Review Article - Imaging in Medicine (2013) Volume 5, Issue 4
Image-guided focused ultrasound: state of the technology and the challenges that lie ahead
Jessica L Foley*1, Matt Eames1, John Snell1,2, Arik Hananel1,3, Neal Kassell1,2 & Jean-Francois Aubry3,41Focused Ultrasound Foundation, 1230 Cedars Court, Suite F, Charlottesville, VA 22903, USA
2Department of Neurosurgery, University of Virginia, Charlottesville, VA, USA
3Department of Radiation Oncology, University of Virginia, Charlottesville, VA, USA
4Institut Langevin, CNRS UMR 7587, Paris, France
- Corresponding Author:
- Jessica L Foley
Focused Ultrasound Foundation
1230 Cedars Court, Suite F
Charlottesville, VA 22903, USA
Tel: +1 434 326 9832
E-mail: jfoley@fusfoundation.org
Abstract
Keywords
focused ultrasound ▪ image-guided n MRI ▪ therapeutic ultrasound ▪ ultrasound imaging
Much like a magnifying glass focusing multiple beams of light onto a single point, focused ultrasound (FUS) concentrates converging beams of ultrasound energy with extreme precision onto a target deep in the body that can be as small as 1 mm in diameter. As individual beams pass through most intervening tissues, there is no effect; however, when the beams converge, there is a profound effect produced by the thermal and/or mechanical mechanisms of FUS. Image-guided FUS has the potential to provide truly noninvasive surgery, an alternative or complement for radiation therapy, the means to dissolve blood clots, or a way to deliver drugs in high concentration to a precise point in the body [1].Treatments can be performed in an outpatient setting without ionizing radiation, general anesthesia, incisions or scars, therefore resulting in minimal pain and discomfort, and recovery with fewer complications compared with conventional surgery or radiation therapy [2,3].
Following more than a decade of basic science research [4], the first clinical use of FUS was in the neurosurgical treatment of movement disorders, neuropathic pain and hypersensitivity in 1958 [5,6]. These early experimental treatments were planned with radiographic imaging of the stereotactic-mounted piezoelectric emitters and executed without real-time image guidance or temperature feedback. The treatment was also invasive and required the drilling of burr holes through the skull, allowing ultrasound transmission into the brain tissue.
The next documented clinical application of FUS was in the treatment of glaucoma in the 1980s [7–9]. These treatments were performed with the first US FDA-approved high-intensity FUS device, which was coupled with a diagnostic ultrasound and fiber optic system for aiming the therapy transducer [10]. The system was not equipped with the capability to monitor temperature.
Vallancien et al. conducted a FUS clinical study to treat bladder cancer in five patients in 1991 [11]. The investigator moved on the following year to treat the prostate, kidneys and liver [12]. The system used diagnostic ultrasound for planning, but lacked real-time monitoring during the therapeutic application. Temperature was monitored by thermocouples inserted under ultrasonic (in the case of the prostate) or endoscopic guidance.
The combined use of therapeutic ultrasound and MRI was demonstrated in vitro in the early 1990s [13–15]. MR provided the ability to monitor FUS-induced temperature change in near real time, permitting greater control of FUS therapy, while the improved soft tissue contrast afforded by MR (compared with the previously used diagnostic ultrasound) aided targeting.
MR-guided FUS was initially applied in vivo to breast fibroadenoma [16,17] with moderate success. The first CE and FDA approved use of MR-guided FUS was for the treatment of uterine fibroids, which was first demonstrated clinically in 2003 [18,19].
Shortly after its initial use in the treatment of uterine fibroids, techniques to correct for skull bone acoustic aberration were formally described and proven [20,21]. This rekindled clinical interest in the original, neurosurgical indications for which FUS saw its first inhuman use. Following the publication of this method, commercial products incorporated the technique and began seeing successful use in clinical trials addressing neuropathic pain in 2009 and other clinical indications in the field of functional neurosurgery [22,23].
FUS mechanisms & bioeffects
Today, FUS is a platform technology with a variety of biological effects in tissue that enable treatment of a wide range of clinical conditions. These biological effects range from thermal ablation to disruption of blood clots to enhanced penetration of drugs through cell membranes or the blood–brain barrier. The varying bioeffects enable clinical applications as diverse as uterine fibroids, prostate and liver cancer, and neurological disorders.
The bioeffects produced by FUS are highly localized in a small region of tissue corresponding to the focal size of the ultrasound beam. The focal size for current FUS systems in clinical use range from 4 to 60 mm in length and 2 to 16 mm in diameter; focal spots – sonications – are typically cylindrical or ellipsoidal in shape. These localized bioeffects are produced by either thermal or mechanical mechanisms of ultrasound interaction with the targeted tissue. These thermal and mechanical effects and their biological outcomes – bioeffects – are determined by the type of tissue (e.g., muscle vs bone) and the acoustic parameters (power, sonicationduration and sonication mode – continuous vs pulsed).
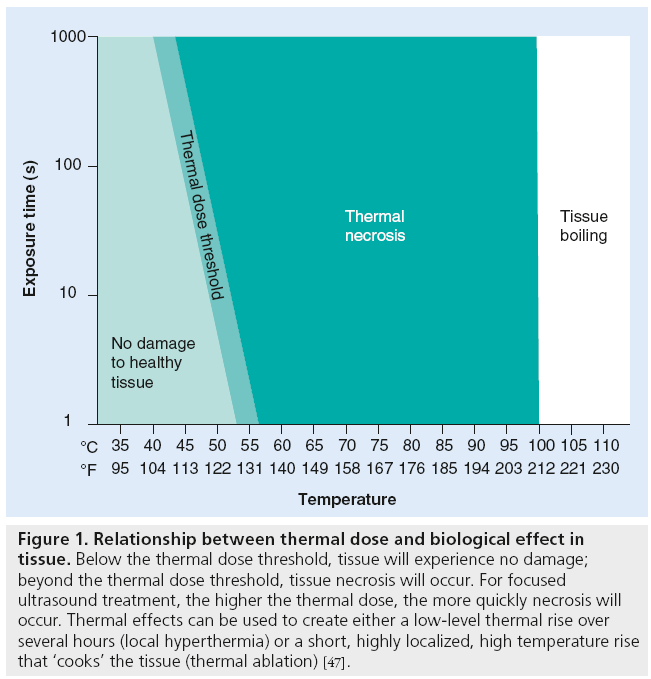
When transmitting the ultrasonic energy continuously, high-intensity FUS can raise tissue temperature at the focal point. The level and duration of this temperature elevation is quantified as the tissue’s ‘thermal dose’. Figure 1 illustrates different levels of thermal dose and their biological outcomes.
Figure 1: Relationship between thermal dose and biological effect in tissue. Below the thermal dose threshold, tissue will experience no damage; beyond the thermal dose threshold, tissue necrosis will occur. For focused ultrasound treatment, the higher the thermal dose, the more quickly necrosis will occur. Thermal effects can be used to create either a low-level thermal rise over several hours (local hyperthermia) or a short, highly localized, high temperature rise that ‘cooks’ the tissue (thermal ablation) [47].
Alternatively, when the acoustic energy is administered using high power and very short pulses, the low amount of energy deposited into the tissue results in a low thermal rise. However, because ultrasound is a mechanical pressure wave that successively increases and decreases pressure in the tissue, a high pressure change due to the use of high-intensity ultrasound can induce various mechanical effects – from vibration of the target to cavitation. Cavitation describes the interaction of the ultrasonic energy with microbubbles in the tissue. Depending on the clinical application, microbubbles can either be injected into the blood stream or injected directly into the tissue. The sharp change in local pressure caused by FUS transmission can also create microbubbles in the tissue [24].
