Special Issue Article - Imaging in Medicine (2011) Volume 3, Issue 5
Image-guided percutaneous breast biopsies
Stephanie K Patterson*
Clinical Associate Professor, Department of Radiology, Division of Breast Imaging, University of Michigan Medical Center, 1500 East Medical Center Drive, MIB Rm C404, Ann Arbor, MI 48109, USA
- *Corresponding Author:
- Stephanie K Patterson
Clinical Associate Professor, Department of Radiology
Division of Breast Imaging, University of Michigan Medical Center
1500 East Medical Center Drive, MIB Rm C404
Ann Arbor, MI 48109, USA
Tel.: +1 734 936 4355
Fax: +1 734 232 5877
E-mail: spatters@umich.edu
Abstract
Diagnosis of breast cancer at its earliest stage affords women the best prognosis. The role of the breast imager has evolved from cancer screening and detection to now include diagnosis and management with the widespread adoption of image-guided core biopsy. This article will briefly discuss the current imageguided biopsy techniques for the diagnosis of suspicious breast findings, specifically, stereotactic, ultrasound and MR-guided, and the evidence in the literature emphasizing proven efficacy for each. For those lesions which cannot undergo percutaneous biopsy, this report will also discuss the use of marker placement at the time of wire localization and excision for ensuring and verifying biopsy of the appropriate lesion.
Keywords
biopsy; breast; breast cancer; breast MRI; false-negative; stereotactic; ultrasound; vacuum-assisted
An estimated 1.6 million breast biopsies are performed in the USA every year. In two studies auditing utilization practices for minimally invasive breast biopsies as the initial diagnostic procedure, it was found that approximately 30–40% of initial breast biopsies performed are actually open surgical biopsies. [1,2]. However, the accuracy of image-guided percutaneous needle or core biopsy is equivalent to open surgical biopsy, and expert opinion states that percutaneous core biopsy should essentially replace open surgical biopsy as the first diagnostic procedure for breast abnormalities [3]. The role of the breast imager has evolved from cancer screening and detection to now include diagnosis and management with the widespread adoption of image-guided core biopsy, primarily by stereotactic guidance or ultrasound (US) guidance, with 95% of percutaneous breast biopsies in the USA performed by radiologists [4].
A major benefit of image-guided percutaneous breast biopsy as the initial procedure, is the ability to establish a benign diagnosis in most cases, and the avoidance of an open surgical procedure. The overall cost of diagnosis is decreased, as well as morbidity. Less than 1% of percutaneous core biopsies are affected by severe complications, compared to 2–10% of open surgical procedures [5]. If the result is malignant, the percutaneous biopsy permits preoperative staging, acquisition of histologic and biomarker data, consultation with appropriate specialists, and planning for surgical resection and axillary nodal sampling [6,7], and overall allows the woman to undergo fewer surgeries during treatment [5].
The operator performing the core biopsy must be familiar with the imaging findings and the level of suspicion of the abnormality in question. Careful review of all prebiopsy images and correlation with the correct lesion is important. To maintain the high sensitivities and low falsenegative rates with any percutaneous core biopsy program, proper preparation, technique, and histologic correlation with appropriate follow-up should be observed. In addition, quality improvement measures, including complication rates, should be monitored and documented.
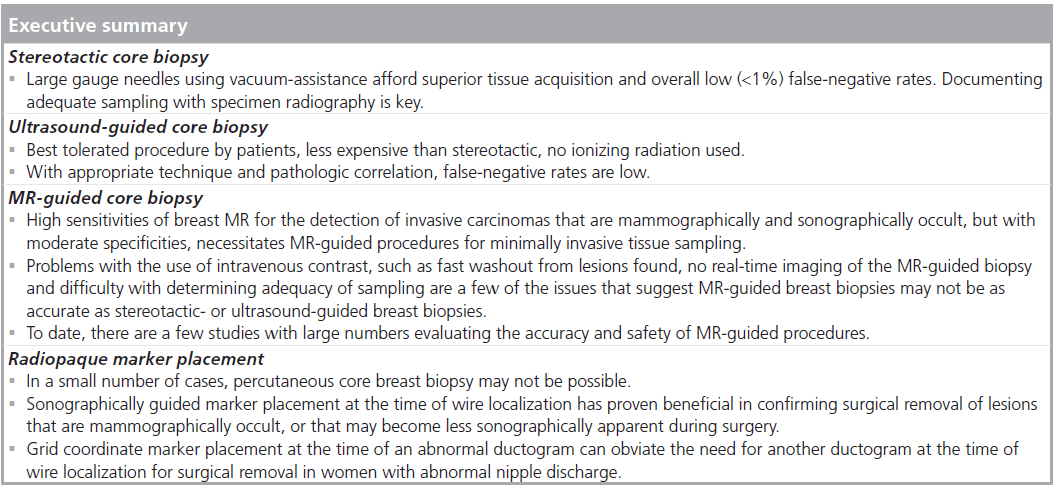
Stereotactic core biopsy
The development of an automated biopsy device approximately 20 years ago that could be used with stereotactic mammography equipment, allowed for conf ident targeting of small lesions [8]. This procedure uses x‑ray guidance to target the lesion, with the patient lying prone, and with her breast suspended through an aperture in the table, or use of an upright table. As with a mammogram, the patient’s breast is in compression. A scout image and two stereotactic images are obtained 15° from midline to target the lesion. Although both masses and calcifications can be biopsied using stereotactic guidance, the majority of imaging abnormalities now biopsied with stereotactic technique contain calcifications [9].
