypoglycemia before dinner and during the night. In addition, since its basal insulin component does not cover 24 h, it is difficult to achieve the fasting blood glucose target of 100 mg/dl. Moreover, the postprandial blood levels are also not at goal because endogenous insulin secretion can’t be improved to required levels. Therefore changing from premixed insulin to IDeg/Asp might solve the problems in those patients with T2DM.
Simply switching to a novel co-formulated short plus long acting insulin analogue combination like IDegAsp can intensify insulin therapy whilst decreasing the risk of hypoglycemia and overcoming the limitations of premixed insulin. The total daily insulin dosage needed could be reduced for IDegAsp, compared to employing premix 30, whilst at the same time achieving the same HbA1c levels and better FPG levels with a lower incidence of nocturnal hypoglycemic events [21,22]. In addition, with IDegAsp there is also no need for reconstitution. On the other hand, it is necessary when employing conventional premixed insulin’s.
▪ Insulin use in T2DM patients with renal impairment
Finally, IDeg is a useful option for patients with renal impairment [23], including patients undergoing dialysis.
In Japan, dialysis is performed in one out of 400 people in the Japanese population. It has taken a tremendous effort to treat patients with insulin therapy who are undergoing dialysis.
However, after the approval of IDeg, we can provide a safer and more efficient therapy with or without using a combination of GLP1RA plus IDeg or GLP1RA plus IDegAsp. First, IDeg is conjugated with fatty acid, and binds to albumin in the blood. Hormones, for example, cortisol, thyroid hormones and testosterone employ carrier proteins and therefore fluctuations in the concentrations of hormones remain stable and minimal. In the same way, IDeg employs albumin as a carrier protein with similar minimal fluctuations. As IDegAsp consists of 70% v/v IDeg as basal insulin, it might act, therefore, in almost the same way.
Secondly, NPH insulin and insulin glargine are precipitated in crystalline form after subcutaneous injection, while IDeg or IDegAsp are not. So, neither of the interstitial fluid volumes before or after dialysis affects the dissolution of IDeg or IDegAsp.
Since body fluid is abundant before dialysis, precipitated insulin enters the blood stream more quickly. On the other hand, since body fluid is reduced after dialysis and subcutaneous interstitial fluid is minimal, precipitated insulin is more stable than usual.
This means that a different dosage is needed on the day of dialysis with conventional insulin. However, with IDeg or IDegAsp there is, in many cases, no need to change dosage with only minimal changes in dosage necessary in some cases.
Thirdly, another important mechanism is a change in the pH of glargine in the body fluid before and after dialysis. Glargine is acidic and soluble at pH 4 in the vial. Glargine is precipitated at the isoelectric point, pH6, in the electrically neutral hypodermis, and is slowly dissolved and absorbed into the bloodstream [24]. In patients undergoing dialysis, the pH of the body fluid is around 7.0 before dialysis and around 7.4 after dialysis.
This causes a difference in the solubility and absorption of glargine.
In fact, the glycemic control of some patients on dialysis who can switch from basal-bolus therapy to once daily IDeg doesn’t worsen.
Conclusion
Novel soluble co-formulation insulin degludec/ insulin aspart, IDegAsp, provides a simple helpful intensification for poorly controlled T2DM patients on basal insulin, those in whom it is difficult to escalate/intensify insulin therapy, and every T2DM patient administrated conventional premix. Furthermore, simple intensification without the addition of another insulin injection and a simple delivery system, i.e., no need for reconstitution before injection, is helpful from a patient’s perspective. Taken together, these result in a patient-friendly intensified insulin therapy.
References
- Heise T, Nosek L, Bøttcher SG et al. Ultra–long–acting insulin degludec has a flat and stable glucose–lowering effect in type 2 diabetes. Diabetes. Obes. Metab.14, 944–950 (2012).
- Heise T, Hermanski L, Nosek L et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady–state conditions in type 1 diabetes. Diabetes. Obes. Metab. 14, 859–864 (2012).
- Ratner RE, Gough SL, Mathieu CP et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre–planned meta–analysis of phase 3 trials. Diabetes. Obes. Metab. 15, 175–184 (2013).