Most FUS clinical applications currently under investigation exploit the thermal mechanism to ablate tissue deep in the body. Depositing a high level of ultrasonic energy causes an intense increase in temperature, thermal coagulation and cell death. Exposing tissue, for even 1 s, to a temperature of 56°C/130°F is enough to induce irreversible thermal damage to cells via protein denaturation. Researchers and physicians use FUS thermal ablation to noninvasively treat a variety of clinical conditions, including symptomatic uterine fibroids, breast [25], liver [26] and prostate cancer [27], low back pain [28] and neurological disorders [23].
Cavitation can also play a role during FUS ablation. The purely thermal and mechanical components of a FUS ablation procedure may be monitored via MR and ultrasound (US) imaging; however, these imaging modalities are both more optimal for monitoring different bioeffects.
Role of imaging in FUS procedures
Imaging is crucial throughout all stages of FUS therapeutic procedures. From precise targeting of the region of interest, to optimal planning of the FUS sonications (e.g., location, size and energy), to real-time treatment monitoring and final confirmation of the treatment (e.g., ablated volume), imaging is critical to the safety and efficacy of the procedure. Both MR and US imaging have been implemented into clinical image-guided FUS devices. Both modalities are effective at guiding and monitoring the treatment; however, they each have different strengths. Two primary stages of FUS treatment procedures where MR and US imaging vary in their utility include definition of the region of interest during construction of a treatment plan and treatment monitoring. Planning and monitoring with US imaging is an inexpensive option that can achieve real-time imaging (more than 50 frames per second) and enable the development of portable devices. Alternatively, MRI offers precise targeting due to its state-of-theart contrast and near real-time temperature monitoring.
■Treatment planning
Ultrasonic planning
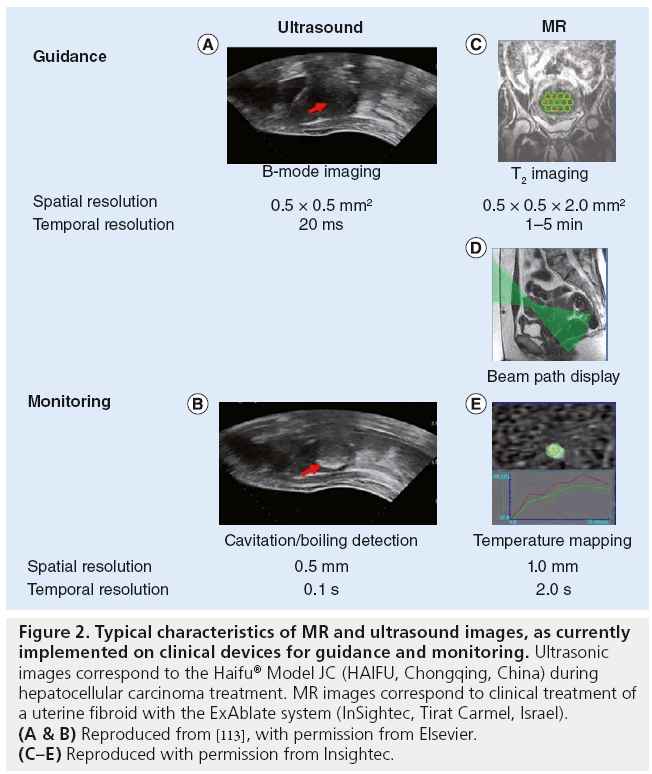
Ultrasound-guided FUS devices take advantage of a diagnostic probe inserted confocally to the therapeutic probe, most generally in the center of the probe [29,30]. The physician defines the location of the sonication target based on the US, B-mode image (guidance-US as shown in Figure 2). Depending on the device, the target is adjusted to the appropriate location by translating the therapeutic probe with step motors (if a single element transducer is used) or by electronic beam steering (if a multi-element transducer is used).
Figure 2: Typical characteristics of MR and ultrasound images, as currently implemented on clinical devices for guidance and monitoring. Ultrasonic images correspond to the Haifu® Model JC (HAIFU, Chongqing, China) during hepatocellular carcinoma treatment. MR images correspond to clinical treatment of a uterine fibroid with the ExAblate system (InSightec, Tirat Carmel, Israel). (A & B) Reproduced from [113], with permission from Elsevier. (C–E) Reproduced with permission from Insightec.
MR planning
MR-guided FUS devices comprise an MR-compatible FUS therapeutic probe, most generally inserted inside the MR bed [18]. As an example of treatment planning on an MR-guided device, the ExAblate system (Insightec, Israel) planning is performed on T2-weighted fast spin echo images (repetition time: 4000 ms; echo time: 102 ms; flip angle 90°; bandwidth 31 kHz; field of view 20 × 20 cm2; and slice thickness 4–5 mm with 1–2 mm gap [31]). After manual selection of a 3D treatment volume on these T2 images, the planning software automatically proposes a set of individual sonication locations. The user is free to move, add or remove sonication locations. Before each sonication, the expected ultrasonic path is superimposed on the T2-weighted fast spin echo images in order to avoid focusing through air cavities, such as the bowels, or being too close to highly absorbing and reflecting bony structures (Figure 2). If needed, the user may rotate or translate the therapeutic probe until an appropriate beam path is found.
■Treatment monitoring
Typical FUS ablation procedures use FUS beams with a frequency of 0.5–1.5 mHz, individual sonication duration (single shot) from 1 to 20 s, and acoustic energy from 300 to 1500 J. Treatment is then achieved by delivering multiple sonications (spot by spot) using mechanical translation or electronic steering. In an effort to reduce the overall treatment time, other strategies have been developed, such as longer sonication time combined with electronic steering to enable continuous sonication with spiral or concentric patterns. Regardless of the specific treatment plan, the biological effects of FUS at focus will be the same. As stated previously, the primary bioeffects to occur during FUS clinical treatments include cavitation and/or thermal ablation. Thermal rises are mainly due to viscous absorption and typically range from 10 to 60°C. Cavitation corresponds to the formation of air bubbles in tissue due to two possible ultrasonic interactions:
▪ Pre-existing nuclei can be activated and grow explosively if the rarefactional pressure exceeds a threshold [32];
▪ The temperature increase may promote so called ‘boiling nucleation’, which corresponds to the growth of nuclei by raising the vapor pressure or the partial pressure of dissolved gas in the medium [33,34].
MR and ultrasound monitoring are based on the ability of each technique to detect either thermal rise or cavitation effects.
Ultrasonic monitoring
Due to the large impedance difference between air and tissue, once the bubbles created by acoustic cavitation are large or numerous enough, a hyperechogenic region can be observed on the B-mode images [24]. The monitoring, US column in Figure 2 illustrates this: as indicated by the red arrow the US image becomes brighter than before sonication. Such monitoring has been widely used clinically on the prostate [35,36], liver [29,37], breast [38], kidney [39,40], pancreas [41] and bone metastasis [42].
MR monitoring
On current clinical devices, such as the Sonalleve system (Philips Medical Systems, The Netherlands) or the ExAblate systems, MR monitoring is achieved by acquiring temperature elevation maps with dedicated temperature sensitive sequences [43]. Others have reviewed different methods used for such MR temperature mapping [44,45]. Briefly, all clinical studies currently use temperature-dependent proton resonance frequency (PRF) shift to estimate in vivo temperature elevation [15]. Temperature elevation induces a phase shift in the MR data. A phase image is thus acquired prior to the ultrasound exposure followed by a series of images during and after the sonication. By subtracting the phase of each voxel from the baseline, a phase-difference image is obtained that is proportional to the temperature elevations. Soft tissues have PRF thermal coefficients that are close to that in water (0.01 ppm/°C) [46].