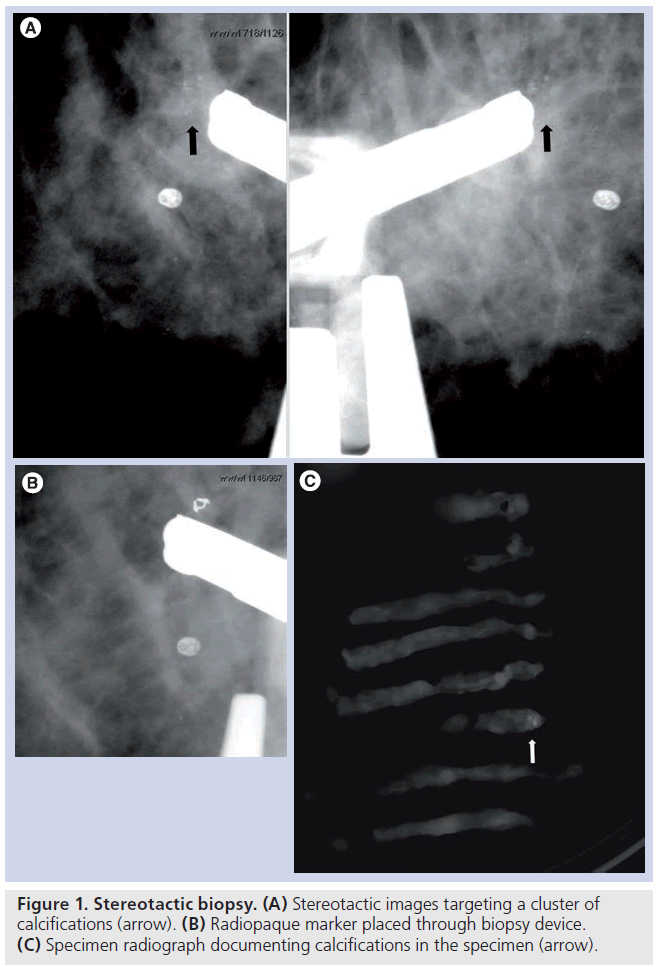
Early experience with automated biopsy needles was without vacuum assistance. However, most are now performed with vacuum assistance given the superior tissue acquisition with these devices. There are seven to 14 gauge needles available and most biopsies are performed with 11 gauge or larger. This is a single pass procedure and samples are taken in a directional fashion, allowing the operator to obtain samples from the lesion where the most diagnostic portion of the lesion may be. As most of the lesions biopsied by stereotactic guidance contain calcifications, documenting adequate sampling with specimen radiography is crucial (Figure 1). In the largest validation study of stereotactic needle core breast biopsies using vacuum assistance, Jackman et al. found a less than 1% overall false-negative rate, with a 4.4% rate for 14 gauge core needles and 0.45% for 11 gauge core needles, demonstrating a particular advantage for larger gauge needles. There was also more than 2 years of follow-up of benign lesions and minimal selection bias of lesions biopsied. Emphasizing the importance of documenting adequate lesion sampling, Jackman et al. found the highest false-negative rate of 25% were in those lesions in which no calcifications were documented in the specimen radiograph [9]. As the mammographic lesion can be completely removed with the biopsy, a radiopaque marker or microclip should be placed after tissue sampling [10], to document appropriate lesion sampling and for targeting of the suspicious area if further intervention is needed.
Figure 1. Stereotactic biopsy. (A) Stereotactic images targeting a cluster of calcifications (arrow). (B) Radiopaque marker placed through biopsy device. (C) Specimen radiograph documenting calcifications in the specimen (arrow).
Limitations for stereotactic breast biopsies include discomfort from compression of the breast, as for a mammogram, and use of ionizing radiation. Lesions located far posteriorly or directly behind the nipple can be difficult to position. Occasionally, the breast may compress too thinly to accommodate the needle. However, manufacturers have developed various tools and different needle geometries to facilitate tissue acquisition under these circumstances.