- Meneghini L, Atkin SL, Raz I et al. The efficacy and safety of insulin degludec given in variable once–daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: A 26–week, randomized, open–label, parallel–group, treat–to–target trial in individuals with type 2 diabetes. Diabetes. Care. 36, 858–864 (2013).
- Kadowaki T, Jinnouchi H, Kaku K et al. Efficacy and safety of once–daily insulin degludec dosed flexibly at convenient times vs. fixed dosing at the same time each day in a Japanese cohort with type–2 diabetes: A randomized, 26–week, treat–to–target trial. J. Diabetes. Investig. 7, 711–717 (2016).
- Havelund S, Ribel U, Hubálek F et al. Insulin degludec (IDeg) and insulin aspart (IAsp) can be co–formulated such that the formation of IDeg multi–hexamers and IAsp monomers is retained upon s.c. injection. Diabetes. 62, A241 (2013).
- Inzucchi SE, Richard MB, John BB et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient–Centered Approach. Diabetes. Care. 35, 1364–1379 (2012).
- DeFronzo RA. The Triumvirate: β–Cell, Muscle, Liver: A Collusion Responsible for NIDDM. Diabetes. 3, 667–687 (1988).
- Müller WA, Faloona GR, EA Parada et al. Abnormal Alpha–Cell Function in Diabetes — Response to Carbohydrate and Protein Ingestion. N. Engl. J. Med. 283, 109–115 (1970).
- Mitrakou A, Kelley D, Veneman T et al. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes. 39, 1381–1390 (1990).
- Franklin I, Gromada J, Gjinovci A et al. β–cell secretory products activate α–cell ATP–dependent potassium channels to inhibit glucagon release. Diabetes 54, 1808–1815 (2005).
- Nathan DM, Buse JB, Davidson MB et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia. 52, 17–30 (2009).
- Holman RR, Turner RC, McCarthy ST et al. Beta–cell function improved by supplementing basal insulin secretion in mild diabetes. BMJ. 1, 1252–1254 (1976).
- Comstock JP, Cunningham GR, Cusi K. Safety and Efficacy of Normalizing Fasting Glucose With Bedtime NPH Insulin Alone in NIDDM. Diabetes. Care. 18, 843–851 (1995).
- Matsuhisa M, Morishima T, Tomita T et al. Augmentation of hepatic glucose uptake by a positive glucose gradient between hepatoportal and central nervous systems. Diabetes. 46, 1101–1105 (1997).
- Tanaka Y, Atsumi Y, Asahina T et al. Usefulness of revised fasting plasma glucose criterion and characteristics of the insulin response to an oral glucose load in newly diagnosed Japanese diabetic subjects. Diabetes. Care. 21, 1133–1137 (1998).
- Meier JJ, Nauck MA, Schmidt WE et al. Chronic Reduction of Fasting Glycemia With Insulin Glargine Improves First and Second Phase Insulin Secretion in Patients with Type–2 Diabetes. Diabetes. Care. 34, 2048–2053 (2011).
- Lankisch MR, Ferlinz KC, Leahy JL et al. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single–dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes. Obes. Metab. 10, 1178–1185 (2008).
- Onishi Y, Ono Y, Rabøl R et al. Superior glycaemic control with once–daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: a randomized, controlled phase 3 trial. Diabetes. Obes. Metab. 15, 826–832 (2013).
- American Diabetes Association. Standards of medical care in diabetes—2017: Summary of Revisions. Diabetes. Care. 40, S4–S5 (2017).
- Kaneko S, Chow F, Choi DS et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre/self-mixed insulin: A 26–week, randomised, treat-to-target trial. Diabetes. Res. Clin. Pract. 107, 139–147 (2015).
- Taneda S, Hyllested J, Gall A et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 twice daily in insulin–experienced Japanese subjects with uncontrolled type 2 diabetes: Subgroup analysis of a Pan–Asian, treat-to-target Phase 3 Trial. J. Diabetes. 9, 243–247 (2017).
- Kiss I, Arold G, Klim S et al. Insulin Degludec: Pharmacokinetics in Patients with Renal Impairment. Clin. Pharmacokinet. 53, 175–183 (2014).
- Gualandi AM, Giorgi G. Insulin formulations––a review. Eur. Rev. Med. Pharmacol. Sci. 5, 73–83 (2001).