Temperature is recorded as a function of time, typically every 3 s, and the curve is integrated in order to calculate the thermal dose as defined by [47]:
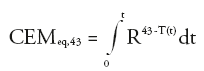
where R = 0.25 if T <43°C or 0.5 if T >43°C. CEMeq,43 is the cumulative number of equivalent minutes at 43°C. The threshold for tissue damage is tissue dependent and has been tabulated [48]. For example, necrosis is induced in the muscle if the CEMeq,43 is greater than 240 min [48].
FUS clinical indications
There are currently several clinical indications for image-guided FUS that have received regulatory approval in at least one region of the world. These indications include symptomatic uterine fibroids, prostate cancer, liver tumors, thyroid nodules, palliative treatment for bone metastases, breast fibroadenoma and functional neurological disorders (essential tremor, neuropathic pain and tremor-dominant Parkinson’s disease). The landscape for FUS treatment of several of these conditions is highlighted below.
■Uterine fibroids
Uterine fibroids are the most common neoplasms of the female pelvis. These benign tumors arise from the smooth muscle cells of the uterus and occur in up to 50% of women of reproductive age. Fibroid symptoms include abnormal bleeding, heavy or painful periods, abdominal discomfort or bloating, urinary frequency or retention and even infertility. Uterine fibroids can be visualized using US or MR imaging. Common treatment options include hysterectomy, myomectomy (surgical removal of the fibroid) and uterine artery embolization.
FUS offers a noninvasive method of treatment for fibroids. Using this treatment modality in conjunction with MR or US image guidance, the physician directs a focused beam of acoustic energy through the patient’s skin, superficial fat layer and abdominal muscles. This thermally coagulates the fibroid tissue, thereby destroying it without damaging intervening or adjacent tissues [18,49–51]. Geographical regions where one or more FUS devices have received regulatory approval to treat symptomatic uterine fibroids to date include: FDA, Europe (CE mark), Korea, Japan, India and Australia. However, there is limited insurance reimbursement for these procedures. To date, image-guided FUS has been used to treat an estimated 20,000 patients worldwide with symptomatic uterine fibroids.
■Prostate cancer
Prostate cancer is the most common type of cancer in men and the second leading cause of death. Common treatment and management options for men with localized prostate cancer include radical prostatectomy, radiation therapy, cryoablation, watchful waiting and active surveillance.
FUS provides a noninvasive, radiation-free option to treat patients with localized prostate cancer or those who need salvage therapy. During the FUS treatment procedure, the physician uses either MR or US imaging to locate the patient’s prostate gland and direct the FUS beam to part of or the entire prostate gland. The FUS energy creates a temperature elevation at the focal point that thermally coagulates the targeted small volume of cancerous cells within seconds. This process is repeated until the entire selected volume (i.e., tumor) or the entire gland is thermally destroyed. Several different systems for image-guided FUS ablation of prostate tissue (using MR or US imaging) are available as research or commercial platforms in various geographical regions [52–56]. Geographical regions where one or more FUS devices have received regulatory approval for the treatment of prostate cancer to date, as either a primary or salvage treatment, include: Europe (CE mark), Canada, Russia, Australia, Korea, Malaysia, Indonesia, South America and the Middle East. In the USA, no device with FDA approval is currently available. However, a complete Premarket Approval (PMA) application for one device is under review with the FDA at this time, therefore, FDA approval may be possible for this indication in the coming year. In the past 15 years, image-guided FUS has been used to treat an estimated 40,000 patients worldwide with lowrisk prostate cancer or those who require salvage therapy [Foley JL et al., Unpublished Data].
■Bone metastases
Bone is the third most common tissue affected by metastatic disease, with breast, lung and prostate cancer as the most frequent primary tumor types. Symptoms of bone metastases include direct pain, fractures, spinal cord compression and high blood calcium levels. Current treatments for patients with bone metastases are primarily palliative and attempt to improve the patient’s quality of life and functional level. Treatment with external beam radiation therapy (EBRT) is the standard of care for patients with localized bone pain it has a success rate (i.e., pain relief) of 70–80%.
Image-guided FUS offers a radiation-free noninvasive method to alleviate pain associated with bone metastases. Under image guidance, the physician targets the FUS beam to the bone and bone–tissue interface affected by the painful metastatic lesion. The FUS-induced temperature rise then thermally coagulates the periosteal membrane surrounding the targeted bone and may also damage tumoral tissue in the targeted area. The destruction of the periosteum, which contains the pain-reporting nerve fibers, provides rapid pain palliation. Although it is not suitable for all patients, FUS treatment for painful bone metastases provides the benefit of fast pain relief within a single session using nonionizing radiation [42,57,58]. Geographical regions where one or more FUS devices have received regulatory approval for treatment of painful bone metastases to date include: FDA, Europe (CE mark), Korea, India and Russia. However, there is limited insurance reimbursement for these procedures. To date, image-guided FUS has been used to palliate pain from bone metastases for several hundred patients worldwide.
■Neurological disorders
The potential benefits of FUS, including its noninvasiveness, lack of ionizing radiation and high precision, coupled with tight focus and sharp ablation margins make it an especially promising modality for brain treatments [59]. FUS is under clinical investigation for thalamotomy treatment of several functional neurological disorders: essential tremor, neuropathic pain and tremordominant Parkinson’s disease. Noninvasive FUS treatment of the brain has been particularly challenging, as the skull presents a significant acoustic barrier to the ultrasonic energy. The variation in skull thickness, along with its heterogeneous internal structure, can defocus the wave propagation and degrade the focal point. This challenge is overcome using 3D CT data of the skull, with a projection algorithm [20] or finite differences simulations [21,60]. The result of the simulation is used as input to calculate the phase shifts that are then applied to the array of transducers to refocus the beam through the skull [61–63].
To date, researchers have used transcranial MR-guided FUS with encouraging results to treat nearly 100 patients with essential tremor, neuropathic pain and tremor-dominant Parkinson’s disease. These treatments have occurred at institutions including: University of Virginia (VA, USA), Sunnybrook Health Sciences Centre (Toronto, Canada), Yonsei University Medical Center (Seoul, South Korea), University Children’s Hospital (Zurich, Switzerland) and the Center for Ultrasound Functional Neurosurgery (Solothurn, Switzerland). The ExAblate transcranial MR-guided FUS device has received regulatory approval in Europe (CE mark) for these indications and is undergoing FDA-approved clinical trials in the USA.
■Liver tumors
Primary and secondary (metastatic) liver tumors constitute a major health concern with an unmet clinical need. The most common type of primary liver cancer, hepatocellular carcinoma, is the fifth most common cancer worldwide. Liver is the second most common site for metastases, with colorectal, breast and lung as common primary tumors.
FUS as a noninvasive treatment option could offer patients another choice when no other treatment is available or when they prefer a nonsurgical approach. However, the technical challenges to FUS ablation of liver tissue are significant and include organ motion during respiration and the inability of ultrasound energy to transmit through the ribs. Various approaches to overcome these obstacles have been tested in the laboratory, in vivo and in clinical studies. Solutions to reduce liver motion include endotracheal intubation with single lung mechanical ventilation, training patients to hold their breath during multiple short energy delivery periods or combining general anesthesia with short induced spells of apnea. To overcome the acoustic obstacle presented by the ribs, some researchers have targeted only the tumors that were accessible below the ribs [64,65]; others have sonicated through a large area of the chest and abdominal wall to minimize energy intensity and heating on the ribs [66,67]. In the past 15 years, FUS has been used to treat an estimated 15,000 patients with primary liver tumors; most of these treatments were performed China using US-guided FUS devices.