US-guided core biopsy
For lesions clearly apparent by US, US-guided biopsy provides a quick and comfortable [11], as well as highly accurate way to obtain a tissue diagnosis of breast lesions. It is also a less expensive alternative to stereotactic core biopsy and excisional biopsy [12]. There is no exposure to ionizing radiation during the procedure and no need for compression of the breast as in stereotactic or MR-guided procedures. Unlike stereotactic core biopsies, US provides access to all areas of the breast, is faster and allows for realtime monitoring of the lesion. The operator can see and document needle placement, which can be particularly important for small, deep, mobile or vaguely palpable lesions. Many palpable breast lesions are biopsied by US guidance for these reasons [13,14]. The two largest validation studies to date of US-guided core biopsies, demonstrated false-negative rates of less than 3% and sensitivities of at least 98% [14,15].
Ultrasound machines with linear near field transducers with high resolution, of at least 7 MHz, should be used. Many machines now have a multidirectional 7–12 MHz transducer. For very deep lesions, a 5 MHz transducer may be helpful. Most of the literature on US-guided core breast biopsies describe using a 14 gauge, 22 mm throw, automated needle device [13–18]. Use of a handheld 11 gauge vacuum-assisted device has also been described [19]. Advantages of the 11 gauge handheld device with vacuumassistance, as with stereotactic core biopsies, is the acquisition of larger tissue samples and the ability to biopsy smaller lesions. However, no significant differences were found in outcomes between the 11 gauge vacuum-assisted biopsy devices and the 14 gauge automated device [20]. For lesions in very dense, glandular breasts, a coaxial system may be helpful to work through the tissue to the lesion, followed by insertion of the biopsy needle through the introducer [21].
Optimal visualization of both the needle and the lesion biopsied is essential for adequate sampling. Given that the most reflected echoes are obtained with the needle perpendicular to the US beam, the long axis of the transducer should remain aligned with the lesion and skin entry site. Very slight sweeping motions of the needle, while the transducer is held in position with the lesion visualized also helps facilitate needle visualization [22]. The transducer can also be angled to make the US beam more perpendicular to the needle, or the biopsy needle can be ‘levered’ down to a more horizontal plane maximizing the number of reflected echoes generated from the needle [22]. Alternatively, some US machines have a steerable beam which can change the incident angle to 90° so the beam will be perpendicular to the needle [23].
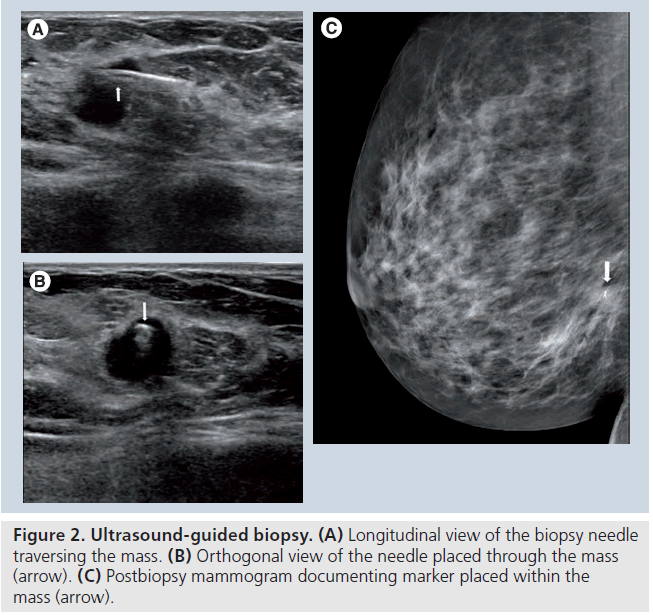
Once the biopsy device has been deployed, an orthogonal view should be taken to ensure the needle has traversed through the lesion. Documenting needle placement decreases the chance of a nondiagnostic biopsy (Figure 2). Fishman et al. analyzed the diagnostic yield for each specimen taken with a 14 gauge core needle, and correlated the findings with mass, procedural and specimen characteristics. A minimum of four specimens, those specimens that were not fragmented and those that sank when placed in formalin correlated with diagnostic tissue [17]. A marker should be placed if the lesion has greatly decreased in size or is no longer visualized. Marker placement also helps correlate with mammographic findings [24].
MRI-guided core biopsy
Figure 2. Ultrasound-guided biopsy. (A) Longitudinal view of the biopsy needle traversing the mass. (B) Orthogonal view of the needle placed through the mass (arrow). (C) Postbiopsy mammogram documenting marker placed within the mass (arrow).
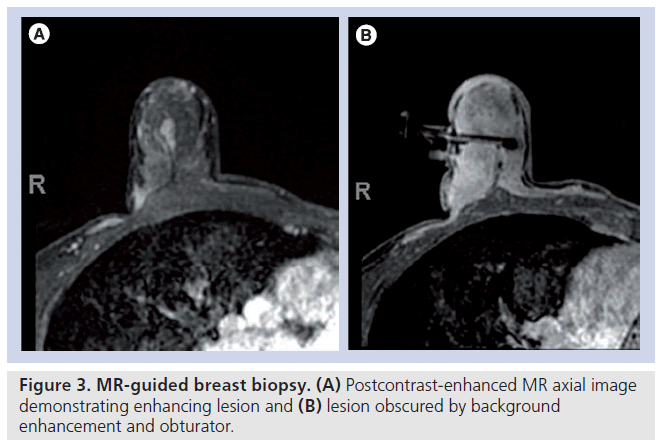
The use of breast MRI in the detection and management of breast cancer is increasing, particularly in women considered high risk (>20% lifetime risk). The sensitivity of breast MRI for the detection of invasive breast cancer has been found to be very high (approaching 100%), but of moderate specificity [25,26]. This high sensitivity allows for the detection of mammographically and sonographically occult cancer. As such, the need for MR-guided breast procedures for minimally invasive tissue sampling is rising. Key differences in MR-guided biopsies include the use of intravenous contrast material, and no real-time monitoring of tissue sampling with only immediate post biopsy MR images to determine adequacy of sampling, as MR specimen imaging is not possible. Although the breast is in compression, as in stereotactic biopsy, compression must be minimal to allow adequate contrast circulation. There can be fast contrast washout from lesions and increasing background enhancement of breast tissue, potentially making localization of lesions found at diagnostic MRI difficult [27], necessitating quick targeting and sampling (Figure 3). Nonvisualization of the lesion on the day of the procedure is well documented (12–13%) [28–32]. Puckering at the needle entry site can occur, changing the calculated depth of the lesion. The lesion may be displaced by hematoma or obscured by the needle or gas artifact [28,33,34]. These issues suggest MR-guided breast biopsies may not be as accurate as stereotactic or US-guided breast biopsies. To date, there are few studies with large numbers evaluating the accuracy and safety of MR-guided breast biopsies [5,29,31,33,35].
Given the larger tissue acquisition with vacuumassisted devices, more recent studies describe the use of 11 gauge or larger needles for MR-guided breast biopsies. Despite the larger gauge needles, there is a relatively high discordancy rate for MR-guided breast biopsy (7% in the USA, and 9% in Europe vs 3% or less for stereotactic or US guidance) [30,32,36]. Of these discordant lesions that went to surgical excision, 36% were malignant, compared with less than 3% reported in the largest, recent studies for stereotactic and US guidance [9,14,15,30]. The higher cancer rate in discordant lesions for MR-guided biopsies may be due to the higher proportion of high risk women in these studies or other technical factors [30]. In the largest multicenter study assessing the accuracy, reliability and reproducibility of MR-guided breast biopsy, a 96% success rate was demonstrated. However, of those described as not successful, no histology or follow-up was given [29]. Therefore, exact false-negative rates cannot be determined. What the appropriate follow-up should be to avoid cancer misses and keep false-negative rates low for MR-guided breast biopsies is evolving. One study recommends for benign, concordant results, follow-up no sooner than 6 months, as cancers missed did not grow before this time, and none were greater than 1 cm or node positive [33]. In the USA, MRI is an expensive resource and insurance reimbursement for follow-up breast MR may be difficult. Optimal timing for postbiopsy follow-up will need further evaluation.
The best timing for breast MR is typically around midcycle for menstruating women, given the potential for increased background enhancement during menstruation [29]. Using the same coil, field strength and patient position as that used for the diagnostic exam is essential. The patient is prone with her breast suspended through a breast coil and in compression. MR compatible equipment is used. Needle grid or pillar and post biopsy devices are used for targeting the lesion. A coaxial sheath is placed into the breast to a calculated depth, the inner stylet removed and a localizing obturator is placed through the sheath. Sagittal or axial sequences are performed to confirm accurate targeting. The obturator is then removed and the biopsy device is inserted through the sheath and tissue sampling is then performed. Placement of MR compatible metallic markers, as with other percutaneous core biopsies, achieves easy subsequent preoperative localization. ‘Second look’ US may prove helpful to find MR detected abnormalities and use for US-guided biopsy. However, careful scanning technique is required as malignant lesions are often subtle [37].
Radiopaque marker placement at time of wire localization
For a small number of cases, percutaneous biopsy of a lesion may not be feasible. The lesion in question could potentially be difficult to biopsy percutaneously due to small size, bloody nipple discharge without clinical or mammographic mass, location in the breast or patient preference for excisional biopsy [38]. Some lesions that are predominantly cystic may be difficult to clearly biopsy if the cystic component is disrupted, leaving no clear soft tissue component to target [39]. Placement of a radiopaque marker or clip at the time of percutaneous US-guided biopsy to facilitate subsequent excision is well described, particularly in lesions less than 7 mm or in those that resolve after aspiration [40]. Sonographically guided marker placement into a lesion prior to preoperative chemotherapy has also proven useful, as the lesion may no longer be detected on imaging after treatment [41]. Sonographically guided marker placement at the time of wire localization for surgical excision has been described for ensuring and verifying biopsy of the appropriate lesion in certain circumstances [42,43]. Finally, marker placement at the time of ductography has been described for ensuring excision of intraductal lesions causing nipple discharge at the time of wire localization [44]. The added cost of marker placement is minimal under these circumstances, especially given the potential larger cost of inadequate excision.