Current & future challenges
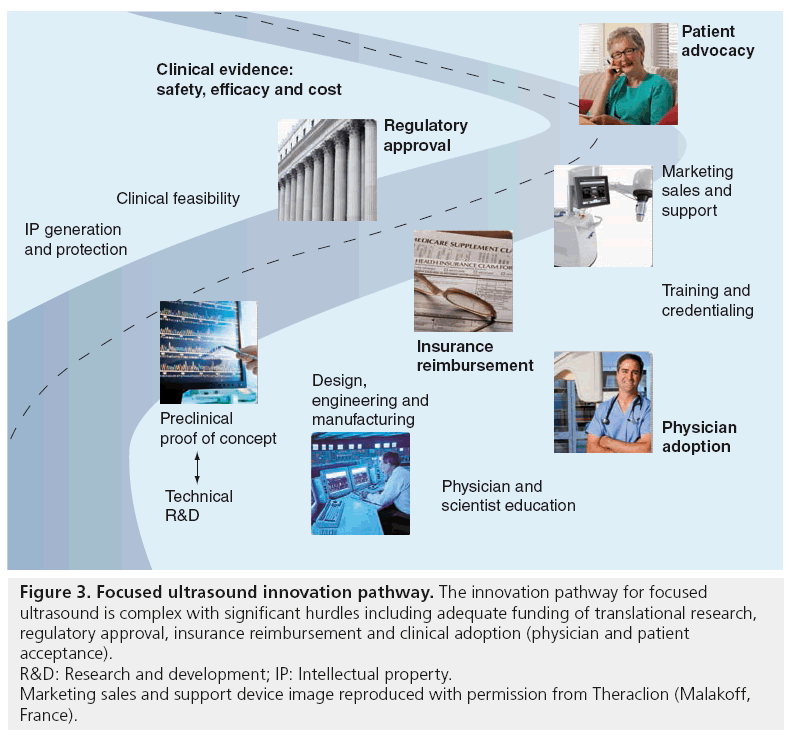
Image-guided FUS has demonstrated its potential as a new therapy for a range of clinical indications; however, there are still challenges that lie ahead. Development and adoption of a new therapeutic medical device is a complex process involving numerous stakeholders with different time constraints and interests. The innovation pathway for image-guided FUS traverses from early-stage research and development – in the imaging/FUS technology as well as into new potential applications – on to commercialization and clinical acceptance of a new noninvasive therapy. Figure 3 shows the complicated innovation pathway for image-guided FUS and highlights the most prominent barriers and hurdles along this pathway from new idea to the patient bedside. For the purpose of this review, the focus will be on the innovation pathway within the USA, viewed by most image-guided FUS device manufacturers as a more challenging pathway than in other regions of the world.
Figure 3: Focused ultrasound innovation pathway. The innovation pathway for focused
ultrasound is complex with significant hurdles including adequate funding of translational research,
regulatory approval, insurance reimbursement and clinical adoption (physician and patient
acceptance).
R&D: Research and development; IP: Intellectual property.
Marketing sales and support device image reproduced with permission from Theraclion (Malakoff,
France).
■Technical research & development
At the current stage of technology development, the safety and efficacy of image-guided FUS for several clinical applications has been demonstrated in numerous in vivo preclinical and clinical studies. As a noninvasive tool for precise ablation, image-guided FUS has demonstrated safety and (at least) preliminary efficacy for a range of clinical conditions. Researchers continue to explore ways to expand the treatment envelope or therapeutic window for FUS treatment within the body. For example, technical advancements are needed to treat tissues that are near to bones or blood vessels that could cause aberrations in energy delivery. Imaging advancements are also enabling new methods for FUS treatment monitoring.
US imaging challenges
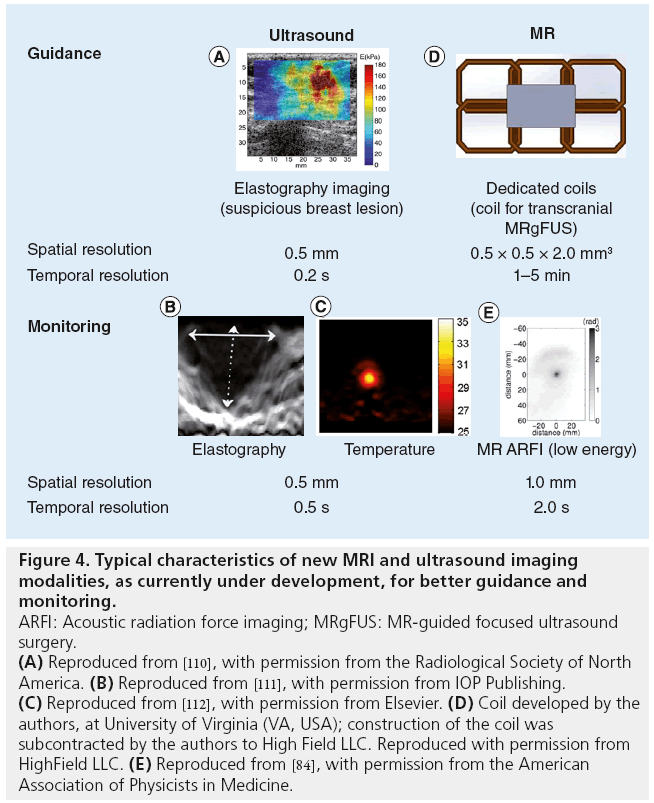
As discussed previously, US-guided monitoring is currently based on imaging of cavitation clouds generated during FUS treatment. The formation of such clouds is most likely due to boiling effects [27]. The hyperechogenic areas around the focus do not appear until long after the thermal dose threshold has been reached. Such monitoring ensures the efficacy of the treatment, but tissues are likely to be overtreated. Recently, novel imaging techniques have been developed in order to detect the early phase of bubble cloud formation [68,69] thanks to the use of novel scanners that are now available (Supersonic Imagine [Aix en Provence, France]/Verasonics [WA, USA] systems), which permit temporal resolution up to 10,000 frames/s. Research is also underway to develop US-based temperature monitoring methods [70,71]. As FUS thermal ablation progresses in tissue, the stiffness of the tissue increases. US elastography has been able to detect this stiffness change during tissue necrosis in vitro [72], as well as the elasticity change during the early subnecrosis stage of the heating process (in vitro [73]) (Figure 4).
Figure 4: Typical characteristics of new MRI and ultrasound imaging
modalities, as currently under development, for better guidance and
monitoring.
ARFI: Acoustic radiation force imaging; MRgFUS: MR-guided focused ultrasound
surgery.
(A) Reproduced from [110], with permission from the Radiological Society of North
America. (B) Reproduced from [111], with permission from IOP Publishing. (C) Reproduced from [112], with permission from Elsevier. (D) Coil developed by the
authors, at University of Virginia (VA, USA); construction of the coil was
subcontracted by the authors to High Field LLC. Reproduced with permission from
HighField LLC. (E) Reproduced from [84], with permission from the American
Association of Physicists in Medicine.