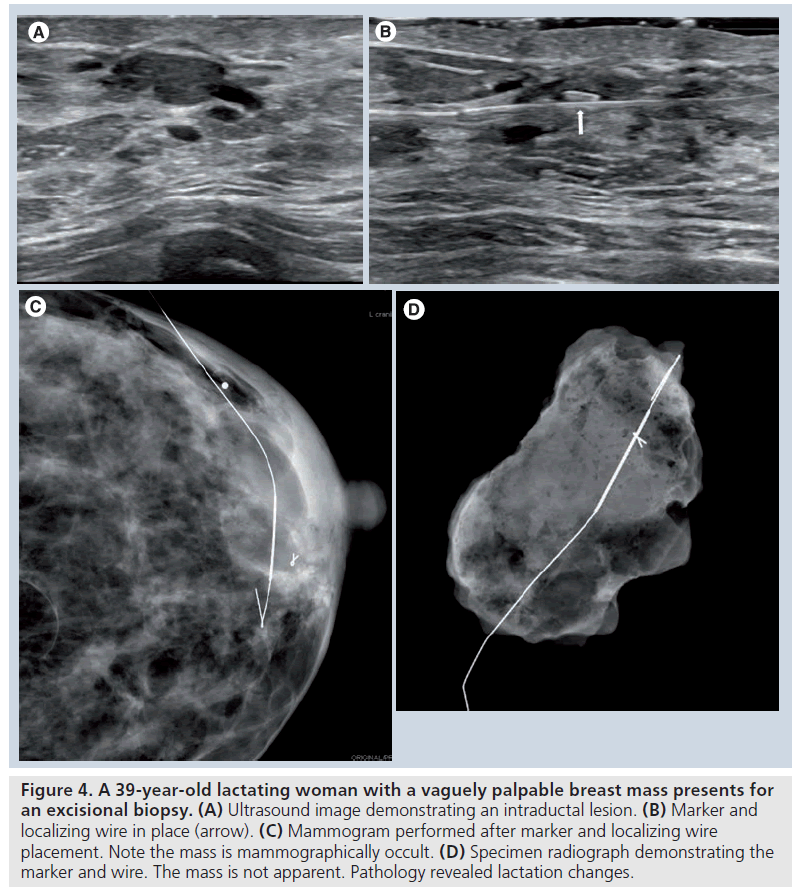
Typically, specimen radiography or specimen sonography will be performed after wire localization and excision to confirm lesion retrieval. However, if the lesion is mammographically occult, confirmation on specimen radiography will be difficult. Also, the breast imaging center may not be in close proximity to the surgical suite, making specimen imaging impossible [42]. There are also additional limitations to specimen sonography. Lesions less than 1 cm, particularly in a fatty background, may lead to false-negative specimen sonography [45]. Certain types of lesions, such as those with a significant fluid component that may be disrupted and disappear in the specimen can make sonographic, as well as radiographic, confirmation difficult [43,45]. Sonographically guided placement of a marker at the same time as wire localization has proven beneficial in ensuring surgical removal of lesions under these circumstances (Figure 4) [42,43].
Figure 4. A 39-year-old lactating woman with a vaguely palpable breast mass presents for an excisional biopsy. (A) Ultrasound image demonstrating an intraductal lesion. (B) Marker and localizing wire in place (arrow). (C) Mammogram performed after marker and localizing wire placement. Note the mass is mammographically occult. (D) Specimen radiograph demonstrating the marker and wire. The mass is not apparent. Pathology revealed lactation changes.
The prevalence of cancer in women who present with pathologic nipple discharge is low, with the most common cause an intraductal papilloma [46]. Yet discharge may be the only symptom [47]. The sensitivity for US detection of intraductal abnormalities in the setting of nipple discharge is variable [48–51], and in two reports with patients with no clinical or mammographic abnormalities, the correct cause of the nipple discharge was sonographically identified in 10–26% of cases, with all malignant cases missed [52,53].
If mammographic and US evaluation are negative in the setting of nipple discharge, a ductogram or galactogram may be requested by the referring surgeon. Although the gold standard for diagnosis is major surgical duct excision, ductography may identify distal ductal abnormalities that may not be excised with routine subareolar duct excision. There are a few series with small numbers of patients describing US-guided or galactographic-guided stereotactic, large gauge vacuum-assisted biopsy of intraductal lesions causing nipple discharge, with limited results or variable follow-up [54,55]. Percutaneous biopsy of intraductal lesions could be attempted using these techniques. The surgeon may request wire localization of the ductographic abnormality for primary excision and treatment, as most will be papillary lesions. Clear guidelines for the management of papillary lesions have not been established and there remains a high association of atypia or malignancy of those diagnosed with percutaneous core biopsy [56]. However, ductography may not be successful for a variety of reasons at the time of surgery, such as inability to cannulate the duct, contrast extravasation, or there may be no discharge at the time of the examination.