MRI challenges
MRI has proven effective to measure the temperature increase during FUS treatment. Currently available FUS systems monitor temperature on a single or small number of 2D slices through the target volume. The acquisition of MR thermometry over 3D volumes is an active topic of research and has important clinical safety and workflow implications [74,75]. However, the use of MR to detect mechanical effects in tissue has been challenging. No MR detection of acoustic nucleation-based cavitation has been reported so far, but Khokhlova et al. have reported in vitro MR detection of boiling cavitation activity [33]. FUS emitted for a few microseconds is known to induce a micrometric tissue displacement by acoustic radiation force, without significant thermal heating. Imaging such a displacement would thus provide the location of the focal spot without heating [76]. Such displacements have been imaged in vivo with US for decades [77–79]. MR acoustic radiation force imaging (MR ARFI) has recently been introduced as a promising method to plan and monitor therapeutic applications of MR-guided FUS (Figure 4). MR ARFI has been performed using customary MR pulse sequences, such as linescan [80], 2D gradient-echo [81] or spin-echo [82]. Moreover, the technique has recently been used in the development of single-shot, echo-planar imaging (EPI) versions in vitro [83] and in vivo [84]. Quantitative displacements obtained with MR ARFI also give an indirect estimation of the FUS intensity at the target, and are thus used as an optimization parameter in MR-guided adaptive focusing procedures [82,84–86]. The clinical setup for various clinical indications can pose challenges for the design of effective MR ARFI coils because of the relative positions of transducers, acoustic coupling medium and patient anatomy. Research toward indication-specific coil design is underway for a variety of devices and indications, including breast and brain [87].
FUS challenges
Bone, such as the skull, has a higher ultrasonic absorption coefficient than soft tissue, and thus can heat significantly during FUS treatment [88]. Thermal conduction can put adjacent tissue, such as the surface of the brain, at risk from damage. To alleviate this risk, transcranial FUS systems are designed to evenly distribute the energy over a large surface of the skull to lower the power density on the skull (and the heat) compared with the focus. The thalamus is an ideal target for current transcranial FUS systems because of its central location in the brain. It is the best case scenario for optimizing the ratio between the acoustic energy at the target compared with energy deposition in the skull bone. However, clinicians are eager to target tissues beyond the thalamus to enable treatment of other clinical conditions. The goal is to extend the treatment envelope to promising sites, such as the anterior cingulate cortex, the amygdala, the hippocampus, the trigeminal nerve and superficial areas of the brain. Simulation-based [89,90] or experimental based phantom approaches are being developed to investigate the possibility of targeting these challenging targets [91].
As described previously, the liver is shadowed by the ribs. It was first suggested in the late 1990s to use phased arrays to sonicate in between the ribs, but experimental testing was not feasible at that time [92,93]. Multi-element phased arrays with hundreds of elements have since been developed [94–96]. Temperature elevation on the surface of the ribs can then be reduced by turning off the elements located in front of rib bones, as shown numerically by Liu et al. [97] and Bobkova et al. [98], and as demonstrated in vivo by Quesson et al. [99]. Aubry et al. implemented a time-reversal based technique in vitro with a 300 element semirandom array to decrease the temperature elevation on the rib surface to a negligible level (a mean of 0.3°C) [100]. This can be achieved automatically and noninvasively by computing the decomposition of the time reversal operator based on the backscattered echoes, as demonstrated in vitro [101,102]. To date, such approaches have not been implemented on clinical devices.
For US-guided FUS, US-based techniques have been able to track the 3D motion of biological tissues locally [103–105]. Such an approach is based on tracking temporal shifts in the backscattered US signals, resulting from the displacements of the tissues. The main advantage of the US-based method is the high penetration rate of US in the human body and its real-time capabilities. Hence, the natural ultrasonic scatterers in biological tissue can be used as markers to track the local motion of tissues located deep within organs. Once the 3D movement of the organ is measured, the FUS beam can be electronically steered in order to compensate for this tissue motion in real time. Marquet et al. achieved motion compensation ten-times per second by interleaving ultrasonic motion detection during the first 20 ms, followed by electronic beam steering calculation and hardware phase adjustment (10 ms) and 70 ms FUS sonication, allowing a 70% duty cycle while tracking pig liver motion in vivo [106]. Several MR-based motion detection techniques have also been proposed [107,108]. Motion detection and beam-tracking are not currently available on clinical devices.
■Financial investment
There is clearly a need for funding at all stages along the pathway in Figure 3. FUS funding has historically been provided by government agencies including NIH and DOD and by private investors through their funding of the device manufacturers. However, federal agencies are more likely to target investments into basic and discovery stage research, a stage that most FUS applications have moved beyond. FUS research requires targeted investments in the translational preclinical and early clinical stage, a funding regime typically filled by industry and venture capitalists. However, given the economic climate, many venture capitalists and industry are more hesitant to invest in potentially risky medical research. The FUS Foundation (FUSF) has also provided seed funding for many translational technical, preclinical and clinical projects to de-risk the technology for other investors. For the future advancement of image-guided FUS technology in the broad range of potential clinical applications, more public and private investment, particularly in translational research, will be necessary. The FUSF is dedicated to developing and fostering unique partnerships with government agencies and other private organizations, including disease-specific foundations, in order to drive the field forward.
■Regulatory approval
The FDA approval process for image-guided FUS devices and treatment procedures can be lengthy, costly and uncertain. The average time it takes for new medical device approvals through the PMA pathway (the pathway taken by most image-guided FUS devices) is 54 months from first communication with the FDA to device approval [109]. The average total cost from new concept to product approval is US$94 million, with approximately US$75 million due to costs associated with FDA approval [109]. Such cost and duration can be prohibitive for many device manufacturers, primarily small businesses with limited resources. Furthermore, the pathway for approval of image-guided FUS therapies is often uncertain and not consistent across device platforms and clinical indications. New PMA applications are required for each image-guided FUS device and for each clinical indication. These factors associated with FDA approval often deter investment by the venture capital community as they deem medical devices to have a lower return on investment than other potential investments. Despite these challenges, many image-guided FUS device manufacturers have found the regulatory process to be improving and that this is not the greatest of their challenges.
■Insurance reimbursement
Rather, perhaps the most significant challenge to image-guided FUS adoption in clinical practice has been that of insurance reimbursement. In part, this is due to lack of the appropriate clinical evidence. The evidence required for regulatory approval is not always sufficient for positive payer decisions. Therefore, extensive postmarket surveillance studies may be needed for new image-guided FUS clinical indications to receive widespread reimbursement. This issue is also clearly tied with physician and patient acceptance of this new technology. Physician acceptance and support by medical societies is critical for payer decisions on reimbursement; however, physicians are less likely to support new treatments if reimbursement is not available. Patients are often drivers in payer acceptance of new treatments; however, it is difficult to accumulate a large patient population if lack of insurance coverage requires patients to pay out of their own pocket.
With recent developments in healthcare, payment models are also changing. Payers are moving towards global payments and bundled payments, rather than the traditional fee-forservice payment structure. This could impact the way that the noninvasive treatment option of image-guided FUS is viewed by payers. Noninvasive outpatient treatment could reduce overall treatment cost for the patient and be desirable to include in a bundled payment for uterine fibroids for example.
■Clinical adoption
Another challenge for image-guided FUS for treating uterine fibroids and many cancers is its nature as a disruptive technology. For example, who should be the treating physician for a patient with uterine fibroids? Should it be the gynecologist or the interventional radiologist? Alternatively, for prostate cancer, the interventional radiologist, urologist or radiation oncologist could all potentially treat the patient with image-guided FUS. It is possible that disagreements over which specialty department should perform image-guided FUS treatments result in less physician acceptance of this new treatment. A potential solution to this issue is the development of training and credentialing programs incorporated into resident programs so that the new generation of clinicians, regardless of their chosen specialty, is capable of performing these procedures.
As image-guided FUS is still not a widespread technology, many physicians and patients are unaware of its potential to treat a wide variety of conditions. As more clinical indications are investigated, the extent of this challenge becomes even greater. Education opportunities are important for the future clinical acceptance of FUS. Patient access to FUS is limited owing to low awareness and reimbursement challenges. Many patients who are aware of this noninvasive treatment alternative and are interested in seeking it out for themselves or their loved ones find that their doctors do not offer the treatment or their insurance provider does not cover it.
■Role of FUSF
The FUSF has a mission to advance the development and clinical adoption of image-guided FUS as a technology platform for the treatment of a variety of clinical conditions. FUSF seeks to identify and address the barriers or choke points along the innovation pathway as described above. In this way, FUSF is able to catalyze this pathway by emphasizing activities that complement or supplement, such as fostering collaborations, funding translational research and influencing the direction of the field. In doing so, FUSF identifies and eliminates rate-limiting steps such as technology shortfalls, gaps in the current funding landscape, lack of evidence, regulatory approval hurdles, and acceptance from patients, physicians and insurers. This 360° perspective is essential to drive the adoption of FUS as a new therapy.
Conclusion
Although image-guided FUS remains an earlystage technology, significant progress has been made in advancing this medical therapy platform towards clinical adoption for the treatment of a wide range of conditions. Current FUS clinical indications throughout the world include uterine fibroids, pain palliation for bone metastases, prostate cancer, essential tremor, neuropathic pain and tremor-dominant Parkinson’s disease. MR and US imaging are critical to enable this noninvasive therapy, particularly for guidance to the region of interest and realtime treatment monitoring. However, there are still significant challenges that remain along the complex FUS innovation pathway. Technical advancements are required to expand the therapeutic window for FUS therapy and to improve treatment monitoring. This would enable safe and effective treatment of conditions, such as brain tumors, epilepsy and liver tumors. Barriers to full clinical adoption of image-guided FUS, including adequate funding of translational research, regulatory approval, insurance reimbursement and physician and patient awareness and acceptance, must also be overcome.
Future perspective
The last several years have shown tremendous progress in the field of image-guided FUS. Since 2006 and the founding of the FUSF, there has been a 70% increase in the number of diseases and conditions being treated or in clinical studies; 220% growth in the number of FUS articles published in professional medical journals; more than a dozen new device manufacturers entering the field; and a tripling of FUS research funding from the NIH, from US$6.5 million in 2006 to US$20.2 million in 2012. FUSF has been critical to this rapid advancement towards clinical adoption, through targeted investments in research, education, advocacy and collaborative efforts. Further public and private investments in image-guided FUS, from a range of stakeholders, should further catalyze the field over the next 5–10 years. In 10 years, several image-guided FUS clinical applications – uterine fibroids, neurological disorders and oncological diseases – should be FDA approved and fully reimbursed. Widespread clinical adoption of image-guided FUS for the treatment of these various clinical disorders will give thousands of patients a noninvasive and radiation-free treatment alternative to improve their health and quality of life.
Financial & competing interests disclosure
N Kassell is an InSightec shareholder; J Foley and A Hananel are former employees of InSightec; J-F Aubry is a former consultant for SuperSonic Imagine. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

References
- Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery – a general review. Expert Opin. Drug Deliv. 1(1), 37–56 (2004).
- Catane R, Beck A, Inbar Y et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases – preliminary clinical experience. Ann. Oncol. 18(1), 163–167 (2007).
- Liberman B, Gianfelice D, Inbar Y et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann. Surg. Oncol. 16(1), 140–146 (2009).
- Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 26(2), 179–193 (1942).
- Fry WJ, Mosberg WH, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J. Neurosurg. 11(5), 471–479 (1954).
- Meyers R, Fry WJ, Fry FJ, Dreyer LL, Schultz DF, Noyes RF. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg. 16(1), 32–54 (1959).
- Coleman DJ, Lizzi FL, Driller J et al. Therapeutic ultrasound in the treatment of glaucoma. I. Experimental model. Ophthalmology 92(3), 339–346 (1985).
- Burgess S, Silverman R, Coleman D et al. Treatment of glaucoma with high-intensity focused ultrasound. Ophthalmology 93(6), 831 (1986).
- Valtot F, Kopel J, Haut J. Treatment of glaucoma with high intensity focused ultrasound. Int. Ophthalmol. 13(1–2), 167–170 (1989)
- Muratore R. A History of the Sonocare CST – 100: the first FDA-approved HIFU device. AIP Conference Proceedings 829(1), 508 (2006).
- Vallancien G, Chartier-Kastler E, Harouni D, Chopin D, Bougaran J. Focused extracorporeal pyrotherapy: experimental results. Eur. Urol. 20, 211–219 (1991).
- Vallancien G, Harouni M, Veillon B, Mombet A, Brisset J, Bourgaran J. Focused extracorporeal pyrotherapy: feasibility study in man. J. Endourol. 6, 173–181 (1992).
- Cline H, Schenck J, Hynynen K, Watkins R, Souza S, Jolesz F. MR-guided focused ultrasound surgery. J. Comput. Assist. Tomogr. 16, 956–965 (1992).
- Hynynen K, Darkazanli A, Unger E, Schenk J. MRI-guided non-invasive ultrasound surgery. Med. Phys. 20, 107–115 (1993).
- Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K. A precise and fast temperature mapping using water proton chemical shift. Magn. Reson. Med. 34, 814–823 (1995).
- Hynynen K, Freund W, Cline H et al. A clinical, noninvasive, MR imagingmonitored ultrasound surgery method.
- Radiographics 16, 185–195 (1996). 17 Hynynen K, Pomeroy O, Smith D et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 219, 176–185 (2001).
- Stewart E, Gedroyc W, Tempany C et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am. J. Obstet. Gynecol. 189, 48–54 (2003).
- Tempany C, Stewart E, Mcdannold N, Quade B, Jolesz F, Hynynen K. MR imagingguided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology 226, 897–905 (2003).
- Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 47(8), 1219–1236 (2002).
- Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J. Acoust. Soc. Am. 113(1), 84–93 (2003).
- Jeanmonod D, Werner B, Morel A et al. Transcranial magnetic resonance imagingguided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 32(1), 1–11 (2012).
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann. Neurol. 66(6), 858–861 (2009).
- Rabkin BA, Zderic V, Vaezy S. Hyperecho in ultrasound images of HIFU therapy: involvement of cavitation. Ultrasound Med. Biol. 31(7), 947–956 (2005).
- Gombos EC, Kacher DF, Furusawa H, Namba K. Breast focused ultrasound surgery with magnetic resonance guidance. Top. Magn. Reson. Imaging 17(3), 181–188 (2006).
- Wu F, Wang ZB, Chen WZ et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason. Sonochem. 11(3–4), 149–154 (2004).
- Rouviere O, Souchon R, Salomir R, Gelet A, Chapelon JY, Lyonnet D. Transrectal highintensity focused ultrasound ablation of prostate cancer: effective treatment requiring accurate imaging. Eur. J. Radiol. 63(3), 317–327 (2007).
- Weeks E, Platt M, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain – case series of an innovative new technique. Eur. Radiol. 22(12), 2822–2835 (2012).
- Wu F, Wang ZB, Chen WZ et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann. Surg. Oncol. 11(12), 1061–1069 (2004).
- Kennedy J, Wu F, Ter Haar G et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 42(1), 931–935 (2004).
- Chapman A, Ter Haar G. Thermal ablation of uterine fibroids using MR-guided focused ultrasound-a truly non-invasive treatment modality. Eur. Radiol. 17(10), 2505–2511 (2007).