If ductography can be performed at the time of wire localization, the suspicious finding can be targeted. However, intraductal lesions causing nipple discharge that can only be detected with ductography will also be very difficult to confirm retrieval on specimen sonography or radiography [44]. Once excision is performed, the contrast material will have been absorbed, or lost and no longer present in the specimen to confirm lesion removal on specimen radiography, and with the fluid component lost, specimen sonography may be negative [45]. Grid coordinate x‑ray guided marker placement at the time of the diagnostic ductogram has been described to obviate the need for repeat duct injection at the time of wire localization (Figure 5). As in x‑rayguided wire localization, the lesion found at the time of ductography can be localized using a grid coordinate technique through a fenestrated paddle. The marker device can be placed through the fenestrated paddle and the depth is determined on the orthogonal mammographic view. In a study describing this technique, no patients were converted to a blind duct excision after marker placement and no surgeries were canceled [44].
Figure 5. Marker placement at the time of ductography. (A) Contrast material from a ductogram outlines an intraductal lesion in a woman with bloody nipple discharge (arrow). Ultrasound at the time of ductography was negative. (B) The lesion is targeted with mammographic guidance using grid coordinate technique (note placement of marker device). (C) The orthogonal view demonstrates the depth for placement of the marker device. Note placement of the tip of the marker device at the level of the intraductal lesion. (D) Mammogram demonstrating marker placement. (E) Specimen radiograph demonstrating marker in place, verifying excision of the occult lesion. Pathology revealed intraductal papilloma.
Conclusion
With an aging population needing screening and evaluation for breast cancer, and over a million breast biopsies performed in the USA every year, there will be a continued need for image-guided breast biopsy procedures, benefiting the patient with a minimally invasive, safe and potentially lower cost work-up. Long term data reveal the safety and efficacy of stereotactic and US-guided core biopsies, with the data for MR-guided breast biopsies evolving. There is a role for percutaneous, sonographically guided marker placement at the time of wire localization for surgical excision under certain circumstances, to verify lesion retrieval and limit the likelihood of inadequate excision of breast abnormalities.
Future perspective
At the beginning of the 20th century, women had to endure more and more invasive and debilitating surgery for the diagnosis and treatment of breast cancer. With an improved understanding of the biology of breast cancer and its behavior, together with the development of medical adjuvant treatments and the refinement of techniques for early detection such as mammography, we are detecting breast cancer at its earliest stages and improving survival. There should be a continued push for minimally invasive approaches for diagnosis, leaving surgery for treatment and not diagnosis. For women with breast lesions detected by MRI, further validation studies are needed to assure the accuracy and efficacy of MR-guided breast biopsies.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
* of interest
References
- Clarke-Pearson EM, Jacobson AF, Boolbol SK et al. Quality assurance initiative at one institution for minimally invasive breast biopsy as the initial diagnostic technique. J. Am. Coll. Surg. 208, 75–78 (2009).
- Gutwein LG, Ang DN, Liu H et al. Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am. J. Surg. 202(2), 127–132 (2011).
- Silverstein M. Where’s the outrage? J. Am. Coll. Surg. 208, 78–79 (2009).
- Bassett LW. The valuable role of the women’s imaging section in AJR: breast imaging. Am. J. Roentgenol. 187, 948 (2006).
- Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann. Intern. Med. 152, 238–246 (2010). & Review of the literature comparing open surgical biopsy and core-needle biopsy, primarily sterotactic and ultrasoundguided, and suggesting these techniques are almost as accurate as open surgical biopsy.
- Silverstein MJ, Recht A, Lagios MD et al. Image-detected breast cancer: state-of-theart diagnosis and treatment. J. Am. Coll. Surg. 209, 504–520 (2009).
- Liberman L, Goodstine SL, Dershaw DD et al. One operation after percutaneous diagnosis of nonpalpable breast cancer: frequency and associated factors. Am. J. Roentgenol. 178, 673–679 (2002).
- Parker SH, Lovin JD, Jobe WE et al. Stereotactic breast biopsy with a biopsy gun. Radiology 176, 741–747 (1990).
- Jackman RJ, Marzoni FA Jr, Rosenberg J. False-negative diagnoses at stereotactic vacuum-assisted needle breast biopsy: longterm follow-up of 1,280 lesions and review. Am. J. Roentgenol. 192, 341–351 (2009). & Largest validation study to date with the majority of benign lesions having more than 24 months follow-up and minimal selection bias of the literature.
- Liberman L, Dershaw DD, Morris EA, Abramson AF, Thornton CM, Rosen PP. Clip placement after stereotactic vacuum-assisted breast biopsy. Radiology 205, 417–422 (1997).