- Neppiras E, Noltingk B. Cavitation produced by ultrasonics: theoretical conditions for the onset of cavitation. Proc. Phys. Soc. 64(12), 1032 (1951).
- Khokhlova TD, Canney MS, Lee D et al. Magnetic resonance imaging of boiling induced by high intensity focused ultrasound. J. Acoust. Soc. Am. 125(4), 2420–2431 (2009).
- Cole R. Boiling nucleation. In: Advances in Heat Transfer. Harnett JP, Irvine TF Jr (Eds). Academic Press Inc., NY, USA, 85–166 (1974).
- Crouzet S, Rebillard X, Chevallier D et al. Multicentric oncologic outcomes of high-intensity focused ultrasound for localized prostate cancer in 803 patients. Eur. Urol. 58(4), 559–566 (2010).
- Crouzet S, Murat FJ, Pasticier G, Cassier P, Chapelon JY, Gelet A. High intensity focused ultrasound (HIFU) for prostate cancer: current clinical status, outcomes and future perspectives. Int. J. Hyperthermia 26(8), 796–803 (2010).
- Illing RO, Kennedy JE, Wu F et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a western population. Br. J. Cancer 93(8), 890–895 (2005).
- Wu F, Ter Haar G, Chen WR. High-intensity focused ultrasound ablation of breast cancer. Expert Rev. Anticancer Ther. 7(6), 823–831 (2007).
- Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim. Invasive Ther. Allied Technol. 15(1), 26–35 (2006).
- Ritchie RW, Leslie T, Phillips R et al. Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up. BJU Int. 106(7), 1004–1009 (2010).
- Orsi F, Zhang LA, Arnone P et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am. J. Roentgenol. 195(3), W245–W252 (2010).
- Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 116(16), 3934–3942 (2010).
- Parker DL, Smith V, Sheldon P, Crooks LE, Fussell L. Temperature distribution measurements in two-dimensional NMR imaging. Med. Phys. 10(3), 321–325 (1983).
- Mcdannold N. Quantitative MRI-based temperature mapping based on the proton resonant frequency shift: review of validation studies. Int. J. Hyperthermia 21(6), 533–546 (2005).
- Rieke V, Butts Pauly K. MR thermometry. J. Magn. Reson. Imaging 27(2), 376–390 (2008).
- Peters RT, Hinks RS, Henkelman RM. Ex vivo tissue – type independence in proton – resonance frequency shift MR thermometry. Magn. Reson. Med. 40(3), 454–459 (1998).
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 10(6), 787–800 (1984).
- Dewey W. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperthermia 10(4), 457–483 (1994).
- Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet. Gynecol. 110(2 Pt 1), 279–287 (2007).
- Okada A, Morita Y, Fukunishi H, Takeichi K, Murakami T. Non-invasive magnetic resonance-guided focused ultrasound treatment of uterine fibroids in a large Japanese population: impact of the learning curve on patient outcome. Ultrasound Obstet. Gynecol. 34(5), 579–583 (2009).
- Rabinovici J, David M, Fukunishi H, Morita Y, Gostout BS, Stewart EA. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil. Steril. 93(1), 199–209 (2010).
- Blana A, Walter B, Rogenhofer S, Wieland WF. High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urology 63(2), 297–300 (2004).
- Blana A, Murat FJ, Walter B et al. First analysis of the long-term results with transrectal HIFU in patients with localised prostate cancer. Eur. Urol. 53(6), 1194–1203 (2008).
- Chopra R, Baker N, Choy V et al. MRI-compatible transurethral ultrasound system for the treatment of localized prostate cancer using rotational control. Med. Phys. 35, 1346 (2008).
- Crouzet S, Murat F, Rouviere O et al. 76 oncological outcomes of high-intensity focused ultrasound for localized prostate cancer in 880 consecutive patients. Eur. Urol. Suppl. 10(2), 51 (2011).
- Thuroff S, Chaussy C. High intensity focused pulsed ultrasound (HIFU) for local ablation of prostate carcinoma. Urologe A. 46(9), 1092 (2007).
- Liberman B, Gianfelice D, Inbar Y et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann. Surg. Oncol. 16(1), 140–146 (2009).
- Turkevich V, Savelyeva V, Kanaev S, Dunaevsky I, Krzhivitsky P. Treatment of painful bone metastases with magnetic resonance guided focused ultrasound. Eur. J. Cancer 47, 227–228 (2011).
- Monteith S, Sheehan J, Medel R et al. Potential intracranial applications of magnetic resonance-guided focused ultrasound surgery: a review. J. Neurosurg. 118(2), 215–221 (2013).
- Marquet F, Pernot M, Aubry JF et al. Non-invasive transcranial ultrasound therapy based on a 3D CT scan: protocol validation and in vitro results. Phys. Med. Biol. 54(9), 2597–2613 (2009).
- Moser D, Zadicario E, Schiff G, Jeanmonod D. Measurement of targeting accuracy in focused ultrasound functional neurosurgery: technical note. Neurosurg. Focus 32(1), E2 (2012).
- Britt RH, Pounds DW, Lyons BE. Feasibility of treating malignant brain tumors with focused ultrasound. Prog. Exp. Tumor Res. 28, 232–245 (1984).
- Chauvet D, Marsac L, Pernot M et al. Targeting accuracy of transcranial magnetic resonance-guided high-intensity focused ultrasound brain therapy: a fresh cadaver model: laboratory investigation. J. Neurosurg. 1–7 (2013).
- Okada A, Murakami T, Mikami K et al. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn. Reson. Med. Sci. 5(3), 167–171 (2006).
- Kopelman D, Inbar Y, Hanannel A et al. Magnetic resonance-guided focused ultrasound surgery (MRgFUS): ablation of liver tissue in a porcine model. Eur. J. Radiol. 59(2), 157–162 (2006).
- Orsi F, Arnone P, Chen W, Zhang L. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J. Cancer Res. Ther. 6(4), 414 (2010).
- Li YY, Sha WH, Zhou YJ, Nie YQ. Short and long term efficacy of high intensity focused ultrasound therapy for advanced hepatocellular carcinoma. J. Gastroenterol.
- Hepatol. 22(12), 2148–2154 (2007). 68 Gateau J, Aubry J, Chauvet D, Boch A, Fink M, Tanter M. In vivo bubble nucleation probability in sheep brain tissue. Phys. Med. Biol. 56(22), 7001 (2011).
- Gateau J, Aubry J-F, Pernot M, Fink M, Tanter M. Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58(3), 517–532 (2011).
- Pernot M, Tanter M, Bercoff J, Waters KR, Fink M. Temperature estimation using ultrasonic spatial compound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 51(5), 606–615 (2004).
- Liu D, Ebbini ES. Real-time 2-D temperature imaging using ultrasound. IEEE Trans. Biomed. Eng. 57(1), 12–16 (2010).
- Bercoff J, Pernot M, Tanter M, Fink M. Monitoring thermally-induced lesions with supersonic shear imaging. Ultrason. Imaging 26(2), 71–84 (2004).
- Arnal B, Pernot M, Tanter M. Monitoring of thermal therapy based on shear modulus changes: I. shear wave thermometry. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58(2), 369–378 (2011).
- Todd N, Adluru G, Payne A, Dibella EV, Parker D. Temporally constrained reconstruction applied to MRI temperature data. Magn. Reson. Med. 62(2), 406–419 (2009).
- Todd N, Vyas U, De Bever J, Payne A, Parker DL. Reconstruction of fully three dimensional high spatial and temporal resolution MR temperature maps for retrospective applications. Magn. Reson. Med. 67(3), 724–730 (2012).