- Mainiero MB, Gareen IF, Bird CE, Smith W, Cobb C, Schepps B. Preferential use of sonographically guided biopsy to minimize patient discomfort and procedure time in a percutaneous image-guided breast biopsy program. J. Ultrasound Med. 21, 1221–1226 (2002).
- Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: use and cost-effectiveness. Radiology 208, 717–723 (1998).
- Liberman L, Ernberg LA, Heerdt A et al. Palpable breast masses: is there a role for percutaneous imaging-guided core biopsy? Am. J. Roentgenol. 175, 779–787 (2000).
- Schueller G, Jaromi S, Ponhold L et al. USguided 14-gauge core-needle breast biopsy: results of a validation study in 1352 cases. Radiology 248, 406–413 (2008). & Large validation study of ultrasound-guided core breast biopsy. 78% of lesions were surgically validated and remainder of the lesions were followed for at least 2 years.
- Youk JH, Kim E-K, Kim MJ, Oh KK. Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. Am. J. Roentgenol. 190, 202–207 (2008). & Large validation study with long-term (at least 2 years) follow-up.
- Pavel C, Koretz M, Shcharynsky S, Makarov V, Strano S. Accuracy of sonographically guided 14-gauge core-needle biopsy: results of 715 consecutive breast biopsies with at least twoyear follow-up of benign lesions. J. Clin. Ultrasound 33, 47–52 (2005).
- Fishman JE, Milikowski C, Ramsinghani R, Velasquez MV, Aviram G. US-guided coreneedle biopsy of the breast: how many specimens are necessary? Radiology 226, 779–782 (2003).
- Parker SH, Jobe WE, Dennis MA et al. US-guided automated large-core breast biopsy. Radiology 187, 507–511 (1993).
- Parker SH, Klaus AJ, McWey PJ et al. Sonographically guided directional vacuumassisted breast biopsy using a handheld device. Am. J. Roentgenol. 177, 405–408 (2001).
- Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuumassisted biopsy methods for sonographically guided core biopsy of the breast. Am. J. Roentgenol. 180, 347–351 (2003).
- Helbich TH, Mayr W, Schick S et al. Coaxial technique: approach to breast core biopsies. Radiology 203, 684–690 ( 1997).
- Youk JH, Kim E-K, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics 27,79–94 (2007). & Discussion of techniques and histologic follow-up of US-guided breast biopsy.
- Baker JA, Soo MS, Mengoni P. Sonographically guided percutaneous interventions of the breast using a steerable ultrasound beam. Am. J. Roentgenol. 172, 157–159 (1999).
- Guenin MA. Clip placement during sonographically guided large-core breast biopsy for mammographic-sonographic correlation Am. J. Roentgenol. 175, 1053–1055 (2000).
- Saslow D, Boetes C, Burke W et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 57, 75–89 (2007).
- Bluemke DA, Gatsonis CA, Chen MH et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA 292, 2735–2742 (2004).
- Lehman CD, DePeri ER, Peacock S, McDonough MD, DeMartini WB, Shook J. Clinical experience with MRI-guided vacuum-assisted breast biopsy. Am. J. Roentgenol. 184, 1782–1787 (2005).
- Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. Am. J. Roentgenol. 185, 183–193 (2005).
- Perlet C, Heywang-Kobrunner SH, Heinig A et al. Magnetic resonance-guided, vacuumassisted breast biopsy. Cancer. 106, 982–990 (2006). & Results from a prospective, European multicenter study of 538 lesions biopsied under MR guidance with vacuum-assistance. Describes a high accuracy rate, yet exact false-negative numbers difficult to ascertain from descriptions of ‘not successful’ biopsies.
- Lee J-M, Kaplan JB, Murray MP et al. Imaging histologic discordance at MRIguided 9-gauge vacuum-assisted breast biopsy. Am. J. Roentgenol. 189, 852–859 (2007). & Study from the USA with large numbers of lesions determining discordancy rate and cancer rates in discordant lesions of MRguided breast biopsy.
- Han B-K, Schnall MD, Orel SG, Rosen M. Outcome of MRI-guided breast biopsy. Am. J. Roentgenol. 191, 1798–1804 (2008).
- Hauth E, Jaeger H, Lubnau J et al. MR-guided vacuum-assisted breast biopsy with a handheld biopsy system: clinical experience and results in postinterventional MR mammography after 24 h. Eur. Radiology 18, 168–176 (2008).
- Li J, Dershaw DD, Lee CH, Kaplan J, Morris EA. MRI follow-up after concordant, histologically benign diagnosis of breast lesions sampled by MRI-guided biopsy. Am. J. Roentgenol. 193, 850–855 (2009).
- Orel SG, Rosen M, Mies C, Schnall MD. MR Imaging-guided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology 238, 54–61 (2006).
- Schrading S, Simon B, Braun M, Wardelmann E, Schild HH, Kuhl CK. MRI-guided breast biopsy: influence of choice of vacuum biopsy system on the mode of biopsy of MRI-only suspicious breast lesions. Am. J. Roentgenol. 194, 1650–1657 (2010).
- Gebauer B, Bostanjoglo M, Moesta KT, Schneider W, Schlag PM, Felix R. Magnetic resonance-guided biopsy of suspicious breast lesions with a handheld vacuum biopsy device. Acta Radiol. 47, 907–913 (2006).
- Abe H, Schmidt RA, Shah RN et al. MRdirected (“second-look”) ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings. Am. J. Roentgenol. 194, 370–377 (2010).
- Lannin DR, Ponn T, Andrejeva L, Philpotts L. Should all breast cancers be diagnosed by needle biopsy? Am. J. Surg. 192, 450–454 (2006).
- Doshi DJ, March DE, Crisi GM, Coughlin BF. Complex cystic breast masses: diagnostic approach and imaging-pathologic correlation. Radiographics 27, S53-S64 (2007).
- Berg W. Image-guided breast biopsy and management of high-risk lesions. Radiol. Clin. N. Am. 42, 935–946 (2004).
- Baron LF, Baron PL, Ackerman SJ, Durden DD, Pope TL Jr. Sonographically guided clip placement facilitates localization of breast cancer after neoadjuvant chemotherapy. Am. J. Roentgenol. 174, 539–540 (2000).
- Mercado CL, Guth AA, Toth HK, Moy L, Axelrod D, Cangiarella J. Sonographically guided marker placement for confirmation of removal of mammographically occult lesions after localization. Am. J. Roentgenol. 191, 1216–1219 (2008). & Describes the benefit of placing a marker under sonographic guidance at the time of wire localization. Enables immediate confirmation of surgical removal, but does not reduce the number of cases with close margins.
- Patterson SK, Joe A, Helvie MA. Sonographically-guided metallic marker placement at time of wire localization for intraductal or cystic lesions: a method to verify lesion retrieval. Acad. Radiol. 15, 1316–1321 (2008). & Describes sonographically guided marker placement at the time of wire localization as an efficient way to confirm retrieval of intraductal or complex cystic lesions.
- Woodward S, Daly CP, Patterson SK, Joe AI, Helvie MA. Ensuring excision of intraductal lesions: marker placement at time of ductography. Acad. Radiol. 17, 1444–1448 (2010). & Describes sonographically guided marker placement at the time of wire localization as an efficient way to confirm retrieval of intraductal lesions causing discharge.
- Mesurolle B, El-Khoury M, Hori D et al. Sonography of postexcision specimens of nonpalpable breast lesions: value, limitations, and description of a method. Am. J. Roentgenol. 186, 1014–1024 (2006).
- Mercado CL, Humele-Bena D, Singer C et al. Papillary lesions of the breast: Evaluation with stereotactic directional vacuum-assisted biopsy. Radiology 221, 650–655 (2001).
- Van Zee KJ, Ortega Perez G, Minnard E, Cohen MA. Preoperative galactography increases the diagnostic yield of major duct excision for nipple discharge. Cancer 82, 1874–1880 (1998).
- Rissanen T, Reinikainen H, Apaja-Sarkkinen M. Breast sonography in localizing the cause of nipple discharge: comparison with galactography in 52 patients. J. Ulrasound Med. 26, 1031–1039 (2007).
- Hild F, Duda VF, Albert U, Schulz KD. Ductal orientated sonography improves the diagnosis of pathological nipple discharge of the female breast compared with galactography. Eur. J. Cancer Prev. 7, S57–S62 (2000).
- Chung SY, Lee KW, Park KS, Lee Y, Bae SH. Breast tumors associated with nipple discharge. Correlation of findings on galactography and sonography. Clin. Imaging 19, 165–171 (1995).
- Nakahara H, Namba K, Watanabe R et al. A comparison of MR imaging, galactography and ultrasonography in patients with nipple discharge. Breast Cancer 10, 320–329 (2003).
- Dillon MF, Mohd Nazri SR, Nasir S et al. The role of major duct excision and micrododechtomy in the detection of breast carcinoma. BMC Cancer 6, 164 (2006).
- Vargas HI, Vargas MP, Eldrageely K, Gonzalez KD, Khalkhali I. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am. Surg. 72, 124–128 (2006).
- Reiner CS, Helbach TH, Rudas M et al. Can galactography-guided stereotactic, 11 gauge, vacuum-assisted breast biopsy of intraductal lesions serve as an alternative to surgical biopsy? Eur. Radiol. 19, 2878–2885 (2009).
- Torres-Tabanera M, Alonso-Bartolome P, Vega-Bolivar A et al. Percutaneous microductectomy with a directional vacuum-assisted system guided by ultrasonography for the treatment of breast discharge: experience in 63 cases. Acta Radiol. 49, 271-276 (2008).
- Valdes EK, Feldman SM, Boolbol SK. Papillary lesions: a review of the literature. Ann. Surg. Oncol. 14, 1009–1013 (2007).