- Ostrovsky L, Sutin A, Il’inskii Y, Rudenko O, Sarvazyan A. Radiation force and shear motions in inhomogeneous media. J. Acoust. Soc. Am. 121, 1324 (2007).
- Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 24(9), 1419–1435 (1998).
- Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med. Biol. 28(2), 227–235 (2002).
- Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 51(4), 396–409 (2004).
- McDannold N, Maier SE. Magnetic resonance acoustic radiation force imaging. Med. Phys. 35, 3748 (2008).
- Huang Y, Curiel L, Kukic A, Plewes DB, Chopra R, Hynynen K. MR acoustic radiation force imaging: in vivo comparison to ultrasound motion tracking. Med. Phys. 36(6), 2016–2020 (2009).
- Larrat B, Pernot M, Montaldo G, Fink M, Tanter M. MR-guided adaptive focusing of ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 57(8), 1734–1737 (2010).
- Larrat B, Pernot M, Aubry JF et al. MR-guided transcranial brain HIFU in small animal models. Phys. Med. Biol. 55(2), 365–388 (2010).
- Marsac L, Chauvet D, Larrat B et al. MR-guided adaptive focusing of therapeutic ultrasound beams in the human head. Med. Phys. 39, 1141 (2012).
- Hertzberg Y, Volovick A, Zur Y, Medan Y, Vitek S, Navon G. Ultrasound focusing using magnetic resonance acoustic radiation force imaging: application to ultrasound transcranial therapy. Med. Phys. 37(6), 2934–2942 (2010).
- Kaye EA, Hertzberg Y, Marx M et al. Application of Zernike polynomials towards accelerated adaptive focusing of transcranial high intensity focused ultrasound. Med. Phys. 39, 6254 (2012).
- Minalga E, Payne A, Merrill R et al. An 11-channel radio frequency phased array coil for magnetic resonance guided highintensity focused ultrasound of the breast.
- Magn. Reson. Med. 69(1), 295–302 (2013). 88 Goss S, Frizzell L, Dunn F. Ultrasonic absorption and attenuation in mammalian tissues. Ultrasound Med. Biol. 5(2), 181–186 (1979).
- Pulkkinen A, Huang Y, Song J, Hynynen K. Simulations and measurements of transcranial low-frequency ultrasound therapy: skull-base heating and effective area of treatment. Phys. Med. Biol. 56(15), 4661 (2011).
- Pinton GF, Aubry J-F, Fink M, Tanter M. Numerical prediction of frequency dependent 3D maps of mechanical index thresholds in ultrasonic brain therapy. Med. Phys. 39(1), 455–467 (2012).
- Monteith SJ, Harnof S, Medel R et al. Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance–guided focused ultrasound: laboratory investigation. J. Neurosurg. 118(5), 1035–1045 (2013).
- Mcgough RJ, Kessler M, Ebbini E, Cain C. Treatment planning for hyperthermia with ultrasound phased arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 43(6), 1074–1084 (1996).
- Botros YY, Ebbini ES, Volakis JL. Two-step hybrid virtual array ray (VAR) technique for focusing through the rib cage. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 45(4), 989–1000 (1998).
- Daum DR, Buchanan MT, Fjield T, Hynynen K. Design and evaluation of a feedback based phased array system for ultrasound surgery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 45(2), 431–438 (1998).
- Pernot M, Aubry JF, Tanter M, Thomas JL, Fink M. High power transcranial beam steering for ultrasonic brain therapy. Phys. Med. Biol. 48(16), 2577–2589 (2003).
- Ebbini ES, Yao H, Shrestha A. Dual-mode ultrasound phased arrays for image-guided surgery. Ultrason. Imaging 28(2), 65–82 (2006).
- Liu HL, Chang H, Chen WS, Shih TC, Hsiao JK, Lin WL. Feasibility of transrib focused ultrasound thermal ablation for liver tumors using a spherically curved 2D array: a numerical study. Med. Phys. 34(9), 3436–3448 (2007).
- Bobkova S, Gavrilov L, Khokhlova V, Shaw A, Hand J. Focusing of high-intensity ultrasound through the rib cage using a therapeutic random phased array. Ultrasound Med. Biol. 36(6), 888–906 (2010).
- Quesson B, Laurent C, Maclair G et al. Real time volumetric MRI thermometry of focused ultrasound ablation in vivo: a feasibility study in pig liver and kidney. NMR Biomed. 24(2), 145–153 (2011).
- Aubry JF, Pernot M, Marquet F, Tanter M, Fink M. Transcostal high-intensity-focused ultrasound: ex vivo adaptive focusing feasibility study. Phys. Med. Biol. 53(11), 2937–2951 (2008).
- Cochard E, Prada C, Aubry J-F, Fink M. Ultrasonic focusing through the ribs using the DORT method. Med. Phys. 36, 3495 (2009).
- Cochard E, Aubry J, Tanter M, Prada C. Adaptive projection method applied to threedimensional ultrasonic focusing and steering through the ribs. J. Acoust. Soc. Am. 130, 716–723 (2011).
- Hsu A, Miller N, Evans P, Bamber J, Webb S. Feasibility of using ultrasound for real-time tracking during radiotherapy. Med. Phys. 32, 1500–1514 (2005).
- Tanter M, Pernot M, Aubry JF, Montaldo G, Marquet F, Fink M. Compensating for bone interfaces and respiratory motion in high intensity focused ultrasound. Int. J. Hyperthermia 23(2), 141–151 (2007).
- Harris EJ, Miller NR, Bamber JC, Evans PM, Symonds-Tayler JRN. Performance of ultrasound based measurement of 3D displacement using a curvilinear probe for organ motion tracking. Phys. Med. Biol. 52(18), 5683–5703 (2007).
- Marquet F, Aubry J, Pernot M, Fink M, Tanter M. Optimal transcostal highintensity focused ultrasound with combined real-time 3D movement tracking and correction. Phys. Med. Biol. 56(22), 7061–7080 (2011).
- Vigen KK, Daniel BL, Pauly JM, Butts K. Triggered, navigated, multi baseline method for proton resonance frequency temperature mapping with respiratory motion. Magn. Reson. Med. 50(5), 1003–1010 (2003).
- De Senneville BD, Ries M, Bartels LW, Moonen CT. MRI-guided high-intensity focused ultrasound sonication of liver and kidney. In: Interventional Magnetic Resonance Imaging. Kahn T, Busse H (Eds). Springer, Berlin, Germany, 349–366 (2012).
- Makower J, Meer A, Denend L. FDA Impact On US Medical Technology Innovation: A Survey Of Over 200 Medical Technology Companies. National Venture Capital Association, VA, USA (2010).
- Athanasiou A, Tardivon A, Tanter M et al. Breast lesions: quantitative elastography with supersonic shear imaging – preliminary results. Radiology 256(1), 297–303 (2010).
- Chenot J, Melodelima D, N’djin WA, Souchon R, Rivoire M, Chapelon JY. Intra-operative ultrasound hand-held strain imaging for the visualization of ablations produced in the liver with a toroidal high intensity focused ultrasound transducer: first in vivo results. Phys. Med. Biol. 55(11), 3131–3144 (2010).
- Liu HL, Li ML, Shih TC et al. Instantaneous frequency-based ultrasonic temperature estimation during focused ultrasound thermal therapy. Ultrasound Med. Biol. 35(10), 1647–1661 (2009).
- Peng S, Xiong Y, Li K et al. Clinical utility of a microbubble-enhancing contrast (‘SonoVue’) in treatment of uterine fibroids with high intensity focused ultrasound: a retrospective study. Eur. J. Radiology 81(12), 3832–3838 (2012).